Published online Aug 7, 2013. doi: 10.3748/wjg.v19.i29.4702
Revised: June 12, 2013
Accepted: June 19, 2013
Published online: August 7, 2013
AIM: To investigate the potential therapeutic effects of mesenchymal stem cells (MSCs) in inflammatory bowel disease (IBD), we transplanted MSCs into an experimental model of IBD.
METHODS: A rectal enema of trinitrobenzene sulfonic acid (TNBS) (100 mg/kg body weight) was administered to female BALB/c mice. Bone marrow mesenchymal stem cells (BMSCs) were derived from male green fluorescent protein (GFP) transgenic mice and were transplanted intravenously into the experimental animals after disease onset. Clinical activity scores and histological changes were evaluated. GFP and Sex determining region Y gene (SRY) expression were used for cell tracking. Ki67 positive cells and Lgr5-expressing cells were determined to measure proliferative activity. Inflammatory response was determined by measuring the levels of different inflammatory mediators in the colon and serum. The inflammatory cytokines included tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-6, IL-17, IL-4, IL-10, and transforming growth factor (TGF-β). Master regulators of Th1 cells (T-box expressed in T cells, T-bet), Th17 cells (retinoid related orphan receptor gamma(t), RORγt), Th2 cells (GATA family of transcription factors 3, GATA3) and regulatory T cells (forkhead box P3, Foxp3) were also determined.
RESULTS: Systemic infusion of GFP-BMSCs ameliorated the clinical and histopathologic severity of colitis, including body weight loss, diarrhea and inflammation, and increased survival (P < 0.05). The cell tracking study showed that MSCs homed to the injured colon. MSCs promoted proliferation of intestinal epithelial cells and differentiation of intestinal stem cells (P < 0.01). This therapeutic effect was mainly mediated by down-regulation of both Th1-Th17-driven autoimmune and inflammatory responses (IL-2, TNF-α, IFN-γ, T-bet; IL-6, IL-17, RORγt), and by up-regulation of Th2 activities (IL-4, IL-10, GATA-3) (P < 0.05). MSCs also induced activated CD4+CD25+Foxp3+ regulatory T cells (TGF-β, IL-10, Foxp3) with a suppressive capacity on Th1-Th17 effecter responses and promoted Th2 differentiation in vivo (P < 0.05).
CONCLUSION: MSCs are key regulators of immune and inflammatory responses and may be an attractive candidate for cell-based therapy of IBD.
Core tip: In this study, the following factors were identified: (1) The differentiation of intestinal stem cells in injured gut was determined by detecting Lgr5+ cells; (2) Th1-Th2-Th17-Tregs-related inflammatory and immune cytokine expressions in serum and local intestinal tissues were determined; (3) A Th2 shift and correction of the imbalanced Th17/Tregs were found; (4) Master regulators of Th1, Th2, Th17 and Tregs were detected in bone marrow mesenchymal stem cells -treated trinitrobenzene sulfonic acid-induced colitis; and (5) The passways of Th1-T-bet, Th2-GATA family of transcription factors 3, Th17-retinoid related orphan receptor gamma(t) and Tregs-Foxp3 which serve as important immunoregulators in the correction of immune disorders and enhance the healing of injured intestinal mucosa were identified.
- Citation: Chen QQ, Yan L, Wang CZ, Wang WH, Shi H, Su BB, Zeng QH, Du HT, Wan J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol 2013; 19(29): 4702-4717
- URL: https://www.wjgnet.com/1007-9327/full/v19/i29/4702.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i29.4702
Inflammatory bowel diseases (IBD) are comprised of Crohn’s disease (CD), ulcerative colitis (UC) and indeterminate colitis, and are found mainly in Western countries[1]. Recent studies indicated that enhanced abnormalities of the immune system, normal gut flora and environmental influences may play central roles in these diseases[2,3]. Conventional therapy for IBD includes corticosteroids, 5-aminosalicylates, 6-mercaptopurine, sulfasalazine, antimicrobial therapy, immunosuppressive agents and monoclonal antibodies (mAbs)[4]. Surgery is indicated for complications and failure of medical treatment[5]. Although the above therapies are effective for maintaining remission, they also have many side effects[6,7]. Several studies have reported that the transplantation of mesenchymal stem cells (MSCs) could temper IBD both in clinical trials and in animal models, which indicated that MSCs may be a promising therapeutic option for IBD[8-12]. However, the molecular mechanisms of this effect are still unclear.
Trinitrobenzene sulfonic acid (TNBS)-induced colitis (CD-like) is a well-established animal model for studying IBD. The model is well characterized by inflammatory cell infiltration accompanied by heightened Th1-Th17 response both systematically and locally in the gut mucosa[13]. T-lymphocytes secrete cytokines (cytokines can be both regulators and effectors) which can be divided into Th1 (characterized by secretion of [tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-2 (IL-2)], Th17 (characterized by secretion of IL-17), Th2 (characterized by secretion of IL-4 and IL-10), and regulatory T cells [Tregs, characterized by expression of forkhead box P3 (Foxp3) and CD25][14]. These T cells can be identified by the expression of specific transcription factors, T-bet for Th1, GATA family of transcription factors 3 (GATA3) for Th2, FoxP3 for Tregs, and retinoid related orphan receptor gamma(t) (RORγt) for Th17 cells, respectively[15]. In this study, we used the TNBS-induced colitis animal model to investigate the therapeutic role of MSCs in CD-like diseases.
Many recent studies have demonstrated that bone marrow mesenchymal stem cells (BMSCs) were seen in injured intestinal mucosa. These cells may repopulate and differentiate into intestinal stem cells (ISCs)[16]. ISCs are the progenitors of various functional intestinal cell types, including intestinal epithelial cells (IECs), interstitial cells, endothelial cells, vascular smooth muscle cells, and ill-defined inflammatory cells[17,18]. Lgr5 gene product can be used as a specific marker for identifying these ISCs[19,20]. However, it is not clear which factors from the transplanted BMSCs promote the repopulation of cells in recipient intestinal tissues. Several experiments have shown that MSCs can release soluble factors (cytokines, chemokines, and growth factors) that result in cell cycle arrest in pro-inflammatory lymphocytes and induce T cell apoptosis[21]. Reports also indicated that the regenerative, immunomodulatory, and anti-inflammatory effects of MSCs may provide a potential remedy for autoimmune diseases, such as focal cerebral ischemia, multiple sclerosis (MS), systemic sclerosis (SSc), type I diabetes and juvenile idiopathic arthritis[22-24]. However, there have been few reports regarding the roles of MSCs in IBD as well as the molecular mechanisms of MSCs in alleviating IBD.
In this study, we focused on the trafficking of transplanted GFP-BMSCs into diseased colon tissues and on their immune modulating effects in TNBS-induced colitis. We investigated the inhibitory effect of BMSCs on Th1 and Th17 mediated inflammatory response. We also examined the enhancement of anti-inflammatory immune activities of Th2 and Tregs by BMSCs in an animal model of TNBS-induced colitis.
Male Green fluorescent protein (GFP) transgenic mice [TgN (β-act-EGFP) Osb; 2-3 wk] on a C57BL/6 background were kindly provided by the Molecular Biology Laboratory of Chinese PLA General Hospital. Female BABL/c mice (6-8 wk) were purchased from the Laboratory Animal Center of the Academy of Military Medical Sciences of China (Beijing). All studies were performed under approval of the Ethics Committee of the Animal Facility of Chinese PLA General Hospital and were in agreement with the Guidance suggestion of caring for laboratory animals[25]. Mice were group-housed under controlled temperature (25 °C) and a 12-h light/dark cycle, fed standard mouse chow and tap water and maintained for 2 wk in our animal facilities before the start of the experiments.
Colitis was induced in female BABL/c mice (6-8 wk, 18-22 g) using 2,4,6-trinitrobenzene sulfonic acid (2,4,6-TNBS; Sigma-Aldrich, Deisenhofen, Germany) in 50% ethanol[26]. Then 0.1 mL of TNBS (100 mg/kg body weight) was administered via a vinyl catheter positioned 2.5 cm from the anus. To ensure distribution of TNBS within the entire colon and rectum, mice were held in a vertical position for 2 min after instillation of the TNBS enema. The control group was administered 0.1 mL of 50% ethanol.
Isolation, culture, and expansion of BMSCs: Male GFP-mice (2-3 wk) were sacrificed by cervical dislocation and their femurs and tibia were carefully flushed with Dulbecco’s modified Eagle’s medium-low glucose (DMEM-LG; HyClone Lab, Inc. Logan, UT) using a 0.45-mm syringe needle until the bones become pale. The released cells were discarded and the bones were dissected into fragments of 1-3 mm3 and digested with collagenase II (Sigma) for 1-2 h in a shaking incubator at 37 °C and a shaking speed of 200 rpm. The collagenase was removed by dilution with DMEM-LG containing 10% heat inactivated fetal bovine serum (FBS; Gibco-Invitrogen, Carlsbad, CA). In addition, the digested bone fragments were washed by centrifuging twice for 5 min at 1000 rpm and then cultivated in a 60 mm dish (Corning International, Tokyo, Japan) with DMEM-LG containing 10% FBS and penicillin/streptomycin (100 U/mL and 100 g/mL; Sigma, St. Louis, MO) at 37 °C in a 5% CO2 humidified incubator. To isolate putative MSCs, after 72 h of culture, nonadherent cells and tissue debris were removed with phosphate-buffered saline (PBS, HyClone), and adherent cells were maintained. On reaching 70%-80% confluence, these adherent cells were replanted using 0.25% (wt/v) trypsin/0.02% (wt/v) EDTA (Gibco) for 2-3 min. The medium was changed every 2-3 d[27].
Flow cytometric analysis: The cultured MSCs were retrieved by trypsin-EDTA digestion. Cell aliquots (1 × 106) were washed with cold PBS and resuspended in 100 μL of PBS per EP tube and stained with FITC-conjugated anti-mouse CD45 (Becton Dickinson), or PE-conjugated anti-mouse CD11b (eBioScience, San Diego, CA) and CD90.2 (eBioScience) at a concentration of 2 μg/mL at 4 °C for 30 min. One tube of unstained cells was prepared as a control for the antibodies. Cells were examined using a Becton Dickinson Fluorescence Activated Cell Sorting (BD FACScalibur) cytometer (Becton Dickinson, San Jose, CA, USA) and analyzed using a FACS Vantage cytometer and CellQuest software (BD).
Differentiation of BMSCs: The potential of BMSCs to differentiate into osteogenic and adipogenic lineages has been examined[27,28]. For osteogenesis, the isolated cells (passage 3) were plated in osteogenic conditioned medium supplemented with 10 mmol/L β-glycerol phosphate (Sigma), 50 μg/mL ascorbate-2-phosphate (Sigma) and 10 nmol/L dexamethasone (Sigma). The culture medium was changed every 3 d for 3 wk. Alizarin red staining was used to examine the culture mineralization. For staining, the cultures were first fixed using 4% paraformaldehyde for 20 min and then subjected to alizarin red solution at 37 °C for 30 min.
For adipogenesis, the cultured cells (passage 3) were incubated in adipogenic medium supplemented with 100 μmol/L indomethacin (Sigma), 1 μmol/L dexamethasone (Sigma), and 0.5 mmol/L 1-methyl-3-isobutylxanth (Sigma). The culture medium was changed every 3 d for 8 d. At the end of this period, the cells were then fixed in 4%paraformaldehyde for 1 h and stained with Oil Red (Sigma) for 1-2 h at room temperature.
GFP-BMSCs were harvested from male GFP-mice as described above. Then 1 × 106 cells in 100 μL PBS were transplanted on the indicated day via the tail vein on the second day after TNBS administration, while control mice received 100 μL PBS without BMSCs. In addition, the mice were killed on day 1, 2, 3, 5, 7 and 9 and their colons were washed with PBS. The colonic tissues were embedded in Tissue-Tek OCT compound (Sakura Finechemical Co., Tokyo, Japan) and circumferential sections 3 μm thick were prepared for fluorescent microscopy examination (Olympus, Tokyo, Japan). Paraffin-embedded tissue slides were stained with hematoxylin and eosin (HE) to assess the intensity of colitis. Additional specimens were frozen in liquid nitrogen for further use.
Isolation of DNA and detection of Y chromosomal DNA by polymerase chain reaction: DNA was isolated from snap-frozen colon specimens using the QIAamp DNA mini kit (Qiagen, CA) and Wizard DNA purification resin (Promega, Madison, WI). Polymerase chain reaction (PCR) was used to investigate the transplanted male donor cells using primers specific for the sex-determining region of the mouse Y-chromosome (Sry). The male group acts as a positive control, while the female group acted as a negative control. PCR amplification was performed as described below. The reaction mixture included 2 μL of genomic DNA, 2 μL Taq DNA polymerase (Takara, Japan), 5 μL of each oligonucleotide primer, 1 μL of a 20 mmol/L solution of each dNTP, 5 μL of 10 × PCR buffer, and 5 μL of 25 mmol/L MgCl2 in a final volume of 25 μL. The primer sequences used were as follows: mouse Sry gene, 5’-GGTGTGGTCCCGTGGTGAGAG-3’ and 5’-ATGGCATGTGGGTTCCTGTCC-3’. PCR was performed in a thermal cycler (PerkinElmer Cetus) for 35 cycles of denaturation (9 °C, 20 s), annealing (64 °C, 20 s), and extension (72 °C, 20 s). The product was analyzed by electrophoresis on a 2% agarose gel followed by ethidium bromide staining.
Clinical activity score of colitis: Mice were observed daily for weight, water/food consumption, morbidity, stool consistency, piloerection, and the presence of rectal bleeding. The clinical activity score of colitis was calculated independently by two blinded investigators using the disease activity index (DAI)[13]. The length of the colon, which was measured from the cecum to the anus, served as an indirect marker of the intensity of inflammation.
Histological scoring of the colon: The sections were HE stained and were used to assess colonic damage microscopically. The criteria were graded as follows: 0 point = no ulcer, no inflammation; 1 point = no ulcer, local hyperemia; 2 points = ulceration without hyperemia; 3 points = ulceration and inflammation at one site only; 4 points = two or more sites of ulceration and inflammation; and 5 points = ulceration extending more than 2 cm.
Immunohistochemistry for Ki67 and Lgr5: The slides of 4 μm colon sections were incubated with a rabbit anti-mouse primary antibody to Ki67 (1:500, ab66155, Abcam, ) or a rabbit anti-human primary antibody to Lgr5 (1:400, LS-A1236, MBL International Co., Woburn, MA) in PBS at 4 °C overnight. After 3 washes in PBS, the sections were incubated with a goat anti-rabbit secondary antibody (1:1000, Beijing Zhongshan Biotechnology, Beijing, China) at 37 °C for 30 min. Finally, the sections were visualized by incubating in 3,3’-diaminobenzidine tetrahydrochloride with 0.05% H2O2 (Liquid DAB + Substrate Chromogen System; Dako) for 3 min to induce a color reaction. The expression and localization of Ki67 and Lgr5 were examined under a light microscope (Olympus, Japan), and a brown color indicated a positive result.
Images were acquired using a digital camera connected to a light microscope (both from Olympus, Tokyo, Japan). Five immunostained sections (1280 × 960 pixels/per section) were selected from each image (× 200, 3 mice per group) for analysis. Ki-67 positivity and mean optical density of Lgr5 (mean optical density = total optical density/area) that best discriminated staining from the background was obtained using the NIH Image J 1.36b imaging software (NIH, Bethesda, MD). For immunohistochemistry analysis, immunostained sections were evaluated by two investigators following the principle of “blinded’’.
Quantification of Th1/Th2/Th17 cytokines by FACS: The BD Cytometric Bead Array Mouse Th1/Th2/Th17 Cytokine Kit (Becton Dickinson, San Jose, CA) was used to quantify TNF-α, IFN-γ, IL-2, IL-4, IL-6, IL-10 and IL-17A secretions in murine peripheral blood and colon proteins according to the manufacturer’s instructions using the BD FACScalibur cytometer (Becton Dickinson). Fifty microliters of supernatant were collected at each of the time points indicated above. Data were acquired on a FACS ARIA and samples were analyzed using FCAP Array Software (BD Biosciences).
RNA extraction and real-time PCR: RNA was extracted from colon (100 mg) using the Total RNA Kit II (Qiagen) following the manufacturer’s instructions. The quantity of extracted RNA was evaluated using the Nanodrop ND1000 (Thermofisher Scientific). Complementary DNA (cDNA) was created using DNase I/RNase-free (EN0521, Fermentas). Single intra-exon gene-specific primers listed in Table 1 were generated using Primer Express Software (Perkin Elmer Applied Biosystems). Applied Biosystems PRISM® 7300 (Applied Biosystems) and SYBR green fluorescent dye were used to detect amplification under the following amplification conditions: (1) 1 warm-up cycle for 2 min at 50 °C; (2) 1 pre-amplification cycle for 10 min at 95 °C, 40 amplification cycles for 30 s at 95 °C and for 31 s at 60 °C; and (3) end-amplification cycle for 15 s at 95 °C, 30 s at 60 °C and 15 s at 95 °C. Quantitative PCR crossing threshold (Ct) values were obtained during the exponential amplification phase using SDS 2.3 Software (Applied Biosystems).
| mRNA target | Forward primer sequences 5’→3’ | Reverse primer sequences 5’→3’ | bp |
| Actin | CGTTGACATCCGTAAAGACC | CTAGGAGCCAGAGCAGTAATC | 111 |
| IL-2 | TGAGTGCCAATTCGATGATGAG | TTATTGAGGGCTTGTTGAGATGAT | 91 |
| IL-4 | CAGCAACGAAGAACACCACAG | CGAAAAGCCCGAAAGAGTC | 148 |
| IL-6 | AATTTCCTCTGGTCTTCTGG | ACTCTGGCTTTGTCTTTCTTGTT | 93 |
| IL-10 | AGTGGAGCAGGTGAAGAGTGATTT | CTATGCAGTTGATGAAGATGTC | 92 |
| IL-17A | GAGAGCTGCCCCTTCACTTTCA | GGCTGCCTGGCGGACAAT | 87 |
| IFN-γ | TGGTGACATGAAAATCCTG | TTGCTGTTGCTGAAGAAG | 141 |
| TNF-α | AGTTCCCAAATGGCCTCCCT | ACTTGGTGGTTTGCTACGAC | 115 |
| GATA3 | AGGTGCATGACGCGCTGGAG | GGAGTGGCTGAAGGGAGAG | 107 |
| Foxp3 | GAAGAATGCCATCCGCCACAA | TGCTCCCTTCTCGCTCTCCA | 70 |
| TGF-β | TGCCCTCTACAACCAACACAAC | GCAGGAGCGCACAATCAT | 142 |
| T-bet | ACCTCTTCTATCCAACCAGTATCC | GAGGTGTCCCCAGCCAGTA | 104 |
| RORγt | GAAGGCAAATACGGTGGTGTGG | GCTGAGGAAGTGGGAAAAGTC | 90 |
Total proteins (100 μg) were added to lysis buffer containing protease inhibitors (Sigma; St. Louis, MO) and boiled at 95 °C for 5 min. The proteins were then routinely processed for Western blotting. Briefly, the proteins were respectively separated on 10% SDS polyacrylamide gels and blotted onto nitrocellulose membranes which were incubated in PBST-milk (PBS buffer containing 0.1% Tween-20 and 5% milk), followed by primary antibodies (1 h) for mouse FoxP3 (1:400 dilution, PM024S, MBL International Co., Woburn, MA); T-bet (1:250 dilution, 14-5825, eBioscience, San Diego, CA); GATA-3 (1:250 dilution, 14-9966, eBioscience); transforming growth factor (TGF-β3) (III) (1:1000 dilution, sc-83, Biotechnology, Santa Cruz, CA); ROR gamma(t) (1:250 dilution, 14-6981, eBioscience). Loading controls were obtained using a goat anti-rabbit antibody (1:3000, CoWin Biotech Co., Beijing, China). Blots were then washed with PBST 3 times (10 min each) and subsequently incubated (1 h) with actin antibody (1:3000, Biotechnology, Santa Cruz, CA) diluted in PBST milk. Each Western blot was repeated at least twice. Bands were detected after exposure to Hyperfilm-MP (1:10000, Amersham International PLC, Buckinghamshire, United Kingdom). The bands were then detected with scanning densitometry using a Desaga Cab UVIS scanner and Desaga ProViDoc software (Desaga, Wiesloch, Germany).
Data are represented as mean ± SD. Statistical comparisons between experimental groups were performed with one-way ANOVA using SPSS 17.0 software. P < 0.05 was considered statistically significant.
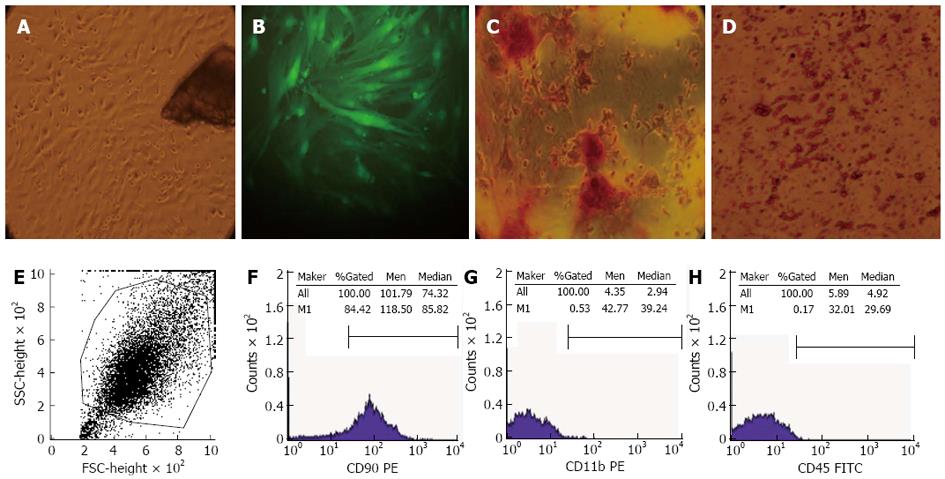
The bone marrow-derived GFP-BMSCs were obtained by cultivation of collagenase II digested bone fragments. At 72 h after initial culture, fibroblast-like cells were observed to migrate out from the bone fragments and adhere to the dish (Figure 1A). Some fibroblast colonies were observed after removing nonadherent cells and tissue debris when changing the culture medium. After 5 consecutive passages, the cell population started to demonstrate clustering and a radial pattern maintaining strong GFP expression (Figure 1B). The differentiation assays showed that these cells could differentiate into osteoblasts and adipocytes. For osteogenic differentiation, alizarin red staining was used after 21 d of culture and mineralized nodules were formed after induction (Figure 1C). For adipogenic differentiation, fat red particles were seen following Oil-red-O-staining after 8 d of culture (Figure 1D). Flow cytometry showed that these cells were homogenously positive for the mesenchymal marker CD90, but negative for hematopoietic markers CD11b and CD45 (Figure 1E-H).
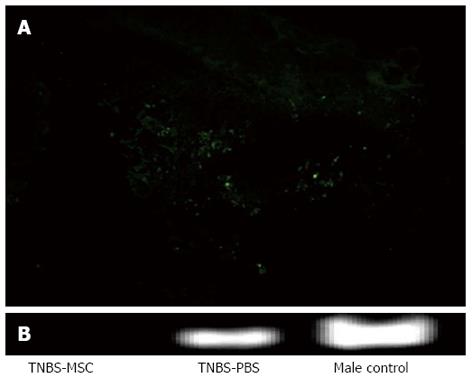
The transplanted GFP-BMSCs were found to be localized in the inflamed colon. Green fluorescence was mainly observed in the lamina propria, near the bottom of crypts 48 h after GFP-BMSCs transplantation (Figure 2A). No GFP-labeled MSCs were observed in non-inflamed tissues. Sry gene was detected in the TNBS-MSC-female group and the male-control group, but not in the TNBS-PBS-female group on day 9 (Figure 2B).
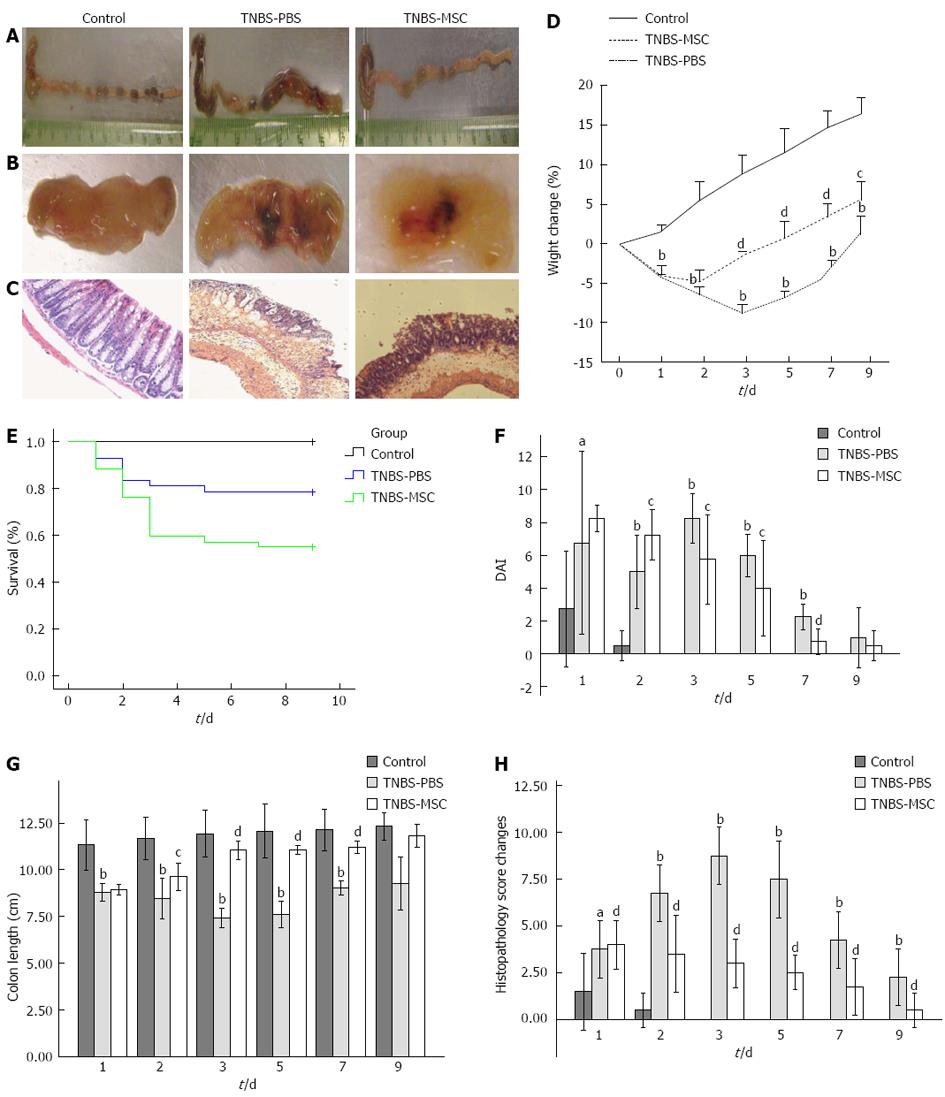
TNBS instillation in 50% ethanol led to a substantial wasting disease caused by severe diarrhea, weight loss, decreased water/food consumption, piloerection and the presence of rectal bleeding with about 55% mortality (Figure 3E). BMSCs significantly reduced colitis intensity in acute TNBS-induced colitis compared with the TNBS-PBS group assessed by DAI scores (Figure 3F). In the TNBS-MSC group, weight loss was improved from day 3 (-1.60 ± 0.45 g vs day 2 -4.71 ± 1.42 g, P < 0.05), but not in the TNBS-PBS group from day 5 (-4.40 ± 0.76 g vs day 3 -8.78 ± 1.08 g, P < 0.01) (Figure 3D). In the TNBS-MSC group, bloody stools decreased and became solid, while food and water intake also improved. We assessed the colon length in these 3 groups on day 0, 1, 2, 3, 5, 7 and 9. On days 3-7, macroscopic findings in the colon in the TNBS-PBS group included severe shortening, and wine-colored tissue with bloody stools compared to the TNBS-MSC group (Figure 3B). On day 3, a significant difference in colon length was observed between the TNBS-MSC group and TNBS-PBS group (11.05 ± 0.31 cm vs 7.43 ± 0.33 cm, P < 0.01) (Figure 3A and G). This significant difference was also seen in the TNBS-PBS group compared with the control group (7.43 ± 0.33 cm vs 11.68 ± 1.01 cm, P < 0.01) (Figure 3A and G).
In addition, histological changes in TNBS-induced colitis were mainly observed in the colon-rectum with severity progressively less towards the proximal site. In the TNBS-MSC group, the histological colitis score significantly decreased compared with the TNBS-PBS group on day 3 (3.00 ± 0.82 vs 8.75 ± 0.96, P < 0.01) (Figure 3C and H). BMSCs treatment reduced the extent of the inflamed area in the intestine. Compared with the TNBS-PBS group, TNBS-MSC reduced TNBS-induced crypt damage and infiltration of inflammatory cells composed mainly of neutrophils and macrophages (Figure 3H).
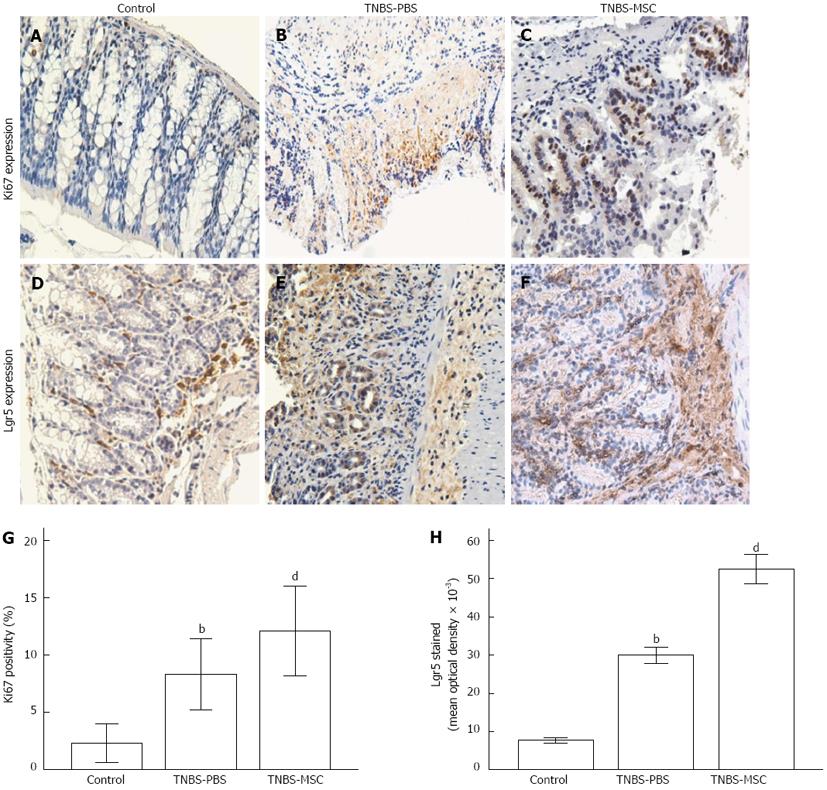
To determine whether administration of BMSCs could promote proliferation and differentiation of intestinal epithelial cells into ISCs, the expression of Ki67 (one of the markers of cell proliferation) and Lgr5 (one of the markers of ISCs), were examined by immunohistochemistry. Four days after transplantation of BMSCs, the expression of Ki67 and Lgr5 in damaged colonic tissues increased significantly compared with TNBS-PBS (Ki67: 12.09% ± 3.95% vs 8.33% ± 3.1%, P < 0.01; Lgr5: 52.54 ± 14.77 × 103vs 30.00 ± 8.08 × 103) (Figure 4).
BMSCs corrected the imbalance in T cell disorders in mice with TNBS-induced colitis. Since BMSCs are known to have in vitro immunosuppressive and anti-inflammatory properties[29], we investigated the potential therapeutic effects of BMSCs in an in vivo experimental model of IBD induced by TNBS. In TNBS-induced colitis, Th1-Th17 cells were activated to promote an exaggerated macrophage and neutrophil infiltration, giving rise to a prolonged severe transmural inflamed intestinal mucosa and immune response, characterized by the production of inflammatory cytokines and their transcription factors[30]. BMSCs can rapidly reduce inflammation by affecting the differentiation of T cells, such as promoting Th2 cells and enhancing regulatory T cell functions.
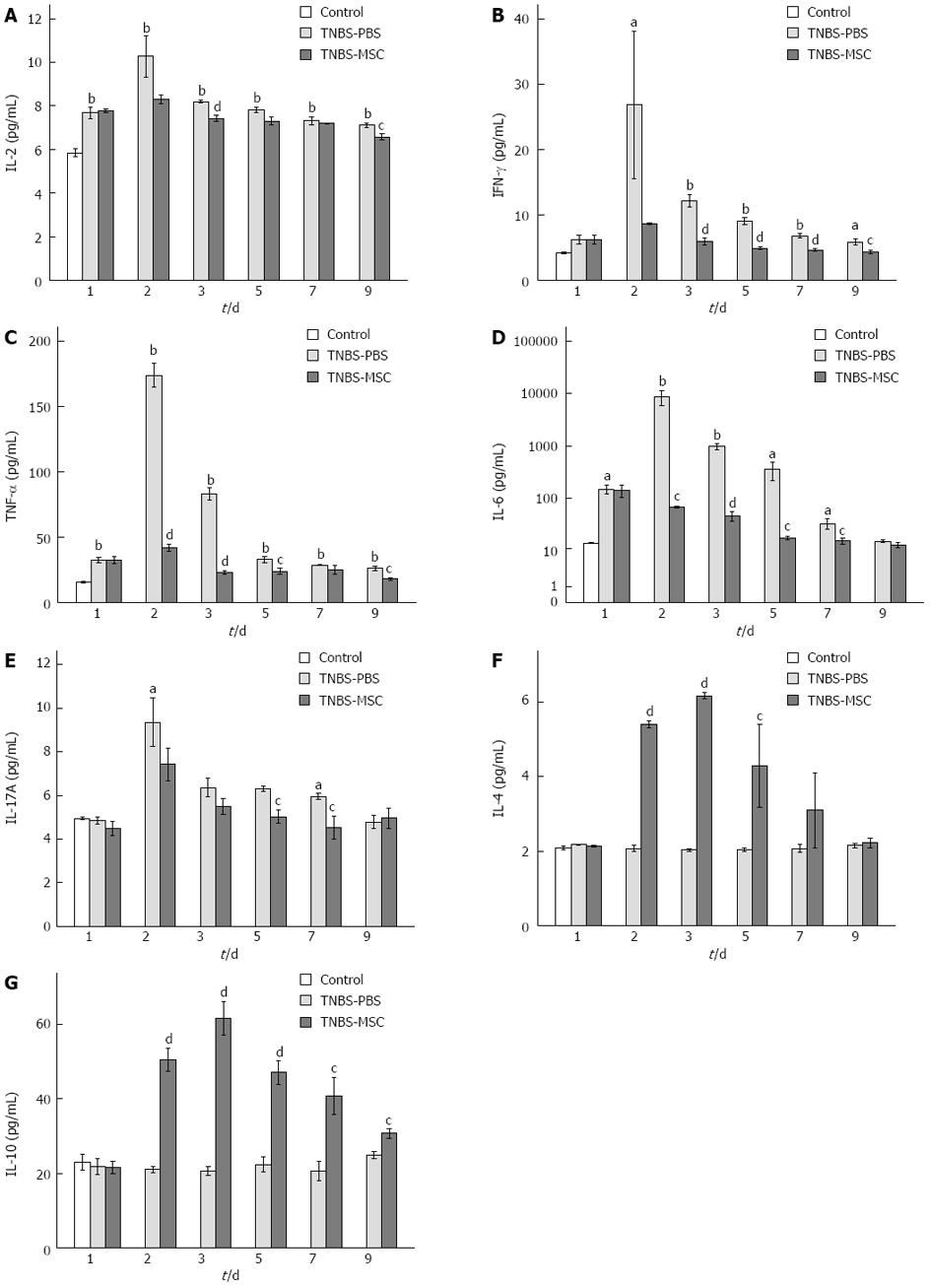
Cytokines are principal mediators of the systemic and local immune responses in immunological or inflammatory diseases. We examined the expression levels of 7 cytokines: IL-2, TNF-α, IFN-γ, IL-4, IL-10, IL-6, and IL-17. In acute TNBS-induced colitis, the level of proinflammatory cytokines (including IL-2, TNF-α, IFN-γ, IL-6, and IL-17) increased significantly (P < 0.01) when compared to the control group (Figure 5A-C, E and F). Following the transplantation of MSCs, the level of proinflammatory cytokines decreased significantly (P < 0.01), while the level of IL-4 and IL-10 increased (P < 0.05) when compared to the TNBS-PBS group (Figure 5A-G).
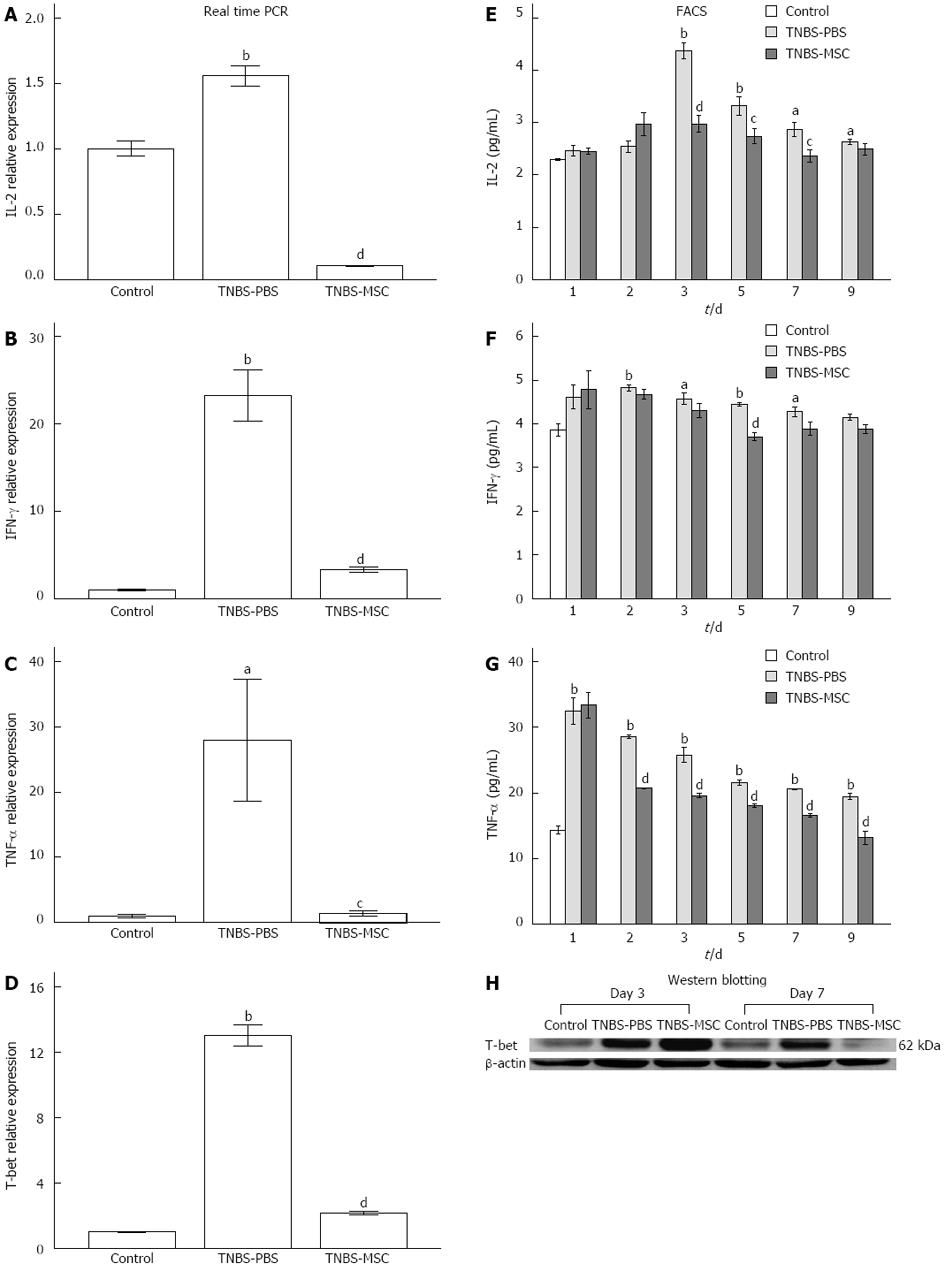
The differentiation of Th1 lymphocytes is known to be associated with a specific transcription factor, T-bet, which is a key component regulating the expression of Th1 cytokines[31]. The up-regulation of Th1 activities in TNBS-induced colitis and the reduction of Th1 activities after BMSCs transplantation were further confirmed by the analysis of T-bet expression using both real-time PCR and Western blotting (Figure 6D and H). In addition, we analyzed the expression of IL-2, IFN-γ, and TNF-α by real-time PCR. There was an increase in Th1 activities in acute TNBS-induced colitis (vs the control group) and a decrease in Th1 activities after treatment with BMSCs (vs the TNBS-PBS group) (Figure 6A-C). FACS analysis of IL-2, IFN-γ and TNF-α proteins (days 1-9) also showed the same trend. In the TNBS-PBS group, IL-2, IFN-γ and TNF-α expressions were significantly up-regulated (vs the control group, IL-2: 4.37 ± 0.27 pg/mL vs 2.30 ± 0.03 pg/mL, P < 0.01; IFN-γ: 4.82 ± 0.11 pg/mL vs 3.64 ± 0.39 pg/mL, P < 0.01; TNF-α 32.45 ± 3.52 pg/mL vs 13.91 ± 0.94 pg/mL, P < 0.01). However, BMSCs treatment led to a distinct reduction in the above factors (vs the TNBS-PBS group, IL-2:2.37 ± 0.20 pg/mL vs 2.87 ± 0.25 pg/mL, P < 0.05; IFN-γ: 3.71 ± 0.17 pg/mL vs 4.44 ± 0.07 pg/mL, P < 0.01; TNF-α: 13.12 ± 1.76 pg/mL vs 19.45 ± 0.82 pg/mL, P < 0.01) (Figure 6E-G).
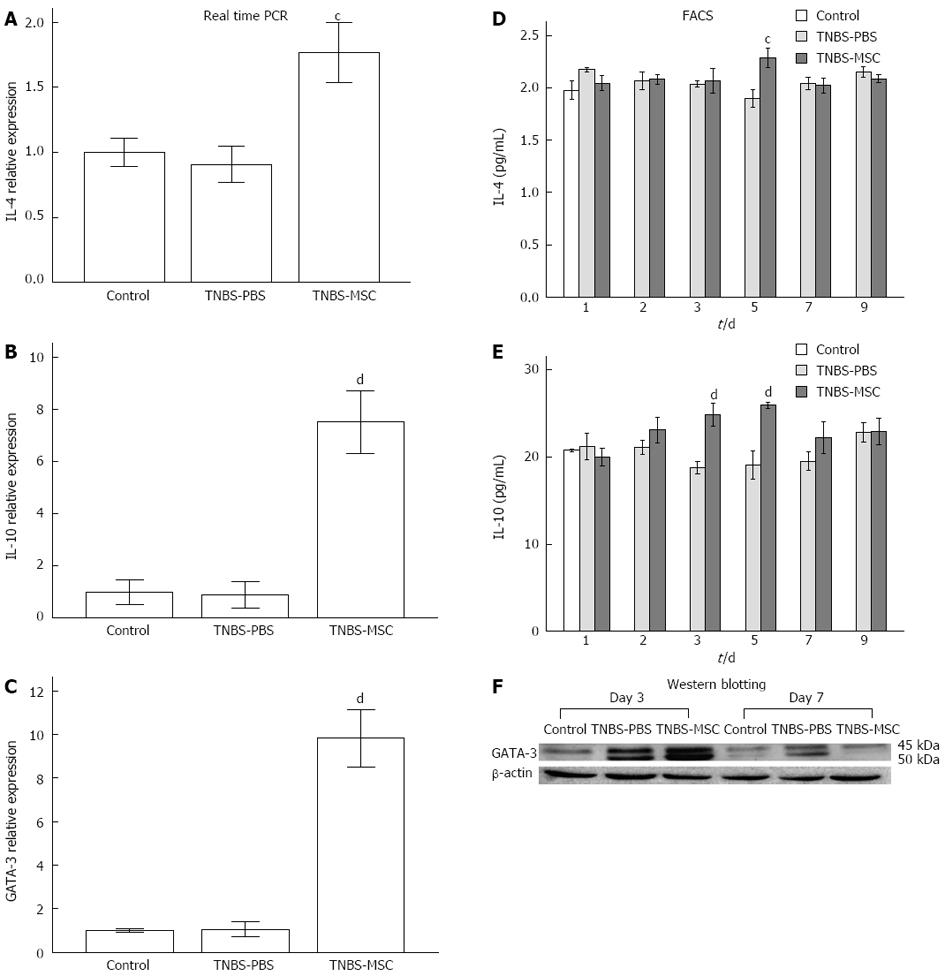
A possible role for BMSCs in promoting the Th2 subset was investigated by analyzing IL-4 and IL-10 production as well as the Th2 lineage transcription factor GATA3. There was no significant change in GATA3 (day 3) expression in the TNBS-PBS group, however, GATA3 expression was increased in the TNBS-MSC group confirmed by either real-time PCR or Western blotting (Figure 7C and F). IL-4 and IL-10 were also up-regulated at both the mRNA and protein levels in the TNBS-MSC group, while no significant changes were observed in TNBS-induced colitis (Figure 7A and B). The expression of IL-4 and IL-10 at the protein level (days 1-9) was further determined by FACS analysis and showed a similar pattern to that of the mRNAs. In the TNBS-MSC group, IL-4 and IL-10 were up-regulated (vs the TNBS-PBS group, IL-4: 2.15 ± 0.16 pg/mL vs 1.90 ± 0.15 pg/mL, P < 0.05; IL-10: 25.93 ± 0.63 pg/mL vs 19.09 ± 2.85 pg/mL P < 0.01). There were no significant changes in IL-4 and IL-10 in the TNBS-PBS group (vs the control group, P > 0.05) (Figure 7D and E).
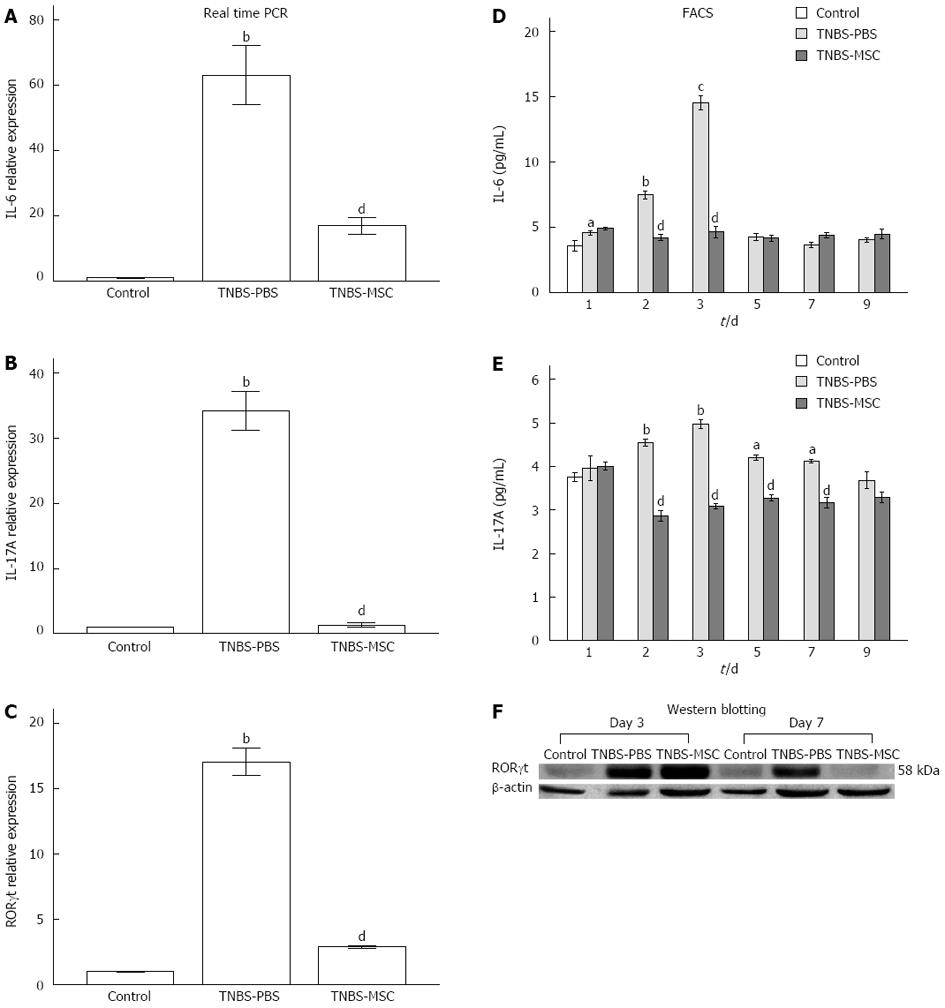
Th17 cell differentiation requires at least two cytokine signals that transmit through the RORγt and Smad-dependent signaling pathways[32]. Real-time PCR and Western blot results of RORγt expression at day 7 revealed that BMSCs clearly inhibited the differentiation of pathogenic Th17 effector cells (Figure 8C and F). In this study, the expressions of IL-6 and IL-17 were significantly up-regulated in the TNBS-PBS group, but were down-regulated in the TNBS-MSC group shown by real-time PCR (Figure 8A and B). The protein expressions of IL-6 and IL-17 (days 1-9) were further determined by FACS and the results showed the same trend as their mRNA expressions. In the TNBS-PBS group, IL-6 and IL-17A expressions were significantly up-regulated (vs the control group, IL-6: 14.52 ± 0.96 pg/mL vs 3.80 ± 0.52 pg/mL, P < 0.01; IL-17A: 4.97 ± 0.19 pg/mL vs 3.61 ± 0.29 pg/mL, P < 0.01). However, BMSCs treatment led to a distinct reduction in the above factors (vs the TNBS-PBS group, IL-6: 4.22 ± 0.40 pg/mL vs 7.50 ± 0.51 pg/mL, P < 0.05; IL-17A: 2.87 ± 0.21 pg/mL vs 4.54 ± 0.14 pg/mL, P < 0.01) (Figure 8D and E).
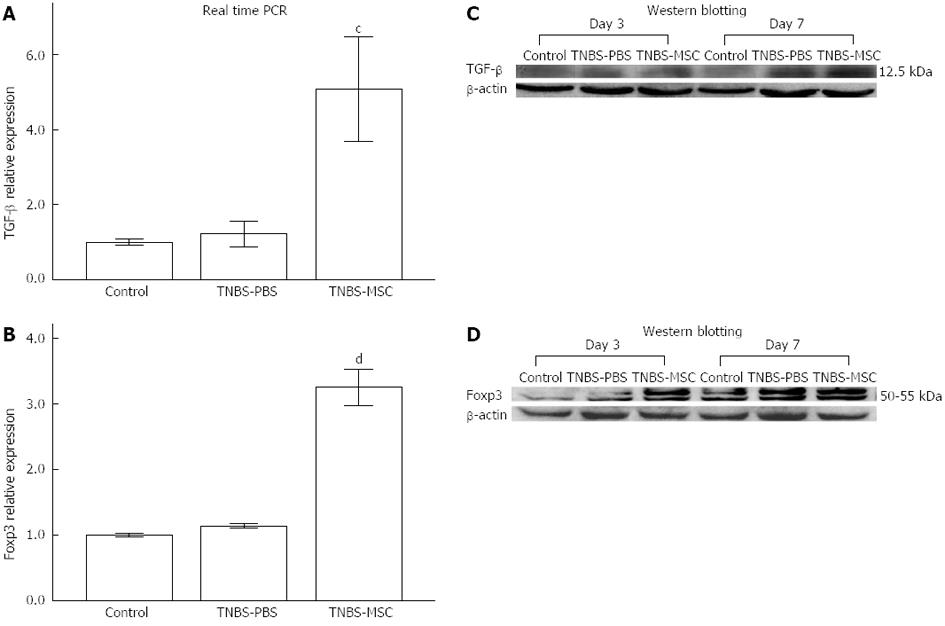
The suppressor cytokines IL-10 and TGF-β are also produced by CD4+Foxp3+ Tregs which are involved in the control of colitis as indicated in recent investigations[10,33]. BMSCs significantly up-regulated IL-10 as well as TGF-β levels (P < 0.01, Figure 7B, E, Figure 9A and C). The expression of Foxp3 also increased in the TNBS-MSC group when compared to the TNBS-PBS group (P < 0.01, Figure 9B and D). However, the production of IL-10, TGF-β and Foxp3 using real-time PCR and Western blotting showed no changes in the TNBS-MSC group compared with the control group (P > 0.05, Figure 7B and E and Figure 9A-D).
Kashyap et al[34] reported a patient with non-Hodgkin lymphoma and CD who was maintained for more than 7 years in clinical remission after autologous hematopoietic stem cell transplantation. Since then, more and more studies have focused on the use of stem cell therapy in IBD. The results from our study were consistent with previous reports, which demonstrated that intravenously transplanted BMSCs may home to injured intestinal tissues, promote intestinal cell regeneration, ameliorate the inflammatory symptoms in an experimental IBD mouse model and increase survival[35,36]. However, our study also identified the following: (1) We showed the differentiation of ISCs in injured gut by detecting Lgr5 positive cells; (2) We examined the expression levels of Th1-Th2-Th17-Tregs-related inflammatory and regulatory cytokines in peripheral blood and in local intestinal tissues; (3) We found a Th2 shift and subsequent correction of imbalanced Th17/Tregs; (4) Master regulators of Th1, Th2, Th17 and Tregs were detected in BMSCs-treated TNBS-induced colitis; and (5) We showed that the signaling pathways of Th1/T-bet, Th2/GATA-3, Th17/RORγt and Tregs/Foxp3 may serve as important immunoregulators in the correction of immune disorders and can enhance the healing of injured intestinal mucosa.
In our study, we established a mouse model of TNBS-induced colitis and transplanted BMSCs into the mice. On day 1, 2, 3, 5, 7 and 9 after transplantation, the mice were sacrificed. Moreover, 48 h after BMSCs transplantation, we found that a small proportion of the infused BMSCs had homed to the inflammatory or injured tissues by observing GFP and detecting Sry gene (only located on the Y chromosome). In addition, we investigated the role BMSCs played in TNBS-induced colitis at different time points by assessing the expression of factors, pathological changes, and clinical symptoms. We found that BMSCs had a strong therapeutic effect on TNBS-induced colitis. We also detected the expression of Ki67 (one of the markers of cell proliferation) and Lgr5 (an intestinal stem cell marker) using immunohistochemistry. The significantly increased expression of Ki67 and Lgr5 after BMSCs transplantation suggests that these BMSCs in recipient colonic tissues may have trans-differentiated into ISCs. Taken together, we showed that there are two possible therapeutic mechanisms of BMSCs treatment. First, some of these BMSCs could directly differentiate into pluripotent stem cells, such as ISCs. Second, MSCs could secrete cytokines or chemokines to influence the imbalanced intestinal micro-environment and stimulate the tissue-specific ISCs to proliferate. However, these experimental findings might be an indirect consequence of the general improvement in tissue regeneration of acute colitis mediated by BMSCs and the detailed mechanism involved requires further clarification.
The mechanism could be as follows: First, when BMSCs were intravenously transplanted into the experimental animal model, they first circulated around the whole body through blood circulation. During this process, BMSCs secrete related cytokines to activate the systemic immune system. Second, these BMSCs migrated, scatter implanted and survived within the injured gut mucosa and subsequently reversed the imbalance in Th1/Th2/Th17/Tregs, which is important in maintaining the intestinal mucosa microenvironment.
In IBD, the ratio of pro-inflammatory (IL-2, TNF-α, IFN-γ, IL-6 and IL-17) and anti-inflammatory cytokines (IL-4 and IL-10) are imbalanced systematically. In our study, we found that intravenously transplanted MSCs were able to modulate the release and/or expression of pro-inflammatory cytokines in the serum by effectively inducing remission and promoting the release and/or expression of anti-inflammatory cytokines in the serum to balance inappropriate immune system disorders systemically. These appropriate responses would finally slow down and/or reverse the natural course of the disease and even prevent complications such as fistulae and colorectal cancer.
Little work has been carried out on how BMSCs display their immune regulatory and anti-inflammatory properties in damaged colonic tissues. According to previous investigations, BMSCs can efficiently suppress the proliferative response of T cells in vitro[37,38]. Our study showed that the complex immune-modulating effects of GFP-BMSCs may result from the differential down-regulation of proinflammatory signaling of Th1 and Th17 lymphocytes as assessed by analysis of IL-2, TNF-α, IFN-γ (Th1-related) and IL-6, IL-17 (Th17-related), and the up-regulation of anti-inflammatory signaling of Th2 activities with IL-4 and IL-10. This also led to a moderate induction of induced/activated regulatory T cells (IL-10, TGF-β), which may influence Th1 and Th17 cell function. Naive T helper cells (Th0) can be induced to differentiate into Th1, Th2, Th17 and regulatory (Treg) phenotypes based on the mode of stimulation, antigen concentration, co-stimulation and cytokine milieu[39]. These factors exert their functions by cross-regulating one another and are selectively expressed in the corresponding cell populations[31,40]. Our experiments showed that when MSCs migrated into the inflamed colon, they began to produce cytokines, chemokines, growth factors and adhesion molecules by themselves or promoted intestinal lymphocytes to regulate the inappropriate inflammatory responses.
In this study, we found significant inhibition of the production of Th1-cytokines such as IL-2, TNF-α, IFN-γ and the Th1-specific transcription factor, T-bet, using real-time PCR, FACs and/or Western blotting, suggesting that MSCs can alter the inflammatory process by down-regulating Th1-driven autoimmune and inflammatory responses. We also found increased expression of IL-4 and GATA-3 using the same methods. In conjunction with the changes in Th1-cytokines, this showed that MSCs may shift the pathways of differentiation towards Th1 and Th2 cells with IL-4 signaling. This is possibly due to the fact that T-bet expression inhibits GATA-3 activity and Th2 cytokines block the differentiation of Th1 cells[41,42]. In this study, the scenarios are very complex. (1) The transplanted MSCs had their own effect in secreting cytokines; (2) Endogenous MSCs can have similar or different effects; (3) The transplanted or endogenous MSCs may migrate to inflamed tissues and recruit Th1/Th2/Th17/Tregs; (4) The transplanted or endogenous MSCs may secrete cytokines/chemokines/growth factors and exert remote effects; (5) Transplanted or endogenous MSCs may affect local ISCs and change the local environment; and (6) Transplanted MSCs could recruit endogenous MSCs or ISCs to the local colon; and even more complex, the combination of all of them or some of them.
In addition to suppressive effects on Th1-activated immune and inflammatory responses, there is ample evidence from our experiments to suggest that MSCs also mediate the modulation of Th17-cytokines such as IL-17 and IL-6 to ameliorate TNBS-induced colitis. Our data showed high mRNA and protein levels of IL-17, RORγt and IL-6 in colonic tissue, but decreased levels in the MSCs-treated group. The reason for this may be that Th17 cells, characterized by expression of IL-17 (also known as IL-17A), differentiate from naive T helper cells in the presence of IL-6 and TGF-β[43]. RORγt is an essential factor for Th17 differentiation[43]. Previous experiments showed that TGF-β and IL-6 enhanced RORγt mRNA expression in naive T cells, which in turn induced IL-17A expression. We also demonstrated that the mRNA and protein levels of IL-10, TGF-β and Foxp3 were increased after administration of MSCs. This indicated that MSCs could restore the balance between Th17 cells and CD4+CD25+Foxp3+ Treg in the intestinal tissues. Foxp3 directly interacts with RORγt to inhibit its function, resulting in decreased IL-17 expression[44,45]. IL-10 produced by regulatory subsets of T cells exerts a variety of anti-inflammatory and immunoregulatory functions linked with Foxp3 in vivo[46].
In conclusion, we have shown that BMSCs have a possible therapeutic effect in Th1- or Th17-driven IBD, including (1) homing to and surviving in the injured location; (2) exerting an immunoregulatory effect and controlling inflammation systematically; (3) accelerating colon mucosa regeneration; and (4) orchestrating a shift from Th1 and Th17 toward Th2 and the enhanced activities of Tregs to suppress local inflammation in colon tissues. Thus, exogenous MSCs transplantation is a novel therapeutic strategy for human IBD. However, for further clinical application, there are some unresolved questions owing to the complexity of human IBD. These include: (1) When is the best time for BMSCs treatment? (2) What dosage of BMSCs should be used to achieve optimal therapeutic results with least side effects? and (3) Should a combination of other drugs be used?
The authors thank the medical professionals in the Molecular Biology Laboratory of Chinese PLA General Hospital and Gastroenterology Laboratory for their guidance on experiments, Dr. Jun Wan and Dr. Li Yan for their valuable advice and suggestions in the study and the manuscript preparation.
Inflammatory bowel disease (IBD) is a chronic disease characterized by severe T-helper cell-driven inflammation and immune disorder. Mounting evidence suggests that mesenchymal stem cells (MSCs) have properties including low immunogenicity, immunomodulation and anti-inflammatory activity both in vitro and in vivo, and especially regulate T-cell responses. However, the molecular mechanisms involved in these effects are still unclear. Therefore, we transplanted MSCs into an experimental model of IBD to investigate their potential therapeutic effects in vivo.
MSCs can release soluble factors (cytokines, chemokines, and growth factors) which result in cell cycle arrest in pro-inflammatory lymphocytes and induce T cell apoptosis. Many studies have shown that these cells are a potential treatment for autoimmune diseases. However, there have been few reports on the roles of MSCs in IBD and the molecular mechanisms of MSCs in alleviating IBD.
The results demonstrated changes in Th1-Th2-Th17-Tregs-related inflammatory and immune cytokine expressions and master regulators of these immune cells in serum and local intestinal tissues after MSCs transplantation. The authors found that MSCs resulted in a Th2 shift and correction of the imbalanced Th17/Tregs to enhance the healing of injured intestinal mucosa.
This data will contribute to future research on the immunomodulatory properties of MSCs and support a rationale for the clinical application of stem cell therapy in IBD.
T-bet is a T-isolated box gene family of transcription factors, which is selectively expressed on Th1 cells. Retinoic acid-related orphan receptor γt (RORγt) is the orphan nuclear receptor that regulates the development of Th17 cells. GATA family of transcription factors 3 (GATA3) plays a central role in Th2 differentiation. Forkhead box P3 (Foxp3) acts as a master switch governing the development and function of CD4+ regulatory T cells.
In this manuscript, the authors studied the influence of bone marrow stem cells on colitis. This is an interesting study showing the mechanisms by which MSCs attenuate colitis. However, the direct contribution of transplanted MSCs in Ki67+ proliferation assay and the data showing an increase in interleukin (IL)-4, IL-10, tumor growth factor-β and Foxp3 in inflamed tissue is are not expressed in transplanted MSCs, but in infiltrating immune cells.
P- Reviewer Bright JJ S- Editor Zhai HH L- Editor A E- Editor Zhang DN
| 1. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3134] [Cited by in F6Publishing: 3259] [Article Influence: 271.6] [Reference Citation Analysis (1)] |
| 2. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1352] [Cited by in F6Publishing: 1444] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 3. | Kabi A, Nickerson KP, Homer CR, McDonald C. Digesting the genetics of inflammatory bowel disease: insights from studies of autophagy risk genes. Inflamm Bowel Dis. 2012;18:782-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Colombel JF, Feagan BG, Sandborn WJ, Van Assche G, Robinson AM. Therapeutic drug monitoring of biologics for inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:349-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | de Buck van Overstraeten A, Wolthuis A, D’Hoore A. Surgery for Crohn’s disease in the era of biologicals: a reduced need or delayed verdict? World J Gastroenterol. 2012;18:3828-3832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 36] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Naganuma M, Kunisaki R, Yoshimura N, Takeuchi Y, Watanabe M. A prospective analysis of the incidence of and risk factors for opportunistic infections in patients with inflammatory bowel disease. J Gastroenterol. 2013;48:595-600. [PubMed] [Cited in This Article: ] |
| 7. | Reenaers C, Belaiche J, Louis E. Impact of medical therapies on inflammatory bowel disease complication rate. World J Gastroenterol. 2012;18:3823-3827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 11] [Cited by in F6Publishing: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Shaker A, Rubin DC. Stem cells: One step closer to gut repair. Nature. 2012;485:181-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Tanaka H, Arimura Y, Yabana T, Goto A, Hosokawa M, Nagaishi K, Yamashita K, Yamamoto H, Sasaki Y, Fujimiya M. Myogenic lineage differentiated mesenchymal stem cells enhance recovery from dextran sulfate sodium-induced colitis in the rat. J Gastroenterol. 2011;46:143-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J Pharmacol Exp Ther. 2008;326:523-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Liang J, Zhang H, Wang D, Feng X, Wang H, Hua B, Liu B, Sun L. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut. 2012;61:468-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 13. | Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 567] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 14. | Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D. Immunomodulatory effects of probiotics in the intestinal tract. Curr Issues Mol Biol. 2008;10:37-54. [PubMed] [Cited in This Article: ] |
| 15. | Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;120:622-635. [PubMed] [Cited in This Article: ] |
| 16. | Karlsson C, Emanuelsson K, Wessberg F, Kajic K, Axell MZ, Eriksson PS, Lindahl A, Hyllner J, Strehl R. Human embryonic stem cell-derived mesenchymal progenitors--potential in regenerative medicine. Stem Cell Res. 2009;3:39-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Beck PL, Li Y, Wong J, Chen CW, Keenan CM, Sharkey KA, McCafferty DM. Inducible nitric oxide synthase from bone marrow-derived cells plays a critical role in regulating colonic inflammation. Gastroenterology. 2007;132:1778-1790. [PubMed] [Cited in This Article: ] |
| 18. | Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011-1017. [PubMed] [Cited in This Article: ] |
| 19. | Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755-1760. [PubMed] [Cited in This Article: ] |
| 20. | Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138:1681-1696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Guo Z, Li H, Li X, Yu X, Wang H, Tang P, Mao N. In vitro characteristics and in vivo immunosuppressive activity of compact bone-derived murine mesenchymal progenitor cells. Stem Cells. 2006;24:992-1000. [PubMed] [Cited in This Article: ] |
| 22. | Vasconcelos-dos-Santos A, Rosado-de-Castro PH, Lopes de Souza SA, da Costa Silva J, Ramos AB, Rodriguez de Freitas G, Barbosa da Fonseca LM, Gutfilen B, Mendez-Otero R. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: Is there a difference in biodistribution and efficacy? Stem Cell Res. 2012;9:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Atkins HL, Muraro PA, van Laar JM, Pavletic SZ. Autologous hematopoietic stem cell transplantation for autoimmune disease--is it now ready for prime time? Biol Blood Marrow Transplant. 2012;18:S177-S183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann N Y Acad Sci. 2012;1266:107-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | The Ministry of Science and Technology of the Peopleâs Republic of China. Guidance suggestion of caring laboratory animals, 2006-09-30. . [Cited in This Article: ] |
| 26. | Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23-33. [PubMed] [Cited in This Article: ] |
| 27. | Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, Zhang Y, Mao N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 28. | Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Sánchez L, Gutierrez-Aranda I, Ligero G, Rubio R, Muñoz-López M, García-Pérez JL, Ramos V, Real PJ, Bueno C, Rodríguez R. Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells. 2011;29:251-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Dutra RC, Claudino RF, Bento AF, Marcon R, Schmidt EC, Bouzon ZL, Pianowski LF, Calixto JB. Preventive and therapeutic euphol treatment attenuates experimental colitis in mice. PLoS One. 2011;6:e27122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Díaz YR, Rojas R, Valderrama L, Saravia NG. T-bet, GATA-3, and Foxp3 expression and Th1/Th2 cytokine production in the clinical outcome of human infection with Leishmania (Viannia) species. J Infect Dis. 2010;202:406-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 33. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 468] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 34. | Kashyap A, Forman SJ. Autologous bone marrow transplantation for non-Hodgkin’s lymphoma resulting in long-term remission of coincidental Crohn’s disease. Br J Haematol. 1998;103:651-652. [PubMed] [Cited in This Article: ] |
| 35. | Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 482] [Cited by in F6Publishing: 482] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 36. | Liang L, Dong C, Chen X, Fang Z, Xu J, Liu M, Zhang X, Gu DS, Wang D, Du W. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transplant. 2011;20:1395-1408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722-3729. [PubMed] [Cited in This Article: ] |
| 38. | Bassi EJ, Aita CA, Câmara NO. Immune regulatory properties of multipotent mesenchymal stromal cells: Where do we stand? World J Stem Cells. 2011;3:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32-46. [PubMed] [Cited in This Article: ] |
| 40. | Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G, Sallusto F. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol. 2003;4:78-86. [PubMed] [Cited in This Article: ] |
| 41. | Wang C, Sanders CM, Yang Q, Schroeder HW, Wang E, Babrzadeh F, Gharizadeh B, Myers RM, Hudson JR, Davis RW. High throughput sequencing reveals a complex pattern of dynamic interrelationships among human T cell subsets. Proc Natl Acad Sci USA. 2010;107:1518-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 42. | Tofukuji S, Kuwahara M, Suzuki J, Ohara O, Nakayama T, Yamashita M. Identification of a new pathway for Th1 cell development induced by cooperative stimulation with IL-4 and TGF-β. J Immunol. 2012;188:4846-4857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 862] [Cited by in F6Publishing: 925] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 44. | Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3754] [Cited by in F6Publishing: 3603] [Article Influence: 240.2] [Reference Citation Analysis (0)] |
| 45. | Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1881] [Cited by in F6Publishing: 2010] [Article Influence: 167.5] [Reference Citation Analysis (0)] |
| 46. | Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |