Published online Aug 7, 2013. doi: 10.3748/wjg.v19.i29.4737
Revised: April 12, 2013
Accepted: June 1, 2013
Published online: August 7, 2013
AIM: To investigate risk factors for hepatocellular carcinoma (HCC) recurrence after living donor liver transplantation (LDLT) and efficacy of various criteria.
METHODS: From October 2000 to November 2011, 233 adult patients underwent LDLT for HCC at our institution. After excluding nine postoperative mortality cases, we analyzed retrospectively 224 patients. To identify risk factors for recurrence, we evaluated recurrence, disease-free survival (DFS) rate, survival rate, and various other factors which are based on the characteristics of both the patient and tumor. Additionally, we developed our own criteria based on our data. Next, we compared our selection criteria with various tumor-grading scales, such as the Milan criteria, University of California, San Francisco (UCSF) criteria, TNM stage, Barcelona Clinic Liver Cancer (BCLC) stage and Cancer of the Liver Italian Program (CLIP) scoring system. The median follow up was 68 (6-139) mo.
RESULTS: In 224 patients who received LDLT for HCC, 37 (16.5%) experienced tumor recurrence during the follow-up period. The 5-year DFS and overall survival rates after LDLT in all patients with HCC were 80.9% and 76.4%, respectively. On multivariate analysis, the tumor diameter {5 cm; P < 0.001; exponentiation of the B coefficient [Exp(B)], 11.89; 95%CI: 3.784-37.368} and alpha fetoprotein level [AFP, 100 ng/mL; P = 0.021; Exp(B), 2.892; 95%CI: 1.172-7.132] had significant influences on HCC recurrence after LDLT. Therefore, these two factors were included in our criteria. Based on these data, we set our selection criteria as a tumor diameter ≤ 5 cm and AFP ≤ 100 ng/mL. Within our new criteria (140/214, 65.4%), the 5-year DFS and overall survival rates were 88.6% and 81.8%, respectively. Our criteria (P = 0.001), Milan criteria (P = 0.009), and UCSF criteria (P = 0.001) showed a significant difference in DFS rate. And our criteria (P = 0.006) and UCSF criteria (P = 0.009) showed a significant difference in overall survival rate. But Milan criteria did not show significant difference in overall survival rate (P = 0.137). Among stages 0, A, B and C of BCLC, stage C had a significantly higher recurrence rate (P = 0.001), lower DFS (P = 0.001), and overall survival rate (P = 0.005) compared with the other stages. Using the CLIP scoring system, the group with a score of 4 to 5 showed a high recurrence rate (P = 0.023) and lower DFS (P = 0.011); however, the overall survival rate did not differ from that of the lower scoring group. The TNM system showed a trend of increased recurrence rate, decreased DFS, or survival rate according to T stage, albeit without statistical significance.
CONCLUSION: LDLT is considered the preferred therapeutic option in patients with an AFP level less than 100 ng/mL and a tumor diameter of less than 5 cm.
Core tip: Liver transplantation (LT) for hepatocellular carcinoma (HCC) is known to be the best therapeutic option. To obtain a good result, it is important to select appropriate LT candidates from among HCC patients. For living-donor liver transplantation (LDLT), investigation of the efficacy of such criteria will facilitate adoption of extended criteria in LDLT. We defined the selection criteria according to risk factors for recurrence based on our results and compared them with other criteria or scoring systems, such as the Milan and University of California, San Francisco criteria, tumor node metastasis and Barcelona Clinic Liver Cancer staging, and the Cancer of the Liver Italian Program scoring system.
- Citation: Choi HJ, Kim DG, Na GH, Han JH, Hong TH, You YK. Clinical outcome in patients with hepatocellular carcinoma after living-donor liver transplantation. World J Gastroenterol 2013; 19(29): 4737-4744
- URL: https://www.wjgnet.com/1007-9327/full/v19/i29/4737.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i29.4737
On the hepatocellular carcinoma (HCC), two alternative treatment options exist with curative intention, such as liver resection and liver transplantation (LT). LT, unlike hepatic resection, has the advantage that it can treat not only the tumor but also the underlying liver cirrhosis. Most LT candidates in the past have been patients with advanced HCC. The high tumor recurrence rates and low survival rates of these patients were discouraging[1,2]. However, LT was established as a suitable treatment for HCC since Mazzaferro et al[3] reported the Milan criteria in 1996. The Milan criteria improved the overall survival and disease-free survival (DFS) rates. Thereafter, LT has achieved the best results in well-selected candidates, and most international transplantation communities have adopted the Milan criteria for the treatment of HCC.
However, unfortunately, about 70% of HCC patients are diagnosed with advanced stage disease[4]. Even among patients who meet the Milan criteria, 20% or more will be removed from the waiting list because progression of HCC excludes them from the Milan criteria[5-7]. Additionally, the treatment outcome of a patient excluded from the waiting list because of HCC progression who selects other treatments is known to be very poor. Furthermore, many centers have reported good results despite expansion of the selection criteria. Therefore, the current Milan criteria are too strict, and most centers agree on the need for their expansion. Additionally, in Asian countries, potential donors for deceased donor liver transplantation (DDLT) are lacking. Therefore, living donor liver transplantation (LDLT) is emerging as an additional therapeutic option. In LDLT, it is possible to match the donor and recipient, so the decision to operate is based on the risk to the donor and the expected benefit to the recipient. Patient selection in LDLT is generally wider compared with that in DDLT. However, ethical issues exist concerning LDLT, such as the risk of the donor and the high recurrence rate of the recipients due to advanced-stage disease. Appropriate criteria are necessary for these issues. Therefore, criteria for LDLT and DDLT that are suitable for each center are necessary.
To date, various groups have attempted to expand the Milan criteria while maintaining long-term survival rates[8-12]. One was the proposal of the University of California, San Francisco (UCSF) criteria by Yao et al[8] in 2001. Most centers agree on expanding the criteria because more patients can benefit from transplantation, and the DFS and overall survival are comparable to the Milan criteria. Other systems include the TNM system that reflects cancer stage, and the Barcelona clinic liver cancer (BCLC) staging and the cancer of the liver Italian program (CLIP) staging systems that are well-known guidelines for therapy and prognosis prediction that are used in the United States and Europe. Regarding LT, investigation of the efficacy of these criteria for therapy of HCC is important.
The purpose of the present study was to evaluate the characteristics of patients and tumors according to recurrence. Subsequently, we defined the selection criteria according to our results and compared our criteria with other criteria or scoring systems, such as the Milan and UCSF criteria, TNM and BCLC staging, and the CLIP scoring system.
From October 2000 to November 2011, 233 patients underwent LDLT for HCC at our center. After excluding nine postoperative mortality cases, 224 patients were evaluated. We defined the postoperative mortality as expire of patients within a month after transplantation. All LDLT patients were adults, and the right lobe was used for transplantation. The Institutional Review Board of our center approved the study design.
All patients with HCC planned for transplantation were evaluated preoperatively by computed tomography (CT) of the abdomen and chest, enhanced magnetic resonance image (MRI), positron emission tomography-CT (PET-CT), bone scintigraphy, gastrofiberscopy, and colonoscopy. Alpha fetoprotein (AFP) and protein induced by vitamin K absence or angiotensin-II (PIVKA-II) were also evaluated as tumor markers. Contraindications of LT in patients with HCC included tumor thrombus in the main portal vein, regional lymph node metastasis, and distant metastasis. We reviewed the tumor diameter and number of tumors based on the pathologic report.
LDLT was performed according to a standard technique using a modified right lobe with middle hepatic vein reconstruction. For ascites, aspiration and cytology were performed before beginning the operation. When lymph node enlargement was present, or in cases with suspicious metastatic disease, an intraoperative biopsy was performed. The operation was performed only in cases with negative biopsy results. Immunosuppression treatment included a regimen of a calcineurin inhibitor (Cyclosporine or Tacrolimus) as part of a dual- or triple-drug regimen with prednisone and mycophenolate mofetil (MMF). An interleukin-2 receptor blocker was administered on both the day of the operation and the fourth postoperative day. Steroids were withdrawn 1 mo after surgery, and MMF was withdrawn 6 mo after surgery. Only a low dose of a single calcineurin inhibitor was administered after this period. The immunosuppression protocol did not differ from other benign diseases. When recurrence was detected during the follow-up period, the immunosuppressive agent was changed or decreased.
For early detection of cancer recurrence, AFP and PIVKA-II were checked monthly during the first year, and then bimonthly thereafter. Abdomen CT, chest CT, and bone scintigraphy were routinely performed every 6 mo during the first 2 years, and then were performed annually. When tumor recurrence was suspected, MRI and/or PET-CT were performed.
We evaluated recurrence, the DFS rate, the survival rate, and various other factors to identify the risk of recurrence. Additionally, based on our data, we developed patient selection criteria suitable for our center. Furthermore, using our LDLT data, we evaluated and assessed criteria, such as the most frequently used Milan criteria, the UCSF criteria, and important therapeutic guidelines such as TNM staging, BCLC staging, and the CLIP scoring system.
Numeric data were presented as means and standard deviations or as medians and ranges. Continuous variables (means, standard deviations, medians, and ranges) were analyzed using an independent t-test or χ2 test. Multiple regression analyses were performed using Cox proportional hazards models for identification of factors independently associated with recurrence in 95%CI. DFS and 5-year survival rates were estimated using the Kaplan-Meier method, and survival curves were compared using the log-rank test. Statistical analysis was performed using SPSS version 19.0 (IBM SPSS statistics 19). Statistical significance was accepted for P values less than 0.05.
The mean age of all patients was 51.9 ± 6.92 years (range, 34-66 years), and 184 patients (82.1%) were male. Underlying liver disease was caused by HBV infection (87.9%) most commonly, followed by HCV infection (5.8%) and other causes (6.3%). The average Child-Pugh score was 8.15 ± 2.40 (range, 5-15), and the average Model for end-stage liver disease (MELD) score was 12.8 ± 7.63 (range, 1-37). The graft vs recipient weight ratio (GRWR) was 1.21% ± 0.27% (range, 0.6%-2.3%). Preoperative treatments were performed in 167 patients (74.6%). Of the 224 patients, 133 (59.4%) met Milan criteria, and 154 patients (68.8%) met UCSF criteria. During the follow-up period, 50 patients expired. The cause of death was HCC recurrence in 31 patients (62%), technical complications in nine patients, (18%), sepsis in five patients (10%), graft failure in three patients (6%), and other causes in two patients (4%). The median follow-up duration was 68 (range, 6-139) mo.
Of the 224 patients, recurrence occurred in 37 (16.5%) during the follow-up period. The number of patients who met the Milan criteria was 16 of 133 (12.0%), and of those who did not meet the Milan criteria 20 of 83 (24.1%) (P = 0.021). Most recurrences occurred within 2 years, with 26 patients (76%) experiencing recurrence within 1 year, and 30 (81%) within 2 years. Two patients experienced recurrence 5 years after transplantation. The primary recurrence site was intrahepatic in 10 patients (27%) and extrahepatic in 27 (73%). Of the extrahepatic metastasis sites, the lung was the most common primary recurrence site [10 patients (27%)]. Other recurrence sites were the brain, bone, adrenal gland, diaphragm, omentum, para-aortic lymph nodes, and neck lymph nodes.
Patient demographics and tumor characteristics were compared between the group without recurrence and the group with recurrence. The levels of AFP and PIVKA-II were compared to the averages of 50, 100 and 200 ng/mL. The AFP level showed a significant difference when compared to that of 100 ng/mL (P = 0.025). Additionally, although the compared number of PIVKA-II cases was small, the comparison itself was significant at 50, 100 and 200 ng/mL. Furthermore, the mean AFP and PIVKA-II levels seemed higher in the recurrent patient group; however, no significant differences were noted (AFP, P = 0.568; PIVKA-II, P = 0.576). The group that had received preoperative treatment showed a higher recurrence rate (P = 0.025). However, age, gender, cause of disease, Child-Pugh scores, MELD scores, and GRWRs showed no statistically significant difference. The mean maximal and total tumor diameters were higher in the recurrent group, being 5.54 ± 5.65 and 7.82 ± 6.56 cm, respectively, in the recurrent group and 2.76 ± 1.98 and 4.21 ± 2.88 cm, respectively, in the non-recurrent group (P = 0.006 and 0.005, respectively). Additionally, when the recurrent and non-recurrent groups were divided according to maximal diameter and total diameter over 5 cm, both groups had significantly higher recurrence rates (P < 0.001 and P = 0.011, respectively). The tumor number was significantly different between the non-recurrent and recurrent groups when the cutoff numbers were 5 and 7 (P = 0.046 and P = 0.049, respectively). Microvascular invasion was significant different between the non-recurrent and recurrent groups (P = 0.039). There was no difference in the Edmondson-Steiner (E-S) grade (P = 0.3; Table 1).
| Variables | Non-recurrent (n = 187) | Recurrent HCC (n = 37) | P-value |
| Patient characteristics | |||
| Age (yr) | 52.05 ± 6.93 | 50.89 ± 6.88 | 0.354 |
| Gender (male: female) | 151 (80.7):36 (19.3) | 33 (89.2):4 (10.8) | 0.221 |
| HBV: HCV: Other cause | 164:11:12 | 33:2:2 | 0.965 |
| Child-Pugh score | 8.15 ± 2.41 | 8.14 ± 2.37 | 0.972 |
| MELD score | 12.76 ± 7.25 | 13.05 ± 9.45 | 0.858 |
| GRWR (%) | 1.20 ± 0.28 | 1.24 ± 0.24 | 0.454 |
| ≤ 1 (n = 29) | 26 (89.7) | 3 (10.3) | 0.327 |
| > 1 (n = 193) | 159 (82.4) | 34 (17.6) | |
| AFP (ng/mL) | 170.5 ± 806.2 | 249.94 ± 481.37 | 0.568 |
| ≤ 100 (n = 163) | 142 (87.1) | 21 (12.9) | 0.025 |
| > 100 (n = 59) | 44 (74.6) | 15 (25.4) | |
| PIVKA-II (mAU/mL) | 189.6 ± 1150.3 | 397.2 ± 674.5 | 0.576 |
| ≤ 100 | 99 (94.3) | 6 (5.7) | 0.018 |
| > 100 | 14 (77.8) | 4 (22.2) | |
| Pre-transplant treatment | 0.025 | ||
| No (n = 57) | 53 (93.0) | 4 (7.0) | |
| Yes (n = 167) | 134 (80.2) | 33 (19.8) | |
| Pathologic characteristics | |||
| Maximal diameter (cm) | 2.76 ± 1.98 | 5.54 ± 5.65 | 0.006 |
| ≤ 5 (n = 196) | 170 (86.7) | 26 (13.3) | < 0.001 |
| > 5 (n = 20) | 10 (50.0) | 10 (50.0) | |
| Total diameter (cm) | 4.21 ± 2.88 | 7.82 ± 6.56 | 0.005 |
| ≤ 9 (n = 185) | 162 (87.6) | 23 (12.4) | 0.001 |
| > 9 (n = 19) | 11 (57.9) | 8 (42.1) | |
| Tumor number | 2.46 ± 2.18 | 3.06 ± 3.11 | 0.282 |
| ≤ 5 (n = 193) | 164 (85.0) | 29 (15.0) | 0.046 |
| > 5 (n = 22) | 15 (68.2) | 7 (31.8) | |
| Microvascular invasion | 0.039 | ||
| No (n = 163) | 140 (85.9) | 23 (14.1) | |
| Yes (n = 44) | 32 (72.7) | 12 (27.3) | |
| E-S grade | 0.300 | ||
| Low (I, II) (n = 108) | 93 (86.1) | 15 (13.9) | |
| High (III, IV) (n = 81) | 65 (80.2) | 16 (19.8) | |
To identify factors related to recurrence, a multivariate analysis of factors that had shown statistical significance in univariate analysis was performed. The prognostic factors affecting recurrence included a serum AFP level > 100 ng/mL, a PIVKA-II level > 100 mAU/mL, a tumor diameter > 5 cm, a total tumor diameter > 9 cm, a tumor number > 5, microvascular invasion, and pretransplantation treatment in univariate analyses. In multivariate analysis, a maximal tumor diameter > 5 cm {exponentiation of the B coefficient [Exp(B)], 11.89; 95%CI: 3.784-37.368; P < 0.001} and an AFP level > 100 ng/mL [Exp(B), 2.892; 95%CI: 1.172-7.132; P = 0.021] had a significant influence on recurrence (Table 2). Based on these data, we set our selection criteria [Catholic Medical Center (CMC) criteria] as a tumor diameter ≤ 5 cm and AFP ≤ 100 ng/mL. When both criteria were met, the case was classified as within criteria (n = 138, 66.0%), and when at least one criterion was not met, the case was classified as beyond criteria (n = 71, 34.0%).
| Variables | P-value | Exp(B) | 95%CI |
| AFP (100 ng/mL) | 0.021 | 2.892 | 1.172-7.132 |
| Pre-transplant treatment | 0.114 | 2.421 | 0.742-7.897 |
| Maximal diameter (5 cm) | < 0.001 | 11.891 | 3.784-37.368 |
| Total diameter (9 cm) | 0.142 | 2.633 | 0.754-9.194 |
| Tumor number (5) | 0.712 | 1.373 | 0.262-7.203 |
| Microvascular invasion | 0.27 | 1.768 | 0.653-4.784 |
We applied our data to various conventional criteria and therapeutic guidelines and compared the results with those of our CMC criteria. The data were applied to the Milan and UCSF criteria, TNM and BCLC stagings, the CLIP scoring system, and our CMC criteria were evaluated in terms of recurrence. In the Milan criteria and UCSF criteria, the patient group not meeting the criteria demonstrated a significantly higher recurrence rate than the group meeting the criteria (Milan, P = 0.021; UCSF, P = 0.002). In the CMC criteria specified above, the within-criteria group showed a recurrence rate of 10.0%, and the beyond-criteria group showed a recurrence rate of 28.4%. The difference was statistically significant (P = 0.001). In BCLC staging, the C stage showed a significantly higher recurrence rate compared with the 0, A, and B stages (P < 0.001); however, the differences among the 0, A, and B stages were not significant. In evaluating the CLIP scoring system, scores of 0 and 1, 2 and 3, and 4 and 5 were classified as three groups. The group with scores of 4 and 5 had a significantly higher recurrence rate than the other two groups (P = 0.023). The group with scores of 0 and 1, as well as that with scores of 2 and 3, showed no difference in recurrence. However, in the TNM staging, although recurrence showed an increasing trend with higher T status, there were no significant differences in recurrence (P = 0.325; Table 3).
| Variables | Non-recurrent | Recurrent | P-value |
| Milan criteria | 0.021 | ||
| Within (n = 133) | 117 (88.0) | 16 (12.0) | |
| Beyond (n = 83) | 63 (75.9) | 20 (24.1) | |
| UCSF criteria | 0.002 | ||
| Within (n = 154) | 136 (88.3) | 18 (11.7) | |
| Beyond (n = 62) | 44 (71.0) | 18 (29.0) | |
| CMC criteria | 0.001 | ||
| Within (n = 140) | 126 (90.0) | 14 (10.0) | |
| Beyond (n = 74) | 53 (71.6) | 21 (28.4) | |
| TNM | 0.325 | ||
| T1 (n = 81) | 71 (87.7) | 10 (12.3) | |
| T2 (n = 127) | 103 (81.1) | 24 (18.9) | |
| T3 (n = 7) | 5 (71.4) | 2 (28.6) | |
| BCLC | < 0.001 | ||
| 0 (n = 36) | 33 (91.7) | 3 (8.3) | |
| A (n = 82) | 73 (89.0) | 9 (11.0) | |
| B (n = 53) | 47 (88.7) | 6 (11.3) | |
| C (n = 45) | 27 (60.0) | 18 (40.0) | |
| CLIP | 0.023 | ||
| 0, 1 (n = 89) | 73 (82.0) | 16 (18.0) | |
| 2, 3 (n = 110) | 97 (88.2) | 13 (11.8) | |
| 4, 5 (n = 12) | 7 (58.3) | 5 (41.7) |
The DFS and overall survival rates according to various criteria were compared. The 5-year DFS and survival rates of total patients were 80.9% and 76.4%, respectively. The Milan criteria showed a statistically significant difference in only the DFS rate (P = 0.009). However, the UCSF criteria showed a statistically significant difference in the DFS rate (P = 0.001) and overall survival rate (P = 0.009). Regarding CMC criteria, the 5-year DFS rate (P < 0.0001) and survival rate (P = 0.0006) showed statistically significant differences. Classification according to T status showed a decreasing trend in the 5-year DFS rate (P = 0.190) and survival rate (P = 0.394) with increasing T stage, albeit without statistical significance (P = 0.190). In the BCLC staging, the 5-year DFS and survival rates among stages 0, A, and B showed no statistically significant differences. However, the 5-year DFS rate (P < 0.001) and survival rate (P = 0.005) of stage C were significantly lower than those of the other stages. The CLIP scoring system showed a statistically significant difference in only DFS (P = 0.011; Table 4).
| Criteria | Disease-free survival | Overall survival | |||||
| 3 yr | 5 yr | P-value | 3 yr | 5 yr | P-value | ||
| Milan | Within (n = 130) | 0.880 | 0.867 | 0.009 | 0.808 | 0.798 | 0.137 |
| Beyond (n = 81) | 0.730 | 0.704 | 0.733 | 0.706 | |||
| UCSF | Within (n = 150) | 0.881 | 0.869 | 0.001 | 0.822 | 0.813 | 0.009 |
| Beyond (n = 61) | 0.681 | 0.645 | 0.677 | 0.643 | |||
| CMC | Within (n = 138) | 0.899 | 0.886 | < 0.001 | 0.840 | 0.818 | 0.006 |
| Beyond (n = 71) | 0.682 | 0.656 | 0.663 | 0.663 | |||
| TNM | T1 (n = 80) | 0.890 | 0.870 | 0.190 | 0.830 | 0.830 | 0.394 |
| T2 (n = 123) | 0.788 | 0.773 | 0.753 | 0.725 | |||
| T3 (n = 7) | 0.571 | 0.571 | 0.556 | 0.556 | |||
| BCLC | 0 (n = 35) | 0.943 | 0.902 | < 0.001 | 0.882 | 0.882 | 0.005 |
| A (n = 80) | 0.878 | 0.878 | 0.805 | 0.787 | |||
| B (n = 52) | 0.854 | 0.854 | 0.878 | 0.826 | |||
| C (n = 44) | 0.578 | 0.544 | 0.549 | 0.549 | |||
| CLIP | 0,1 (n = 88) | 0.795 | 0.771 | 0.011 | 0.776 | 0.776 | 0.272 |
| 2,3 (n = 106) | 0.885 | 0.870 | 0.819 | 0.792 | |||
| 4,5 (n = 12) | 0.556 | 0.556 | 0.509 | 0.509 | |||
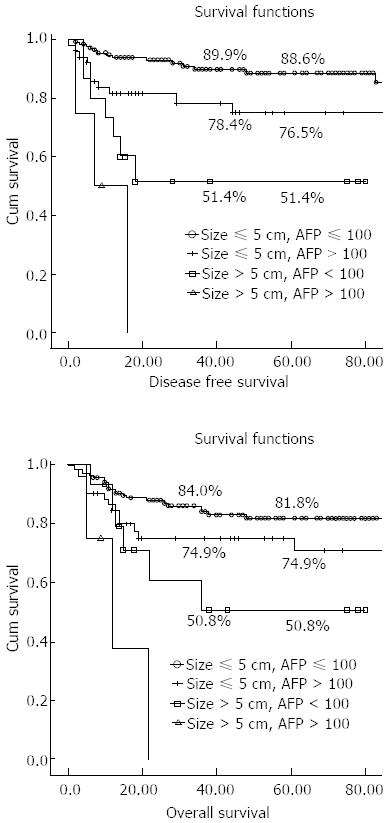
The Milan criteria showed significant differences regarding DFS, but not survival rate. We subdivided each factor of the Milan criteria and compared them with respect to the recurrence rate, and DFS and survival rates. Additionally, we subdivided factors of the CMC criteria and compared them with those of the Milan criteria (Table 5). Of the total, 133 (59.4%) patients were within the Milan criteria, and 140 (62.5%) were within the CMC criteria. When applying Milan criteria, the difference was significant when the cutoff value was a single tumor under 5 cm but was not significant when the cutoff value was less than three tumors, or two or three tumors less than 3 cm. When factors of the CMC criteria were subdivided, the recurrence rate, DFS, and overall survival rates were all significantly different. Although the number was small, most cases with a tumor size over 5 cm and an AFP level over 100 ng/mL recurred with a very poor prognosis; no patients survived beyond 3 years (Figure 1).
| Criteria | Recurrence rate | P-value | 5-yr DFS | P-value | 5-yr survival | P-value | |
| Milan criteria | |||||||
| Single | ≤ 5 cm (n = 91) | 13% | < 0.001 | 0.862 | < 0.001 | 0.829 | < 0.001 |
| > 5 cm (n = 12) | 61.5% | 0.333 | 0.333 | ||||
| No. 2 or 3 | ≤ 3 cm (n = 39) | 9.8% | 0.889 | 0.878 | 0.891 | 0.711 | 0.251 |
| > 3 cm (n = 22) | 8.7% | 0.848 | 0.747 | ||||
| Number | ≤ 3 (n = 164) | 15.4% | 0.209 | 0.824 | 0.228 | 0.781 | 0.316 |
| > 3 (n = 47) | 21.7% | 0.737 | 0.698 | ||||
| CMC criteria | |||||||
| Size ≤ 5 cm | AFP ≤ 100 (n = 138) | 9.6% | 0.001 | 0.886 | < 0.001 | 0.818 | < 0.001 |
| Size ≤ 5 cm | AFP > 100 (n = 52) | 20.0% | 0.765 | 0.749 | |||
| Size > 5 cm | AFP ≤ 100 (n = 15) | 38.9% | 0.514 | 0.508 | |||
| Size > 5 cm | AFP > 100 (n = 4) | 75.0% | 0 | 0 | |||
LT is a curative treatment modality for HCC. LT is particularly important in cases where resection is impossible due to factors such as liver cirrhosis. In countries with a shortage of deceased donor organs, LDLT can be the mainstay of therapy rather than DDLT. However, there is concern that LDLT has disadvantages regarding HCC recurrence compared with DDLT. The LDLT selection criteria are likely applied more widely than DDLT. Due to the short waiting time, LDLT candidates have no opportunity to be screened for an aggressive tumor biology. Because of their relatively small size LDLT grafts are subject to additional mechanical injury at the start of reperfusion, and angiogenesis and cell division signaling pathways may be initiated more frequently. The rapid graft regeneration in LDLT may also be associated with acceleration of tumor growth[13]. In fact, some studies have reported higher recurrence rates of LDLT compared with DDLT[14,15]. However, a recent meta-analysis concluded that the DFS rates of LDLT and DDLT do not differ significantly[16]. In our study, the recurrence rate was 16.5%, and for DDLT performed on patients within the Milan criteria, the recurrence rate was 12.0%. The total 5-year DFS rate and overall survival rate were 80.9% and 76.4%, respectively, and these results were generally acceptable.
The Milan criteria (MC) are often used to determine which patients will benefit from LT. However, when LT is strictly confined to those within MC, many patients who may benefit from LT will be lost. In advanced-stage III HCC patients, survival rates are about 59% after LT, which is comparable to patients with benign disease (65%)[17]. LT is undoubtedly superior to transarterial chemoembolization or chemotherapy in patients beyond MC, who may still benefit from transplantation. Unlike DDLT, LDLT is private, not public, and is performed in beyond-MC patients more easily. As reported by four major LT centers, 29.6% of LDLT procedures were performed in beyond MC patients[18]. At our institution, 37.5% of LDLT patients exceeded MC. Many centers have center-based criteria for selection of patient who exceed MC. The UCSF, Tokyo, and up-to-seven criteria are based on HCC size and number, which are surrogate markers of tumor volume[8-10]. Recently, the expression level of pre-operative serum tumor markers that affect HCC recurrence, such as pre-operative AFP and PIVKA-II levels, have become a hot topic of research. Some authors have reported that an AFP level prior to LT of > 200 or > 1000 ng/mL affected HCC recurrence[8,19,20]. Conversely, the Kyoto and Kyushu University criteria included the PIVKA-II level as a factor affecting tumor recurrence[11,12].
According to our results, a largest tumor diameter > 5 cm and AFP level C 100 ng/mL significantly influenced recurrence. Tumor number, which is important in the Milan criteria, did not show statistical significance. Indeed, many tumors discovered postoperatively are around 1 cm in size and are difficult to identify preoperatively. A discrepancy between the preoperatively and postoperatively discovered tumor numbers exists. Therefore, using tumor number as an important criterion may be problematic. Our results showed significant differences with the Milan criteria regarding recurrence and DFS; however, there was no significant difference regarding the survival rate, and so we subdivided each factor. There was a significant difference with a “single tumor smaller than 5 cm”, a finding that ascribes importance to size. However, no significant difference was found regarding the two other factors, a finding that confers importance on the tumor number. These findings correlate with the finding that tumor number was not a significant factor in multivariate analysis. Additionally, biologic factors are more important than morphologic factors in terms of predicting tumor behavior. Thus, inclusion of AFP, which is a biologic factor rather than representative of the tumor number, seems to be more appropriate. Therefore, we decided that, for our criteria (CMC criteria), the largest diameter of the tumor should be less than 5 cm, and the AFP level should be less than 100 ng/mL.
Next, we applied our data to current HCC guidelines, staging systems, and the Milan and UCSF criteria, which are relatively widely used LT criteria, and compared the recurrence, DFS, and overall survival rates with those of our criteria. The Milan and UCSF criteria both adequately reflected recurrence, DFS, and survival. However, when compared with only our data, the UCSF criteria included more patients and showed a better correlation between criteria and prognosis. CMC criteria showed statistically significant differences in recurrence, DFS, and overall survival rates. Notably, although the case number was small, in cases beyond both criteria, the recurrence rate was as high as 75%, and the 5-year DFS and overall survival rates were 0%, suggesting a contraindication to LT.
Many HCC staging systems are extant; however, the most commonly used is TNM staging, the 7th edition of which was recently published by the American Joint of Committee on Cancer (AJCC). The TNM system effectively stratifies post-hepatectomy HCC patients into stages I, II, and III[21]. According to this system, we analyzed our results to evaluate the efficacy of the TNM system for LDLT. The recurrence, DFS, and overall survival rates worsened with increasing T stage; however, no statistically significant difference was found. T3 cases seemed to show a stronger trend, although the number was small. A statistically significant difference may be found if additional cases are examined.
Because HCC patients also have liver cirrhosis, not only the stage of HCC but also liver function and the general condition of the patient must be considered when deciding the treatment modality. Many HCC guidelines exist, but the most commonly used are the BCLC and CLIP scoring systems. The BCLC scoring system is used frequently in the United States and Europe for HCC therapy and considers factors such as the patient’s general condition, Child-Pugh score, portal pressure, and tumor size and number. LT is recommended only for early stage A (single, 3 nodules, < 3 cm). Because LT can be performed regardless of Child-Pugh score or portal pressure, only the tumor factor is considered. Regarding transplantation, it is important to investigate the results according to BCLC stage. Vitale et al[22] reported that LT showed a survival benefit for patients with HCC and advanced liver cirrhosis (BCLC stage D) and in those with intermediate tumors (BCLC stage B-C). In our study, the recurrence, DFS, and overall survival rates showed no significant differences among BCLC stages 0, A, and B. However, these rates showed significant differences between stage C, which includes gross portal vein thrombosis, and the other stages. Therefore, although LT is recommended only for stage A according to the BCLC guidelines, LT may be performed on stage 0, A, and B patients with comparable results. Additionally, our study showed a stage C recurrence rate of 40.0% and 5-year DFS and survival rates of 54.4% and 54.9%, respectively. Although those data indicate a poor prognosis, as the recommended minimum prerequisite 5-year survival is 50%, LDLT may be performed in select stage C patients when the patient and family agree.
The CLIP scoring system was established in 1993. It considers Child-Pugh stage, tumor morphology, AFP level, and degree of portal vein thrombosis, and is considered an important index of prognosis. The CLIP scoring system for HCC is accurate and easy to implement[23]. However, to our knowledge, few studies have investigated the association between the CLIP scoring system and LDLT. In our study, we divided patients into three groups according to CLIP scores. In the lower-scoring groups (scores 0-3), recurrence rates, DFS rates, and overall survival rates showed no statistically significant differences. However, the group with scores of 4 and 5 showed a significant difference compared with the lower-scoring groups. Although it is difficult to reach a definite conclusion due to the small number of cases, patients with a CLIP score of 3 or lower may be suitable for LT, whereas those with a score of 4 or 5 may demonstrate worse results.
In conclusion, the recurrence rate was 16.5%, and the 5-year DFS and overall survival rates were 80.9% and 76.4%, respectively, after performance of LDLT in HCC patients. Factors influencing recurrence were a maximal tumor diameter greater than 5 cm and an AFP level greater than 100 ng/mL. When both criteria are not met, LT is contraindicated. Milan criteria, UCSF criteria, TNM staging, BCLC staging, and the CLIP scoring system showed different outcomes depending on the degree of criteria. Therefore, all of these staging and scoring systems are useful for determining LDLT for HCC patients. These findings should be confirmed by future prospective studies that include larger numbers of cases.
Liver transplantation (LT) for hepatocellular carcinoma (HCC) is known to be the best therapeutic option. To obtain a good result, it is important to select appropriate LT candidates from among HCC patients. Currently, many centers have their own criteria or treatment guidelines, which generally consist of factors that influence survival.
The Milan criteria is a well-established tool for assessing the prognosis of HCC. We expanded the criteria while maintaining long-term survival rates. Authors decided that, for our criteria (CMC criteria), the largest diameter of the tumor should be less than 5 cm, and the alpha fetoprotein (AFP) level should be less than 100 ng/mL. When both criteria are not met, authors think LT is contraindicated.
Most centers agree on expanding the criteria because more patients can benefit from transplantation. Other systems include the TNM system that reflects cancer stage, and the Barcelona Clinic Liver Cancer (BCLC) and the Cancer of the Liver Italian Program (CLIP) staging systems that are well-known guidelines for therapy and prognosis prediction that are used in the United States and Europe. Regarding LT, investigation of the efficacy of these criteria for therapy of HCC is important. Authors defined the selection criteria according to risk factors for recurrence based on authors’ results and compared them with other criteria or scoring systems, such as the Milan and UCSF criteria, TNM and BCLC staging, and the CLIP scoring system.
For living donor liver transplantation (LDLT), investigation of the efficacy of such criteria will facilitate adoption of extended criteria in LDLT.
It is an important study which establishes a new and useful scoring system for HCC patients undergoing living donor liver transplantation. The paper is interesting, as it introduces a new (biochemical) parameter for the evaluation of HCC candidates to LDLT.
P- Reviewers Cappelli A, Marino IR, Sipos F S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 413] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R. Hepatic resection vs transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221-228; discussion 221-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 459] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5110] [Cited by in F6Publishing: 5002] [Article Influence: 178.6] [Reference Citation Analysis (0)] |
| 4. | Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer. 1996;77:2217-2222. [PubMed] [Cited in This Article: ] |
| 5. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection vs transplantation. Hepatology. 1999;30:1434-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1331] [Cited by in F6Publishing: 1393] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 6. | Keeffe EB. Summary of guidelines on organ allocation and patient listing for liver transplantation. Liver Transpl Surg. 1998;4:S108-S114. [PubMed] [Cited in This Article: ] |
| 7. | Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 326] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1594] [Cited by in F6Publishing: 1586] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 9. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1267] [Cited by in F6Publishing: 1428] [Article Influence: 89.3] [Reference Citation Analysis (1)] |
| 10. | Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Takada Y, Ito T, Ueda M, Sakamoto S, Haga H, Maetani Y, Ogawa K, Ogura Y, Oike F, Egawa H. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteria. Dig Dis. 2007;25:299-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Taketomi A, Sanefuji K, Soejima Y, Yoshizumi T, Uhciyama H, Ikegami T, Harada N, Yamashita Y, Sugimachi K, Kayashima H. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation. 2009;87:531-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, Sterling RK, Fulcher AS, Posner MP. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000;69:1375-1379. [PubMed] [Cited in This Article: ] |
| 14. | Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor vs deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007;94:78-86. [PubMed] [Cited in This Article: ] |
| 15. | Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS, Ghobrial RM, Fair JH, Olthoff KM, Kam I. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7:1601-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Liang W, Wu L, Ling X, Schroder PM, Ju W, Wang D, Shang Y, Kong Y, Guo Z, He X. Living donor liver transplantation vs deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:1226-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Goodman J, Glasgow SC, Schnitzler M, Lowell JA, Shenoy S, Jendrisak MD, Desai N, Lisker-Melman M, Crippin J, Chapman WC. Liver transplantation for hepatocellular carcinoma: expanding special priority to include stage III disease. Arch Surg. 2005;140:459-464; discussion 464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Hwang S, Lee SG, Joh JW, Suh KS, Kim DG. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl. 2005;11:1265-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Pérez-Saborido B, de los Galanes SJ, Menéu-Díaz JC, Romero CJ, Elola-Olaso AM, Suárez YF, Valencia VB, Moreno-González E. Tumor recurrence after liver transplantation for hepatocellular carcinoma: recurrence pathway and prognostic factors. Transplant Proc. 2007;39:2304-2307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Figueras J, Ibañez L, Ramos E, Jaurrieta E, Ortiz-de-Urbina J, Pardo F, Mir J, Loinaz C, Herrera L, López-Cillero P. Selection criteria for liver transplantation in early-stage hepatocellular carcinoma with cirrhosis: results of a multicenter study. Liver Transpl. 2001;7:877-883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Cheng CH, Lee CF, Wu TH, Chan KM, Chou HS, Wu TJ, Yu MC, Chen TC, Lee WC, Chen MF. Evaluation of the new AJCC staging system for resectable hepatocellular carcinoma. World J Surg Oncol. 2011;9:114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Vitale A, Morales RR, Zanus G, Farinati F, Burra P, Angeli P, Frigo AC, Del Poggio P, Rapaccini G, Di Nolfo MA. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol. 2011;12:654-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Levy I, Sherman M. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:881-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |