Published online Aug 7, 2013. doi: 10.3748/wjg.v19.i29.4651
Revised: May 29, 2013
Accepted: June 18, 2013
Published online: August 7, 2013
Mutation of the p53 gene is a key event in the carcinogenesis of many different types of tumours. These can occur throughout the length of the p53 gene. Anti-p53 auto-antibodies are commonly produced in response to these p53 mutations. This review firstly describes the various mechanisms of p53 dysfunction and their association with subsequent carcinogenesis. Following this, the mechanisms of induction of anti-p53 auto-antibody production are shown, with various hypotheses for the discrepancies between the presence of p53 mutation and the presence/absence of anti-p53 auto-antibodies. A systematic review was performed with a descriptive summary of key findings of each anti-p53 auto-antibody study in all cancers published in the last 30 years. Using this, the cumulative frequency of anti-p53 auto-antibody in each cancer type is calculated and then compared with the incidence of p53 mutation in each cancer to provide the largest sample calculation and correlation between mutation and anti-p53 auto-antibody published to date. Finally, the review focuses on the data of anti-p53 auto-antibody in colorectal cancer studies, and discusses future strategies including the potentially promising role using anti-p53 auto-antibody presence in screening and surveillance.
Core tip: Anti-p53 auto-antibodies are commonly produced in response to p53 mutations. Anti-p53 auto-antibody titres generally increase with tumour load, but not all patients who are initially sero-negative develop an auto-antibody response despite disease progression and metastases. Conversely, sero-positive patients do not lose their anti-p53 auto-antibodies despite the cancer being completely excised. In general, cancers with the highest p53 mutation rate, e.g., oesophageal and ovarian, demonstrate the highest anti-p53 auto-antibody rates; conversely, melanoma and testicular carcinoma with the lowest mutation rate have the lowest serum auto-antibody levels. Measurement of anti-p53 auto-antibodies may be useful in screening or monitoring for tumour recurrence.
-
Citation: Suppiah A, Greenman J. Clinical utility of anti-
p53 auto-antibody: Systematic review and focus on colorectal cancer. World J Gastroenterol 2013; 19(29): 4651-4670 - URL: https://www.wjgnet.com/1007-9327/full/v19/i29/4651.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i29.4651
The p53 gene is located on the distal band of the short arm of chromosome 17p13[1,2]. It consists of approximately 20000 base pairs spread over 11 exons[2-5]. p53 was initially discovered in 1979 as a protein binding to a viral oncogene, Simian Vacuolating 40 (SV40) large T-antigen, and hence was thought to be an oncogene itself[6-8]. It has since been established that it has a critical role as a tumour-suppressor gene[9-11]. p53 inactivation predisposes cells to malignant transformation in rodent models and in human clinical diseases such as Li-Fraumeni syndrome; the latter being characterized by germline mutations of p53[12-14]. The tumour suppressive role of p53 is so crucial that it is referred to as “the guardian of the genome”[15,16]. It is the most common mutation found in cancers and is present in half of all solid tumours thus emphasising its importance in protecting cells from carcinogenesis[3]. The frequency of mutation varies in individual cancers ranging from 5%-12% in cervical and haemopoietic malignancies to 40%-50% in colorectal and ovarian cancer[1,5]. Additionally, the remaining cancers with no detectable p53 mutation are still thought to have dysfunctional p53 caused by mechanisms other than mutation[9,17-20]. The most recent advances in colorectal cancer (CRC) treatment have been in the field of immunology with the use of antibodies against potent growth factors including epidermal growth factor receptor (EGFR) and vascular endothelial growth factors (VEGF)[21,22]. As such, p53, with its diverse immuno-regulatory role maintains a vital role in future of management of cancer and benign diseases. This review begins with the description of the normal p53 gene function and mechanisms of p53 inactivation in cancer, followed by a systematic review of the association between the anti-p53 auto-antibody response and underlying p53 mutations, and finally a clinical focus on the current evidence and potential future role of anti-p53 auto-antibody in colorectal cancer.
p53 acts as a tumour suppressor by preventing propagation of defective cells. It is up-regulated by various upstream factors in response to cellular stress or damage such as DNA damage, hypoxia, telomere shortening and oncogenic stimulation or radiation[2,11]. Activated p53 modifies downstream gene expression and co-factor transcription, which in conjunction with p53, lead to growth arrest (e.g., via p21WAF1) or apoptosis (e.g., p53-upregulated modulator of apoptosis, PUMA)[19,23,24].
The p53 gene encodes for a 393 amino-acid, 53 kDa, phospho-protein which is divided into 3 domains-an amino (-NH2) terminal region (approximately amino acids 1-100), a central “core” domain (amino acids 100-300) and a terminal carboxyl (-COOH) region (amino acids 320-360)[25-27]. Almost all mutations are harboured in the central “core” which contains the DNA-binding regions. Thus p53 dysfunction is most likely caused by mutations that alter DNA binding behaviour. However, most anti-p53 auto-antibodies do not recognise central core mutations but rather recognise epitopes in the 2 terminal regions. An interesting observation is that these terminal regions which contain the least mutations are also found on the wild-type and the mutant p53 protein[25,27,28]. This suggests anti-p53 is not only produced in response to mutation but also elevated levels of normal p53. This is discussed later (see Anti-p53 Auto-antibody).
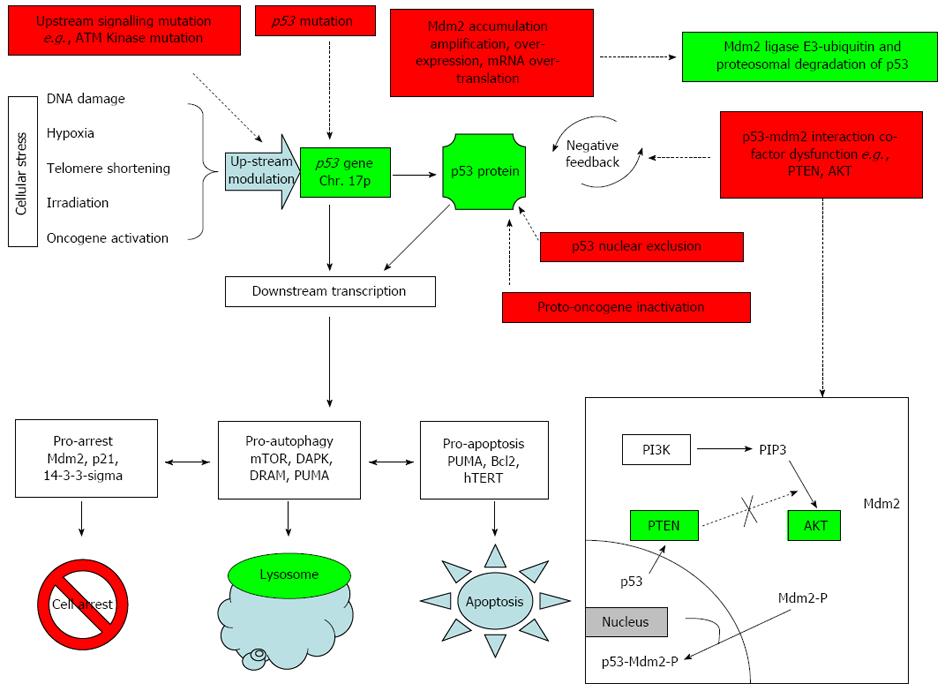
Wild-type p53 protein expression is intra-nuclear with a half-life of 5-30 min and is subject to complex regulation[29]. The most important regulator is thought to be Murine Double Minute 2 (Mdm2)[29,30]. Mdm2 is an ubiquitin-dependant E3 ligase which targets wild-type p53 protein for nuclear and cytoplasmic proteasome-mediated degradation[31,32]. When up-regulated p53 binds to the Mdm2 promoter leading to increased levels of mdm-2 transcription; the Mdm2 gene product then inhibits p53 thus creating a negative feedback loop. This feedback process is complex and regulated by multitude of factors. Mdm-2 in itself is subject to modifications mainly self-degradation by (1) auto-ubiquitination[33,34]; (2) small Ubiquitin-like Modifier (SUMO)-ylation[35]; (3) acetylation[36]; (4) post-translational upstream kinases (e.g., ATM kinase phosphorylation of Mdm2 and Mdm-X)[37-39]; (5) Mdm-2 in conjunction with Mdm-X (also known as Mdm-4) can form a Mdm2-MdmX-p53 complex which represses p53 activity; and (6) Mdm-X can furthermore act independently of Mdm-2 and repress p53-bound chromatin, without Mdm2, most likely by direct binding. The combinations of these mechanisms, regulate p53 accumulation in response to various cellular stresses (Figure 1).
p53 increases in response to cellular stress caused by a variety of insults including DNA damage, oncogene activation, ribosomal stress and hypoxia[11] by several mechanisms: (1) increased transcription; (2) increased intra-nuclear accumulation of active p53; (3) increased extra-nuclear export of Mdm-2[40,41]; (4) down-regulation of Mdm2-Mdmx which usually represses chromatin-bound-p53[24]; (5) various downstream post-translational modifications of both p53 and its regulators e.g., Mdm2[17]; and (6) raised cytosolic p53[42-45].
Active p53 has tumour suppressive activity by causing cell cycle arrest, apoptosis and autophagy. Cell cycle arrest initially provides additional time for the cell to repair damaged DNA. However, cells unable to repair damage are directed towards apoptosis by shifts in the balance between pro-arrest, pro-autophagy and pro-apoptotic factors severe cellular damage, the cell pushed directly towards apoptosis by the relative increase in pro-apoptotic markers relative to cell-cycle arrest promoters[19].
Autophagy is an evolutionary catabolic process of mass lysosomal self-degradation of cytosol/proteins/organelles which are sequestered into a double membrane vesicle which is then fused with lysosomes for bulk degradation[42,43]. p53 plays a dual role in activating and/or inhibiting autophagy by transactivating numerous autophagy regulators including mammalian target of rapamycin (mTOR)[46], activated protein kinase (AMPK) and tuberous sclerosis protein (TSC2)[43,47]. p53 is also able to influence the decision between apoptosis and autophagy by selectively activating pro-autophagy proteins such as AMPK, death-associated protein kinase 1 (DAPK-1) and damage-regulated autophagy modulator (DRAM)[48]. Alternatively, p53 also promotes apoptosis by activating pro-apoptotic markers such as B-cell Lymphoma 2 (Bcl-2), Bcl-2 associated death protein (BAD), Bcl-2 associated X-protein (BAX), p53-upregulated modulator of apoptosis (PUMA)[49] and autophagy inhibitors such as TP-53 induced glycolysis and apoptosis regulator proteins (TIGAR)[42].
Disruption of p53 gene transcription function and subsequent production of an inactive mutant p53 protein allows cells to escape the cellular arrest/apoptosis controls. This allows unregulated propagation of abnormal cells and a predisposition to malignant transformation. It is important to be aware that in vivo p53 behaviour can be different from in vitro response. This could be due to different stress types[50,51], cell types[52] and immune responses[24,53,54].
The most common cause of p53 inactivation is mutation which most frequently occurs within the p53 core, and furthermore 70% occur at “hot spots”-amino acids 132-142, 151-159, 172-179, 237-249 and 272-286[26,55]. The International Agency for Research on Cancer (IARC) TP53 database similarly reports the most frequent p53 mutations at codons 175, 245, 248, 249, 273 and 282; which is further corroborated by the UMD-TP53 mutation database, another international database that spans over 25 years[1,3,56]. The most common type of mutation is mis-sense (73%) followed by frame shift (9%), non-sense (8%), silent (4%) and others (6%)[1,3,57].
p53 is also inactivated by mechanisms other than p53 gene mutation and are described below (Figure 1).
Proto-oncogenes are normal proteins that become oncogenic with relatively minor modifications[6]. These proto-oncogenes are usually important cell cycle regulators (e.g., p14ARF)[58]. Human papilloma virus (HPV) 16/18 E6 protein which causes cervical cancer is able to inactivate p53 without mutation. This explains the relatively low incidence of p53 mutation in cervical cancer[59,60]. The SV40 large-T-antigen is another viral oncogene which is able to inactivate wild-type p53[61].
Mdm2 is a negative regulator of p53 and reduces the cell’s ability to trigger the pro-arrest/apoptotic pathway in the event of cellular damage[62,63]. Mdm2 over-expression can occur by gene amplification, gene over-expression or mRNA over-transcription[20,56]. Mdm2 over-expression is classically observed in soft tissue sarcomas[64,65]. Interestingly, instead of a decrease in p53 expression, the levels of both Mdm2 and p53 expression are increased. This suggests Mdm2 may have an additional p53-independent oncogenic mechanism (in addition to p53 suppression by negative feedback) which can promote tumour growth.
Mutations of in the p53-Mdm-2 feedback loop such as AKT Kinase, Phosphatidylinositol-3-kinase (PI3K), Phosphatase and Tensin Homolog (PTEN) and Ataxia Telangiectasia Mutated (ATM)-Kinase can inappropriately influence levels of p53 (see detailed description below). p53 disruption has also been associated with inactivation of other tumour suppressors e.g., BRCA1, Bcl-2, transforming growth factor (TGF)-β. AKT-kinase not only influences p53 levels but forms an apoptotic pathway with mTOR, an autophagy marker, in the PI3K/AKT/mTOR pathway demonstrating the complex interplay between p53 and the relative levels of its regulators in deciding cell fate[19,42,43]: (1) AKT-kinase phosphorylates Mdm2 and induces migration of phosphorylated-Mdm2 into the nucleus where it inactivates p53. AKT over-expression has been shown to occur in cancer cells[66,67]; (2) PTEN is tumour-suppressive and activated in response to stress leading to p53 up-regulation. Wild-type PTEN inhibits AKT-kinase phosphorylation of Mdm-2 and thus, intra-nuclear Mdm2 migration which suppresses p53 activity[29,68,69]. In contrast mutated PTEN is unable to inhibit AKT-kinase which leads to continuous Mdm2- phosphorylation and Mdm-2 intra-nuclear migration leading to reduced p53 tumour suppressive ability[69,70]; and (3) Cell stress (e.g., irradiation) activates factors up-stream of p53 such as ATM kinase and checkpoint Kinase-2[54,65]. Mutated ATM-kinase is unable to activate p53 in response to radiation-induced stress.
Extrusion of p53 into cytoplasm has been observed in certain tumours such as breast[71], colon[72], neuroblastoma[73] and malignant melanoma[74]. Nuclear extrusion prevents p53 from performing its intra-nuclear interactions.
Mutant p53 has an impaired ability to regulate cell cycle which is referred to as “loss of function”[3,6,75]. In addition to this, mutated p53 can also exhibit conformational changes which result in acquisition of new pro-oncogenic abilities; this is known as “gain of function”. Such functions include increased transcription of tumour-promoting factors such as MYC and VEGF[76] and disruption of protective pro-apoptotic factors such as p73[7,77].
An anti-p53 auto-antibody response was first reported by Crawford et al[78] in 1982 in 9% (14/155) of patients with breast cancer. Further interest in this anti-p53 auto-antibody response declined due to the lack of accurate quantification methods and no observable clinical relevance. Research into the auto-antibody was invigorated in the 1990s when the critical role of p53 gene in carcinogenesis was recognized. The exact cause of induction of anti-p53 auto-antibody production is unknown but is thought to be associated with the presence of p53 mutation and p53 protein over-expression.
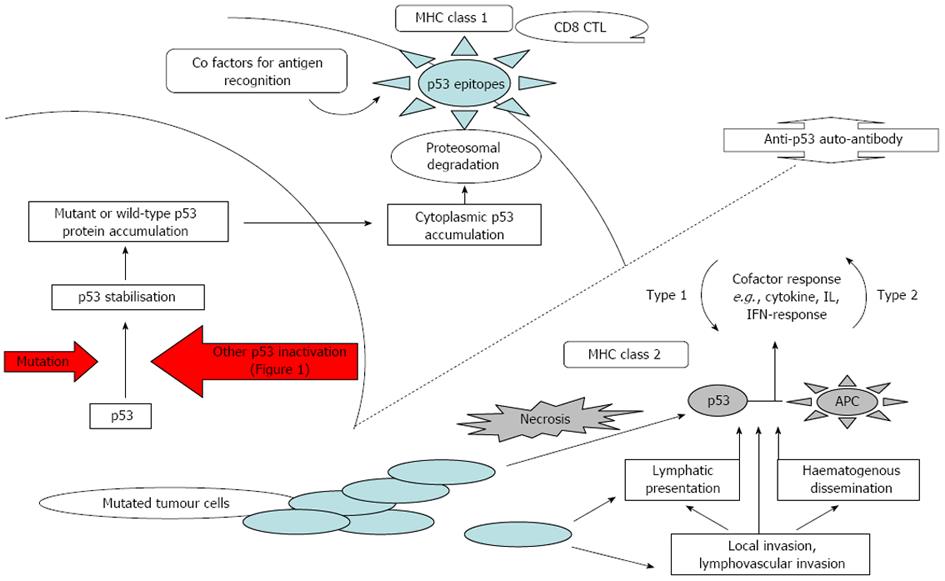
An anti-p53 auto-antibody is not normally produced wild-type p53 protein induces tolerance of the host[32,79]. In abnormal cells, mutant p53 protein is stabilised as discussed above which cause relatively high intra-nuclear p53 protein accumulation which then escapes into the cytoplasm. The resulting high cytoplasmic p53 levels increase the likelihood of p53 protein being degraded by proteasomes and presented on cell surfaces to be recognised by T-cells in a MHC I response[16]. Auto-antibodies recognises epitopes on the terminal regions of the protein, and hence auto-antibody production can theoretically be triggered by either the wild-type or the mutant p53, provided sufficiently high levels of these immuno-dominant epitopes are present at the cell surface[80]. Another probable antigen presentation mechanism is where cancer cells containing high cytoplasmic concentrations of p53 undergo necrosis and release p53 into the blood and lymphatic system where appropriate B-cells can interact. These antigens are also captured by Antigen Presenting Cells (APC) in their normal scavenging role and are presented in association with MHC class II response causing a Th2-like cell response[16] (Figure 2).
P53 mutation alone is insufficient to trigger anti-p53 auto-antibody production as evidenced by several observations. Firstly, only 20%-50% of patients with detectable p53 mutations produce detectable auto-antibodies[81,82]. This is attributed to the type of mutation, e.g., mis-sense mutations are associated with higher auto-antibody production compared with other mutations[5,56,57,83]. This is probably because mis-sense mutations are more likely to produce a stable mutant p53 protein which is more likely to accumulate to sufficient levels to increase the likelihood of antigen presentation. Other mutations such as non-sense, frameshift and deletions often lead to truncated mRNA and unstable protein sequences which are less likely to accumulate, and thus less likely to induce auto-antibody production[84]. Secondly, anti-p53 auto-antibodies most frequently recognise terminal epitopes but not the central domain with the majority of mutations[25,27,28,81]. Thirdly, large SV40 T-antigen stabilises p53 protein leading to accumulation of the wild-type protein which also induces auto-antibody production. Together these observations suggest that humoral response is triggered by elevated p53 protein levels per se (mutated and/or wild-type) rather than specifically directed at a mutated sequence.
There are discrepancies between the presence of p53 mutation, p53 protein product expression and anti-p53 auto-antibody production. This is largely attributed to the methodological differences of detection. Initial gene screening studies reported that most p53 mutations were localised to exons 5-8 and to a lesser extent 4, 9, 10. Subsequent studies then only tended to screen these regions leading to substantial screening bias. It is now known that at least 10% of p53 mutations occur outside these areas[84,85]. Another source of methodological difference is p53 protein detection which is inherently subject to tissue sampling and biopsy errors. Older studies (pre-1999) had different immuno-histochemical, fixation, paraffinization, antigen and antibody retrieval and observer scoring techniques. Finally, the antibody used to detect the mutant p53 protein affects sensitivity of p53 protein detection in IHC and also the detection of anti-p53 auto-antibody in ELISA as described below.
Historically, the auto-antibody was initially detected using immunoblots or in-house enzyme linked immunosorbent assay (ELISA). These ELISA used different cut-off values leading to a vast range of reported frequencies of anti-p53 auto-antibody within individual cancers in many older studies. Although standardised commercial ELISA are now widely available leading to an increase in anti-p53 ELISA studies (2000 onward), auto-antibody detection can still vary depending on different manufacturers’ product[86]. Most importantly, these ELISAs only measure an antibody response against those p53 epitopes, which are expressed by the recombinant proteins used as the coating antigen. This may account for the reason that there are minor variations in commercial ELISA studies in different populations but when the same ELISA is used in the same population, inter and intra-coefficient of variations of 0.3%-2.7% are extremely reliable[82].
Finally, the differences in individual’s immune systems cannot be ignored. The humoral response is dependent on an individual’s unique MHC presentation as shown by several observations. Firstly, patients with similar cancers containing the same p53 mutations do not necessarily mount the same immune response[81]. Secondly, whilst anti-p53 auto-antibody titres increase in response to tumour load, all patients who are initially sero-negative do not develop an auto-antibody response despite disease progression and metastases. Conversely, patients who are sero-positive at diagnosis do not sero-convert to a negative anti-p53 auto-antibody status even after the cancer is completely excised. It seems that once the patient’s immune system has been primed, there is sufficient p53 antigen available to maintain a long-term anti-p53 humoral response[28,87,88].
Literature searches were performed using Medline and PubMed up to January 2012. Keywords used were “p53”, “anti-p53”, “antibody”, “auto-antibody”, “cancer” and combinations. No language or time restrictions were applied. All abstracts were reviewed and the relevant articles retrieved. The results of all published anti-p53 auto-antibody cancer studies were accumulated and compiled in Table 1 with relevant key findings. The anti-p53 auto-antibody frequency from all published studies in each cancer type was calculated in this review. This calculated anti-p53 auto-antibody frequency was then correlated with reported p53 mutation rates to determine the associated between anti-p53 auto-antibody presence and mutation in each cancer (Figure 3).
| Group | Ref. | Anti-p53 positive | Summary of study and tumour type |
| Park et al[107] | 4/79 (5) | Comparative study with lung cancer | |
| Healthy/Benign | Wu et al[133] | 9/879 (1) | Case-control study of anti-p53 in various cancers |
| Kulić et al[134] | 1/20 (5) | Comparative study with breast carcinoma | |
| Suppiah et al[130] | 0/28 (0) | Comparative study with colorectal carcinoma | |
| Cai et al[125] | 0/30 (0) | Comparative study with oesophageal carcinoma | |
| Atta et al[135] | 5/29 (17.2); 13/26 (50)1 | Comparative study with hepatocellular carcinoma | |
| Mattioni et al[136] | 0/64 (0) | Comparative study with gastric carcinoma | |
| Akere et al[137] | 4/45 (8.9) | Comparative study with hepatocellular carcinoma | |
| Müller et al[123] | 0/57 (0); 0/379 (0)2 | Single study of anti-p53 in various cancers | |
| Chang et al[85] | 0/40 (0) | Comparative study with colorectal carcinoma | |
| Fonseca et al[95] | 0/15 (0) | Comparative study with glioma | |
| Shimada et al[82] | 10/205 (6.3); 13/189 (7)3 | Multi-institutional study of anti-p53 in various cancers | |
| Neri et al[138] | 0/51 (0) | Comparative study with lung carcinoma | |
| Numa et al[139] | 0/9 (0) | Comparative study with uterine, ovarian, cervical carcinoma | |
| Mack et al[140] | 1/46 (2.2) | Comparative study with SCLC | |
| Chow et al[141] | 1/28 (3.6) | Comparative study with head and neck carcinoma | |
| Moch et al[142] | 2/130 (1.5) | Comparative study with skin carcinoma (SCC/BCC) | |
| Hofele et al[143] | 0/80 (0) | Comparative study with oral SCC | |
| Hagiwara et al[144] | 0/13 (0) | Comparative study with oesophageal carcinoma | |
| Ralhan et al[145] | 4/50 (8) | Comparative study with lung carcinoma | |
| Bielicki et al[111] | 0/28 (0) | Comparative study with colorectal carcinoma | |
| Soussi[90] | 35/2404 (1.5) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 102/4924 (2.1) | ||
| Blanchard et al[146] | 24/97 (28) | Correlates with decreased overall and disease free survival | |
| Oesophageal | Wu et al[133] | 4/29 (13.8) | Case-control study of anti-p53 in various cancers |
| Cai et al[125] | 18/46 (39.1) | Correlates with advanced histological grade, stage, lymph node metastases and decreased tumour response following radiotherapy | |
| Müller et al[123] | 10/50 (20) | No correlation with stage or prognosis | |
| Bergström et al[147] | 31/42 (73.8) | No correlation with clinico-pathological parameters, tumour size or survival | |
| Shimada et al[82] | 90/301 (29.9) | Multi-institutional study of anti-p53 in various cancers | |
| Kozłowski et al[148] | 20/75 (26.6) | No correlation with stage, lymph node metastases or size. | |
| Shimada et al[99] | 14/35 (40) | Correlates with tumour p53 protein expression but not clinico-pathological parameters | |
| Hagiwara et al[144] | 13/46 (28) | Correlates with increased stage and tumour p53 protein expression but not prognosis | |
| Ralhan et al[145] | 36/60 (60) | Correlates with tumour p53 protein expression and missense mutations but not clinico-pathological parameters. | |
| Soussi[90] | 85/274 (31) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 345/1055 (32.7) | ||
| Head/Neck16 | Wu et al[133] | 1/20 (5.0) | Case-control study of anti-p53 in various cancers |
| Shimada et al[82] | 10/31 (32.3) | Multi-institutional study of anti-p53 in various cancers | |
| Chow et al[141] | 23/75 (31) | Correlates with nodal metastases but not prognosis | |
| Total | 34/126 (27.0) | ||
| Oral | Wu et al[133] | 5/15 (33.3) | Case-control study of anti-p53 in various cancers |
| Hofele et al[143] | 19/102 (18.6)4; 12/24 (50)5 | Correlates with poor prognosis | |
| Castelli et al[149] | 3/61 (18.7); 9/13 (69.2)3 | Serum anti-p53 is useful as a screening tool in pre-malignant lesions | |
| Soussi[90] | 309/1062 (29.1) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 348/1219 (28.5) | ||
| Ovary | Wu et al[133] | 5/12 (41.6) | Case-control study of anti-p53 in various cancers |
| Qiu et al[150] | 36/92 (39.1) | Correlates with p53 expression, not clinico-pathological parameters | |
| Shimada et al[82] | 2/27 (7.4) | Multi-institutional study of anti-p53 in various cancers | |
| Numa et al[139] | 8/30 (27) | Correlates with p53 tumour expression and poor prognosis | |
| Abendstein et al[151] | 28/113 (25); 21/113 (19)6 | Correlation between serum and ascitic anti-p53. No correlation with stage or grade. Anti-p53 in ascites associated with poor prognosis | |
| Soussi[90] | 140/635 (22) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 219/909 (24.1) | ||
| Colorectal(detailed results in Table 2) | Wu et al[133] | 11/66 (16.7) | Case-control study of anti-p53 in various cancers |
| Suppiah et al[130] | 20/92 (21.7) | No correlation with stage or prognosis | |
| Nozoe et al[97] | 17/36 (47.2) | Correlates with advanced lymph node status and stage | |
| Müller et al[123] | 63/197 (32)7; 7/46 (15.2)8 | No correlation with stage or prognosis | |
| Chang et al[85] | 47/167 (28.1) | p53 mutation, not anti-p53, correlates with poor prognosis | |
| Lechpammer et al[88] | 40/220 (18.2) | ? Correlation with stage or prognosis in Dukes’ A/B1 | |
| Shimada et al[82] | 46/192 (23.9) | Multi-institutional study of anti-p53 in various cancers | |
| Forslund et al[84] | 24/88 (27.3) | Correlates with p53 mutation | |
| Tang et al[89] | 130/998 (13) | Correlates with advanced lymph node involvement but not prognosis | |
| Broll et al[152] | 20/130 (15.4) | No correlation with stage or prognosis | |
| Takeda et al[98] | 17/27 (63) | 95% negative sero-conversion within 3 wk post-surgery | |
| Shiota et al[112] | 18/71 (25.4) | Correlates with advanced stage and poor prognosis | |
| Bielicki et al[111] | 30/145 (20.7) | ? Correlation with Dukes’ A →B | |
| Soussi[90] | 307/1244 (24.7) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 797/3719 (21.4) | ||
| HCC | Wu et al[133] | 15/93 (16.1) | Case-control study of anti-p53 in various cancers |
| Atta et al[135] | 28/41 (68.3) | Correlates with advanced stage and shorter survival. | |
| Akere et al[137] | 5/41 (12.2) | Correlates with increased Okuda stage | |
| Müller et al[123] | 19/80 (23.8) | Non-significant trend towards poor prognosis | |
| Charuruks et al[153] | 26/141 (18.4) | Correlates with stage but not tumour p53 protein expression | |
| Tangkijvanich et al[154] | 16/121 (13.2)17 | Preliminary report of Charuruks et al (2001). No correlation with severity, stage or prognosis. Survival too short for survival analysis (3 mo vs 4 mo) | |
| Sitruk et al[155] | 19/159 (12) | Correlates with multinodular, infiltrative tumour but not survival | |
| Soussi[90] | 82/387 (1.2) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 210/1063 (19.8) | ||
| Wu et al[133] | 0/11 (0) | Case-control study of anti-p53 in various cancers | |
| Bladder | Müller et al[123] | 3/24 (12.5) | No correlation with prognosis |
| Watanabe et al[156] | 17/63 (27)9 | Correlates with higher grade, stage, lymph node metastases and tumour p53 protein expression, but not prognosis | |
| Gumus et al[157] | 14/80 (17.5) | Correlates with tumour p53 protein expression and poor prognosis. | |
| Gumus et al[158] | 25/76 (33) | Negative sero-conversion post-treatment (35%, 8/23) associated with good prognosis. | |
| Shimada et al[82] | 4/33 (12.1) | Multi-institutional study of anti-p53 in various cancers | |
| Morita et al[159] | 12/100 (12) | Correlates with stage, and p53 protein expression but not prognosis | |
| Wunderlich et al[160] | 4/32 (12.5) | Correlates with tumour protein p53 expression but not stage. | |
| Soussi[90] | 8/29 (27.6) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 70/385 (18.2) | ||
| Lung | Park et al[107] | 28/82 (34.1) | Sensitivity study with other markers for lung cancer |
| Wu et al[133] | 13/95 (13.7) | Case-control study of anti-p53 in various cancers | |
| Bergqvist et al[161] | 14/84 (16.6) | No correlation with tumour volume. Correlates with survival in adenocarcinoma, but not SCC | |
| Bergqvist et al[162] | 12/58 (20.7) | No correlation with tumour volume or lymph node metastases | |
| Neri et al[138] | 2/30 (6.7)10; 8/48(16.7)11 | No correlation with stage, histology or prognosis. Non-significant increased survival in LC but not MM | |
| Cioffi et al[163] | 35/109 (32.1) | Low sensitivity, but high specificity (100%) and accuracy (69%). Only 14% agreement with other tumour markers (CEA/TPA, CYFRA21-1, NSE.) | |
| Zalcman et al[126] | 20/97 (20.6) | Correlates with poor prognosis in limited stage SCLC, but not all SCLC | |
| Mack et al[140] | 4/35 (11.1)12; NSCLC 13/99 (13.3)13 | Correlates with stage and prognosis in NSCLC but not SCLC | |
| Shimada et al[82] | 18/125 (14.4) | Multi-institutional study of anti-p53 in various cancers | |
| Soussi[90] | 219/1282 (17.1) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 373/2049 (18.2) | ||
| Cervix | Shimada et al[82] | 10/53 (18.9) | Multi-institutional study of anti-p53 in various cancers |
| Numa et al[139] | 12/86 (14) | No correlation with tumour p53 protein expression or prognosis | |
| Total | 22/139 (15.8) | ||
| Wu et al[133] | 7/43 (16.3) | Case-control study of anti-p53 in various cancers | |
| Gastric | Qiu et al[150] | 19/61 (31.1) | Correlates with tumour size but not prognosis. |
| Mattioni et al[136] | 17/111 (15.3) | Correlates with tumour p53 protein expression, prognosis and survival | |
| Lawniczak et al[164] | 16/71 (22.5) | Correlates with tumour type and age, but not stage or prognosis | |
| Müller et al[123] | 14/122 (11.5) | No correlation with prognosis | |
| Shimada et al[82] | 13/123 (10.6) | Multi-institutional study of anti-p53 in various cancers | |
| Nakajima et al[165] | 13/81 (16) | Correlates with lymph node metastases but not stage or prognosis | |
| Maehara et al[166] | 23/120 (19.2) | Correlates with increased stage and tumour p53 protein expression but not prognosis | |
| Soussi et al[90] | 105/727 (14.1) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 227/1459 (15.6) | ||
| Nozoe et al[167] | 15/42 (35) | Correlates with grade 3 and triple negative cancer | |
| Breast | Wu et al[133] | 9/25 (16) | Case-control study of anti-p53 in various cancers |
| Kulić et al[134] | 21/61 (35) | Correlates with decreased 5 year survival | |
| Müller et al[123] | 17/50 (34) | Non-significant trend towards poor prognosis | |
| Gao et al[168] | 31/144 (21.5) | Correlates with stage, lymph node metastases, ER negative, c-erb-2 and tumour p53 protein expression | |
| Shimada et al[82] | 13/71 (18.3) | Multi-institutional study of anti-p53 in various cancers | |
| Volkmann et al[169] | 18/165 (10.9) | Poor concordance between recombinant/native p53 ELISA, immunoblot and immunofluorescence | |
| Metcalfe et al[87] | 155/1006 (15.4) | No correlation with stage and prognosis | |
| Soussi[90] | 296/2006 (14.8) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 539/3467 (15.5) | ||
| Uterus | Wu et al[133] | 1/13 (7.7) | Case-control study of anti-p53 in various cancers |
| Shimada et al[82] | 5/22 (22.7) | Multi-institutional study of anti-p53 in various cancers | |
| Numa et al[139] | 5/41 (12) | No correlation with tumour p53 expression/prognosis (see Cervix, Ovary) | |
| Total | 11/79 (13.9) | ||
| Pancreas | Wu et al[133] | 0/17 (0) | Case-control study of anti-p53 in various cancers |
| Müller et al[123] | 5/22 (22.7) | Increase sensitivity in conjunction with CA19-9. No correlation with prognosis. | |
| Shimada et al[82] | 3/28 (10.7) | Multi-institutional study of anti-p53 in various cancers | |
| Ohshio et al[170] | 19/82 (23.2) | No correlation with tumour p53 expression or prognosis | |
| Soussi[90] | 60/650 (9.2) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 87/799 (10.9) | ||
| Lymphoma | Messmer et al[171] | 19/120 (15.8) | Associated with 17p deletions |
| Wu et al[133] | 0/18 (0) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Soussi[90] | 19/248 (14.3) | Case-control study of anti-p53 in various cancers | |
| Total | 38/386 (9.8) | ||
| Biliary tract16 | Wu et al[133] | 1/8 (6.3) | Correlates with tumour p53 protein expression but not stage |
| Limpaiboon et al[172] | 6/49 (12.2) | Multi-institutional study of anti-p53 in various cancers | |
| Shimada et al[82] | 1/6 (16.7) | Correlates with tumour p53 mutation | |
| Tangkijvanich et al[173] | 6/82 (7.3) | ||
| Total | 14/145 (9.7) | ||
| Haematological | Wu et al[133] | 8/33 (25) | Case-control study of anti-p53 in various cancers |
| Shimada et al[82] | 32/364 (6.3)14 | Multi-institutional study of anti-p53 in various cancers | |
| Soussi[90] | 14/428 (3.3)15 | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 54/825 (6.5) | ||
| Glioma | Wu et al[133] | 1/24 (4.2) | Case-control study of anti-p53 in various cancers |
| Fonseca et al[95] | 5/24 (20.8) | No correlation with p53 protein but increased in patients < 16 years | |
| Shimada et al[82] | 2/31 (6.5) | Multi-institutional study of anti-p53 in various cancers | |
| Soussi[90] | 6/144 (4.2) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 14/223 (6.3) | ||
| Prostate | Wu et al[133] | 1/8 (12.5) | Case-control study of anti-p53 in various cancers |
| Shimada et al[82] | 4/23 (17.4) | Multi-institutional study of anti-p53 in various cancers | |
| Soussi[90] | 4/148 (2.7) | Literature review of anti-p53 in various cancers (1979-1999) | |
| Total | 9/179 (5.0) | ||
| Skin | Moch et al[142] | 3/105 (2.9) | No difference between controls and patients. Increased in aggressive SCC (8%) vs slow-growing BCC (1.5%) |
| Testicular | Soussi[90] | 0/144 (0) | Literature review of anti-p53 in various cancers (1979-1999) |
| Melanoma | Soussi[90] | 0/58 (0) | Literature review of anti-p53 in various cancers (1979-1999) |
| Total | 3419/18595 (18.4) | All cancers (1979-2012) |
| Ref. | Method and manufacturer | Samples | Follow-up | Key findings |
| Suppiah et al[130] | ELISA (p53 ELISAPLUS, Calbiochem, Darmstadt, Germany) | 20/92 (21.7); 0/20 (0)10/8 (0)2 | Median 97 mo | No correlation with tumour stage, differentiation or location. Multivariate analysis show only Stage (Dukes’ and TNM) to be independent prognostic factors |
| Nozoe et al[97] | ELISA (Pharmacell, France) | 17/36 (47.2) | Not stated | Anti-p53-ab (+) associated with greater lymphatic invasion (94.1%; 16/17 vs 68.4%; 13/19), nodal involvement (70/6%; 12/17 vs 17.6%; 3/17) and advanced stage (P = 0.02). Anti-p53 frequency higher in p53 protein expressing tumours (74%; 14/19 vs 18%; 3/17). Only 3 patients with Dukes’ A CRC, all sero-negative |
| Muller et al[123] | Immunoblot | Colon 63/197 (32); Rectum 7/46 (15.2); 0/57 (0)1 0/379 (0)2 | CRC patients enrolled into trial with 5 year follow-up | No correlation with clinico-pathological parameters or prognosis. Trend toward higher anti-p53 sero-positivity in N2/3 disease, poor differentiation and metastases. There were no patients with Dukes’ A in this study. Anti-p53 independent of CEA and CA19-9 with 16% information gain. This is the only study to report negative to positive sero-conversion (3.6%, 11/303) |
| Chang et al[85] | ELISA (p53-AK, Dianova, Hamburg, Germany) | 47/167 (28.1); 0/40 (0)1 | Median 36.3 mo (4-58) | Anti-p53 correlates with p53 mutation (43% vs 18%) but not tumour p53 expression, clinico-pathological features or prognosis. p53 mutations, advanced stage and pre-operative CEA > 5 ng/mL were independent prognostic factors (in that order). p53 mutation strongly associated with advanced stage and poor differentiation |
| Lechpammer et al[88] | ELISA (ELISAPLUS Oncogene Research Products, Cambridge, United States) | 40/220 (18.2); 0/42 (0)1 | 40 patients up to 20 wk; 8 patients up to 48 wk | Anti-p53 had higher tumour p53 expression (70% vs 52%). Anti-p53 frequency shows highest increase in Dukes’ A (0%, 0/28) →Dukes’ B: (24%, 21/87) but no increase in progression to Dukes’ C (18%,19/105). No correlation with overall tumour grade or metastases. Anti-p53 reflects tumour load following surgery, during chemotherapy and with disease recurrence |
| Shimada et al[82] | ELISA (Anti-p53 EIA Kit II, MESACUP anti-p53 Test; MBL; Nagoya, Japan) | 46/192 (23.9); 10/205 (4.9)1; 13/189 (6.9)2 | Not reported | Validation study for MESACUP ELISA using prevalence of anti-p53 in various cancers. Good intra- and inter-assay coefficient of variation of 1.85-2.37% and 0.3-3.2% respectively. Demonstrates stability of anti-p53 titres at room temperature for 7 d and following 10 freeze-thaw cycles. No comment on correlation with clinico-pathological parameters or prognosis |
| Forslund et al[84] | ELISA (Dianova, Hamburg, Germany) | 24/88 (27) | Not reported | Cross-sectional study on relationship between p53 mutations and anti-p53 presence. Frequency of p53 mutation higher in anti-p53 sero-positive group (92%, 22/64 vs 34%, 22/64) Correlation with clinico-pathological and survival parameters not reported |
| Tang et al[89] | ELISA (Calbiochem-Novabiochem, Darmstadt, Germany) | 130/998 (13);2/211 (1)3 | Not reported | Anti-p53 sero-positivity increases in progression from N2→N3 (2.9%-10.6%); but not N0→N1 (11.7%-12.3%), N1→N2 (12.3%-10.6%) or M0→M1 (12%-17%). No correlation with CEA, overall TNM stage or metastases. Anti-p53 associated with shorter survival in uni- but not multi-variate analysis. Largest study on anti-p53 in CRC |
| Broll et al[152] | ELISA (p53-autoantikorpfer ELISA, Dianova, Hamburg, Germany) | 20/130 (15); 0/44 (0)1 | Median 25.5 mo | Anti-p53 positive predictive value of 100%, but accuracy 37% and negative predictive value 29% due to poor sensitivity (15%). Anti-p53 correlated with p53 expression (P < 0.05), but not TNM stage, grade or location (exact numbers not shown). Approximately 70% of series Stage I/II CRC |
| Takeda et al[98] | Anti-p53 EIA(PharmaCell, Paris, France) | 17/27 (63); 1/38 (2.6)3 | Up to 2 yearsMedian not reported | Anti-p53 correlates with p53 protein expression and independent of CEA and CA-19-9. Sero-conversion in 94% (16/17) within 3 wk of endoscopic resection. No correlation with clinico-pathological parameters or prognosis/recurrence as all patients had early superficial CRC (23 mucosal, 4 submucosal invasion). This study reports exceptionally high anti-p53, especially considering very early CRC |
| Takeda et al[174] | ELISA (anti-p53-EIA kit, Pharmacell, Paris, France) | 40 patients with anti-p53 ab from previous studies | Up to 29 mo | No correlation between post-operative anti-p53 sero-positivity and histological (depth, lymphatic or venous invasion) or clinico-pathological features of lymph node or liver metastases. High (96%; 27/28) sero-conversion in patients with complete tumour resection. No sero-conversion in patients with residual disease. |
| Shiota et al[112] | ELISA (GIF, Munster, Germany) | 18/71 (25); 1/18 (6)3 | Not stated, median survival 56 mo anti p53 ab negative | Anti-p53 correlates with TNM stage (Stage I-IIIb: 9%, 4/45 vs IV: 56%, 14/25), Dukes’ stage (A-C: 9%, 4/45 vs D: 56%, 14/25), CEA, CA19-9 and tumour p53 protein expression. Anti-p53 associated with shorted survival (56 mo vs 20 mo) and is weak poor prognostic indicator. Anti-p53 prognostic significance secondary to other factors, including weak factors e.g., CEA and CA19-9. Only small number of Stage I-IIIb patients |
| Bielicki et al[111] | ELISA (Dianova, Hamburg, Germany) | 30/145 (21); 0/20 (0)2; 0/8 (0)3 | Not stated. Cross sectional study | No correlation with Dukes’ Stage (A/B: 22%, 16/73 vs C/D 19% 14/72), size, location, CEA. Highest increase in anti-p53 frequency from Dukes’ A (0%, 0/6) to Dukes B1 (28%, 5/18) but no further difference in progression to Dukes’ C (19%, 7/36). Only 6 Dukes’ A patients in study, all sero-negative |
| Soussi[90] | ELISA/WB/IP | 307/1244 (24.7) | ELISA/WB/IP | Review combining all studies with different methodologies from 1979-1999. Range of sero-positivity (12.5%-68% in 11 studies) |
| Total (1999-2009) | 479/2409 (19.9) | All modern studies (1999 onwards) using commercial ELISA only, with one exception using Immunoblot (Muller et al, 2006) | ||
| Review Total (1979-2009) | 786/3653 (21.5) | All studies on anti-p53 in CRC (1979-2009) |
All published studies on anti-p53 auto-antibody (1979-2012) were retrospective or cross-sectional case control series with relatively small sample size (27-220 subjects tested) with a heterogeneous mix of cancer stages. The largest single study was published by Tang et al[89] that included a cohort of 998 CRC patients with anti-p53 present in only small numbers (n = 130) for stage-specific analysis. An earlier non-systematic review by Soussi in 2000 recruited large numbers from various anti-p53 studies but was study quality was limited by different cancers at various stages and different auto-antibody detection methods[90]. The primary outcome was not stated in most studies, and none was powered appropriately for survival outcomes.
The reported frequency of anti-p53 auto-antibody in individual cancer studies vary significantly due to small sample sizes, stage bias (usually a greater proportion of advanced stage tumours were included and different detection methods used. Anti-p53 auto-antibody is usually measured in patients’ sera but has also been measured in ascitic fluid of patients with ovarian cancer[68], saliva of patients with oral cancer[91] and in pleural effusions (12.5%) associated with lung, colon and pancreatic cancer[92]. In a landmark review, Soussi compiled results of 80 anti-p53 auto-antibody studies in 18 cancer types over a 20 year (1979-1999) period[90]. The mean serum sero-positivity across all cancer types was 16.9% (1600/9489 patients, range 0%-31%) compared with 1.45% (35/2404) in controls thus demonstrating remarkable specificity (98%) but poor sensitivity. The specificity would be even higher as half the false positive subjects (17 out of 35) were from a single study reporting an extra-ordinarily high sero-positivity (24%, 17/70)[93](Table 1). When this study was excluded anti-p53 auto-antibody specificity is near 100% for any cancer, which is confirmed by most recent reports.
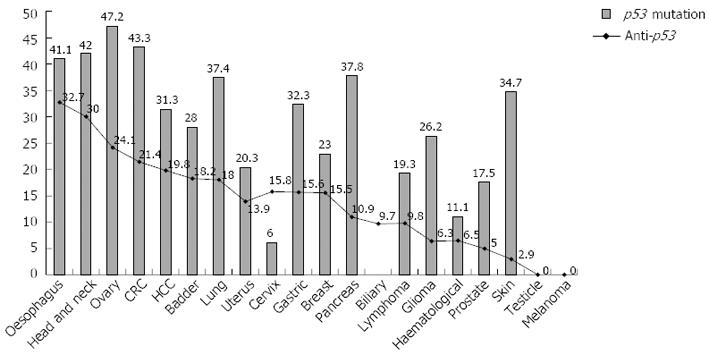
The 30-year cumulative sera anti-p53 auto-antibody frequencies in individual cancers were calculated in this review to provide the most comprehensive anti-p53 auto-antibody frequency in each cancer to date (Table 1). The auto-antibody frequencies are plotted (point) against the p53 mutation rate (bars) as reported by the IARC TP53 Mutation Database to ascertain a relationship between anti-p53 auto-antibody and p53 mutation rates in each cancer.
The graph shows moderate correlation (r2 = 0.45, correlation 0.59) between anti-p53 auto-antibody and p53 mutation (Figure 3). In general, cancers with the highest p53 mutation rate such as oesophageal, head and neck, and colorectal demonstrate highest anti-p53 auto-antibody rates[82,90]. Conversely, melanoma and testicular carcinoma with the lowest mutation rate have the lowest serum anti-p53 auto-antibody rates (< 1%)[90]. The two exceptions are gliomas and skin cancers which have moderate p53 mutation rate and low anti-p53 auto-antibody rate (Figure 3). Proposed reasons for the low anti-p53 auto-antibody production are poor brain antigenicity, poor p53 antigen-presentation across the blood-brain barrier, and use of immuno-suppressive steroids (dexamethasone) in the majority of glioma cases[90,94,95]. Similar arguments about poor antigen presentation across an epithelial barrier are made for the majority superficial skin cancer. In summary, anti-p53 auto-antibody has up to 35% sensitivity, depending on cancer type, nearly 100% specific for any malignancy but varies in individual cancer types; and demonstrates moderate correlation with p53 mutation rate of each cancer.
CRC has the second highest anti-p53 auto-antibody sero-positivity rates due in part to the high frequency of p53 mutation. Pre-1999, eight studies used an “in-house” developed ELISA, 1 used Western blotting (WB), 1 used immuno-precipitation (IP) and another used all 3 detection methods (ELISA, WB, IP)[90]. Despite these methodological differences, most studies, 10 out of 11, reported a sero-positivity rate between 12.5% and 32%. The only study to report a discrepant and much higher sero-positivity rate (68%) used WB thus demonstrating the potential bias caused by non-standardised detection methodology[96]. New standardised commercial ELISA kits have since been developed with less variation in sero-positivity (13%-27%) with intra- and inter-assay coefficient of variation of 1.85%-2.37% and 0.3%-3.32% respectively (MESACUP anti-p53 Test; Medical and Biological Laboratories, MBL, Nagoya, Japan)[82].
The mean sero-positivity from ELISA-only CRC studies calculated in this review was 19.9% (479/2409) with individual studies reporting of 13%-27% (Table 2). Only two studies reported inconsistently high rates of 47% and 63% in patients, and also in controls (2.6%), which suggests a lower cut-off value was used[97,98]. The same authors then reported an unusually high (40%) sero-positivity in superficial oesophageal carcinoma in another series, compared with 20%-30% in the majority of other studies. These studies used the same ELISA (Pharmacell, France)[99]. Interestingly, when the same authors later used a different ELISA (anti-p53 EIA Kit II, MESACUP) in a similar population, they reported a much lower sero-positivity of 30% (oesophageal cancer) and 24% (CRC) which was more consistent with other published ELISA studies[82]. This highlights potential methodological biases with anti-p53 auto-antibody quantification even with commercially standardised ELISA kits.
Cancer screening is used when early detection and intervention can lead to improved outcome for example CRC where 5 year survival in Dukes’ A is 95%-100% compared with 5% in Dukes’ D. Colonoscopy is the current gold-standard diagnostic tool but is painful, expensive and is associated with life-threatening complications such as colonic perforation (0.01%-0.3%) and haemorrhage (0.6%)[100,101]. The United Kingdom Flexible Sigmoidoscopy Screening Trial has provided evidence that one-off screening flexible sigmoidoscopy between age 55 and 64 was beneficial in reducing CRC incidence by 23%-33% and reducing mortality by 31%-43%[102,103]. Similar mortality reduction has been reproduced in other screening trials such as Norwegian Colorectal Cancer Prevention (NORCCAP)[104]. A recent meta-analysis similarly confirmed benefits of screening (endoscopy or stool-based screening) over an unscreened population in increasing detection and prognosis[105].
There are intuitive benefits of screening with serum anti-p53 auto-antibody compared to CT, barium enemas and colonoscopy. The titre is not subject to tumour sampling error, is quicker, cheaper, easier and less traumatic, thus making it more repeatable in the general population. The auto-antibody titre itself is remarkably stable, showing no significant change when stored at room temperature for up to 7 d, or when stored at -80 °C for 3 years[81]. Repeated freeze-thaw cycles (up to 10 cycles) have minimal or no effect on serum levels as immunoglobulins are generally robust proteins[82]. Also, anti-p53 auto-antibody appears to be independent of other conventional CRC tumour markers such as carcino-embryonic antigen (CEA) which means it could detect CRC in CEA-negative patients. The combined advantages of serum testing and the characteristics of anti-p53 auto-antibody (serum stability, 95%-100% specificity, independent of current tumour markers), makes anti-p53 auto-antibody a potentially valuable screening modality.
The role of p53 in screening is promising its specificity for cancer, but this enthusiasm is tempered by a low sensitivity (20%-30%). It would thus be required as part of a panel of tumour markers. This panel could then be used to guide more invasive investigations such as colonoscopy. Combined serum immuno-testing for 6 markers (CEA, anti-p53 auto-antibody, CYFRA 21-1, osteopontin, separase and ferritin) has been reported to have comparable sensitivity (> 80%) to faecal immuno-testing[106]. A similar tumour marker panel using CYFRA-21, CEA and anti-p53 has been used in lung cancer also with 80% sensitivity[107]. Combined biomolecular and endoscopic strategies[108] are being investigated, and in conjunction with other new diagnostic non-invasive modalities (e.g., CT-colonography)[109] may be able to further broaden the screening programmes for CRC and other cancers in the general population.
p53 mutation is usually a late event in the adenoma-carcinoma sequence and hence anti-p53 auto-antibody is unlikely to be present in early pre-invasive lesions where p53 mutations have not occurred[110]. The largest study reports 1% (2/211) sero-positivity in adenomas which increased to 6% in carcinoma in-situ[89]. This 1% could be due to undetected microfoci of invasive cancer within adenoma or changes that predate microscopic detection. The increase prevalence of anti-p53 auto-antibody to 6% in carcinoma in situ can be expected in these tumours which are at the end of the adenoma-carcinoma sequence with greater proportion of p53 mutation. This would then suggest that anti-p53 auto-antibody should increase with further growth (CRC stage) but this is not seen. Almost all studies reported no association between anti-p53 auto-antibody and CRC stage (Tables 1 and 2). This was reported in the largest cross-sectional series, and confirmed by other long-term follow-up studies[89] (Table 2). Only a handful of studies have suggested an association between anti-p53 and T-stage[88,111], selected nodal disease[89] and metastases[112]
Two studies reported increased anti-p53 in progression from Dukes’ A to B, but not with progression from Dukes’ B to C[88,111]. Lechpammer et al[88] reported 0% (0/28) anti-p53 in Dukes’ A which increased significantly to 9.6% (21/87) in Dukes’ B, but did not increase further with Dukes C (8.6%, 19/105). Bielicki et al[111] similarly reported increased anti-p53 auto-antibody from Dukes’ A (0%) to Dukes’ B (28% Dukes’ B1, 22% Dukes’ B2); but no increase in progression to Dukes C (19%). This suggests auto-antibody production is stimulated by early (Tis to T2) local invasion such as microvascular basement membrane invasion leading to antigen presentation; but not further progression. Further studies are required to understand the precise series of events in anti-p53 auto-antibody production.
Anti-p53 auto-antibody is produced in part due to response to p53-antigen presentation. Thus nodal involvement should also increase anti-p53 auto-antibody production by increasing probabilities of antigen presentation to the humoral system. However, there is no correlation between anti-p53 auto-antibody and nodal involvement in any of the studies (Table 2). Tang et al[89] suggested increased anti-p53 with “advanced” nodal disease (N3: > 10 regional nodes or systemic nodal metastases) compared to N0-2 CRC in selected analysis. We re-classified the data into node “positive” and “negative” disease and found no difference in sero-positivity of 12% vs 14% with nodal involvement (calculation not shown).
It would be expected that haematogenous cancer cell dissemination should invoke a further immune response but there has been no association between anti-p53 auto-antibody status and metastatic disease except one study[112]. In this study, anti-p53 auto-antibody had extremely high prevalence (56%, 14/25) in Stage IV disease, and unusually low prevalence in Stage I-III (9%, 4/45) leading to strong anti-p53 bias towards Stage IV disease. This is the only study to report anti-p53 auto-antibody association with stage and adverse prognosis which is discussed later (anti-p53 auto-antibody in prognosis in CRC).
Anti-p53 auto-antibody production is initially most likely to be produced in the final stages the adenoma-carcinoma sequence (in keeping with p53 mutation being a relatively late event. It is likely that anti-p53 auto-antibody production is no longer dependant on antigen-presentation, but rather now dependant on immune-recognition by (1) tumour factors e.g., p53 mutation type and conformation, presence of co-factors; and (2) patients’ immune-specific factors such as MHC expression required for recognition. This response is not sufficiently consistent to justify a separate clinico-pathological parameter of its own. In the future, anti-p53 auto-antibody may have some benefit in refining CRC stage if there is influence on prognosis or treatment, similar to k-RAS status in anti-EGFR and anti-VEGF therapy for CRC and liver metastases, or oestrogen- or progesterone-status in breast cancer.
CEA is the most common serum tumour marker used in CRC. It is a 180 kDa serum glycoprotein which is present at low levels in normal cells but over-expressed in adenocarcinoma, especially of the colon, rectum, breast and lung[113]. Pre-operative CEA presence has been associated with aggressive CRC and poor prognosis[114,115]. CEA has also been used as an adjunct in CRC screening, monitoring for disease recurrence following resection, or as part of tumour marker panel for metastases of unknown primary origin. CEA has high specificity (80%) with false elevations in smokers, inflammatory diseases, cirrhosis, obstructive jaundice, gastric ulcers, emphysema, diabetes and collagen vascular diseases[116-118].
CEA in isolation is not recommended for screening or detection of recurrence due to its variable sensitivity (30%-80%)[114,119]. CEA sensitivity can be modulated by changing the cut-off values for “positivity” but sensitivity has still remained low despite variations in the cut-off value used[120,121]. Despite this, The American Society of Surgical Oncology (ASCO) guidelines suggest serial CEA measurements every 3 mo in Stage II/III CRC for at least 3 years following diagnosis, and during treatment of metastatic disease[122].
Tumour markers used in conjunction with CEA could increase the efficacy of CRC screening in selected populations. Such tumour markers should be independent of CEA as to detect the CEA-negative CRC population and thus increase sensitivity of the tumour marker panel. The majority of studies have shown that anti-p53 auto-antibody is independent of CEA (Table 3). The two studies which report a positive correlation had results inconsistent with other studies, with the first study having an unusually strong association between anti-p53 auto-antibody and Stage IV disease (as discussed earlier)[112] and the second reporting the highest anti-p53 auto-antibody frequency (68%) and used WB, not ELISA[96]. Methodological difference and sample bias are most likely responsible for the results observed.
In this review, information is compiled from all studies reporting CEA and anti-p53 in Table 4. This shows that when used in isolation, anti-p53 auto-antibody can CEA can detect CRC in 17% and 42% respectively. If both tumour markers are used, the sensitivity increases to 51% (as both markers are absent in 48.9%). This results in information/sensitivity gain of +9% (compared with CEA alone); and +34% (compared with anti-p53 auto-antibody alone). The only other study to report “information gain” using anti-p53 auto-antibody in CRC confirmed reported mean increased sensitivity of 16% with individual increased sensitivity from 55% to 71% in colon cancer and 78% to 83% in rectal cancer[123]. This report is consistent with our calculation using data from all other published anti-p53 auto-antibody and CEA studies in CRC (Tables 3 and 4).
| CEA normal | CEA elevated | |
| Anti-p53 ab present | 112 (9.4) | 90 (7.6) |
| Anti-p53 ab absent | 584 (48.9) | 406 (34.1) |
The clinical utility of this “information gain” requires examination. CRC has low prevalence in the general population and thus pick-up rates would remain low despite the use of both tumour markers. Both markers also have preponderance towards later stage CRC (as opposed to Stage I) which reduces impact of earlier detection and thus screening efficacy. It is thus likely that these 2 markers alone are insufficient and additional markers would be required, i.e panel of 6 tumour markers was used to form a panel with sensitivity similar to faecal occult testing in population screening[108]. In post-operative surveillance, small studies have demonstrated overall 4 tumour markers (CEA, TPA, CA19-9, CA72.4) panel sensitivity of 81% compared with 9%-45% using individual markers[124]. Hence, the optimal strategy would be to use other markers in addition anti-p53 and CEA to select patients for investigations. The cost-effectiveness of these immunological-targeted strategies for general population screening, high risk population screening or post-operative surveillance requires further evaluation.
Anti-p53 auto-antibody may have its most promising role in post-operative monitoring for disease recurrence or distant metastases. Several, but not all, studies have demonstrated that anti-p53 reflects tumour load, with increasing serum titres corresponding with disease recurrence/progression and decreased titres following surgery/chemotherapy[81,88,120]. Lechpammer et al[88] produced the most convincing series demonstrating clear decreases with post-surgery and during chemotherapy. More importantly, increases, especially during chemotherapy, predated clinical diagnosis of recurrence. Smaller subset analysis in other studies has also demonstrated fluctuations in serum titres with disease load. Similar fluctuations with resection and radiotherapy have been reported in oral, oesophageal, lung, ovarian and breast cancer[125-127].
In almost all cases, the anti-p53 auto-antibody persists but at a much lower level. Only one study has reported complete absence of the anti-p53 auto-antibody in a series of patients with superficial (mucosal and submucosal) CRC treated with endoscopic resection[98]. This may be because the early stage CRC had a smaller mutant p53 load which may not have adequately stimulated the humoral system to produce a prolonged immune response following CRC removal. The other studies had more advanced CRC where there would have been prolonged antigen exposure to the humoral system[81,88,98,128].
This cost efficacy of serial anti-p53 auto-antibody for surveillance must be considered in the light of only 20%-30% prevalence at presentation and subsequent sero-conversion (Table 5). Assuming 1% future sero-conversion and 3-monthly serum measurements for 3 years as per ASCO recommendations, this would result in 20-30 initial positives at diagnosis; and an additional 1 positive over the subsequent 3 years. This results in an initial yield 20-30 positives followed by only 1 positive out of 960 samples over next 3 years (remaining 80 patients × 4 samples per year × 3 years). We then consider this 1 positive sero-conversions out of 960 samples may not alter treatment as serum measurements may predate clinical evidence of disease, and treatment cannot be offered based anti-p53 auto-antibody titres alone.
| Ref. | Patients, method | Follow-up | Findings |
| Müller et al[123] | 303 patients, 197 colon, 46 rectal | Median 6 mo | All cancers: 3.6% (11/303) sero(-)→ (+); 3.6% (11/303) sero(+)→ (-); Total 7.2% (22/303) sero-conversion. |
| Colon cancer: 3% (4/137) sero(-)→ (+); 3.6% (5/137) sero(+)→ (-); Total 6.6% (9/137) sero-conversion. | |||
| Rectal cancer: 6.5% (2/31) sero(-)→ (+); 3.2% (2/31) sero(+)→ (-); Total 12.9% (4/31) sero-conversion | |||
| Lechpammer et al[88] | Immunoblot 32 , ELISA (Oncogene, Research Products, Cambridge, United States) | Up to 20 wk; 8 patients-48 wk | Non-significant decrease at 4 wk (pre-first cycle chemo) and significant decrease at 12 wk post-surgery |
| Significant decreases during chemotherapy and 2 patients with anti-p53 increase at 12 wk (during chemotherapy) developed recurrence | |||
| 8 patients with extended follow-up: 7/8 had decreased anti-p53 with no recurrence. 1/8 anti-p53 decrease post-surgery/chemotherapy but increased at 12 wk corresponding with liver metastases. Anti-p53 fluctuates in response to tumour load but does not disappear. Anti-p53 levels reflects tumour load even during chemotherapy | |||
| Takeda et al[174] | 30 CUR A, 5 CUR B, 5 CUR C, anti-p53 EIA, Pharmacell | Median 26 mo (13-144) | CUR A (n = 30): 28/30 sero(+)→(-) in 6 mo; 2 no sero-conversion: 1 recurrence |
| CUR B (n = 5): 2 sero(+)→(-) no recurrence. 3 no sero-conversion, 2 had metastases | |||
| CUR C: No sero-conversion | |||
| Correlation between post-operative negative conversion and operative curability | |||
| Takeda et al[98] | 17 mucosal/submucosal, ELISA (anti-p53 EIA, Pharmacell, France) | Up to 2 years | 94%, 16/17 sero(+)→(-) within 3 wk post-surgery |
| No recurrences as early stage tumours and hence not able to comment on anti-p53 and recurrence rates | |||
| Polge et al[128] | 10, ELISA (Dianova, Hamburg, Germany) | Up to 6 mo | 8 followed-up: 5/8 remained sero(+) post-operatively. All developed metastases |
| 3/8 decreased anti-p53 titres. No metastases or recurrence. | |||
| Anti-p53 titres decreased within 1 mo of surgery/chemotherapy but no sero-conversion to anti-p53(-) | |||
| Angelopoulou et al[81] | 6, “In house” immunofluorometric assay | Up to 17 mo | Anti p53 decreases with surgery/chemotherapy but persists at low levels |
| Anti-p53 increases with recurrence | |||
| Anti-p53 reflects tumour load more sensitively than CEA (n = 5) and in non-CEA producing tumour (n = 1) | |||
| Hammel et al[175] | 12, “In house” ELISA | Up to 20 mo | Anti-p53 in 5/8 patients decrease by > 25% within 1 mo. |
| At 1 year, 3 with normal anti-p53 levels and 3 with substantial decrease in anti-p53 remain disease-free | |||
| 2 patients with post-operative increased anti-p53: 1 developed recurrence and 1 developed metastases | |||
| Anti-p53 decreased again following surgery in both patients. CEA and CA19-9 were normal in both cases |
An alternative more cost-effective strategy of screening would be to screen all patients for anti-p53 auto-antibody at diagnosis with further serial measurements only in patients sero-positive at diagnosis. Post-operative patients with rising titres could be selected for expedited investigations and thus increase diagnostic yield, compared to current blanket strategy of routine investigations for surveillance at fixed time intervals. Preliminary studies in small groups using tumour marker panel (CEA, TPA, CA19-9 and CA72.4) demonstrated 81% sensitivity for recurrence with mean lead times of 5.3 mo prior to radiological confirmation of recurrence[124]. A cost efficacy study would be required to ascertain the ability of anti-p53 auto-antibody as part of a tumour marker panel to guide post-operative surveillance, improve resource allocation and prolong survival.
p53 mutations have been associated with poor prognosis, possibly in part due to chemo-resistance against p53-dependant chemotherapy (e.g., 5-fluorouracil) but reports of its prognostic significance are inconsistent[90,129]. As the anti-p53 auto-antibody response has been associated with p53 mutations and serum testing is easier than DNA sequencing, studies have focused on using anti-p53 auto-antibody to predict prognosis. The majority of studies report that anti-p53 auto-antibody response has no independent prognostic value. This was confirmed in the study with the longest follow-up which reported CRC stage, but not anti-p53 auto-antibody, to be an independent prognostic marker in multivariate analysis[130], and also by the study with the second longest follow-up but larger sample size[85] (Table 6).
| Ref. | n (%) | Follow-up | Findings |
| Suppiah et al[130] | 20/92 (21.7) | Median 97 mo | No difference in overall survival (62 mo vs 60 mo) or disease-free survival (73 mo vs 82 mo) |
| Müller et al[123] | 70/243 (28.8) | 5-year trial protocol | No survival difference with anti-p53 in CRC and other cancers. Trend towards decreased survival in anti-p53 positive patients with HCC and breast carcinoma |
| Tang et al[89] | 130/998 (13) | Recruitment 1995-2000 | Anti-p53 associated with decreased survival in univariate analysis but not multivariate analysis. Anti-p53 associated with advanced nodal disease (Stage N2→N3) and metastases (M1) |
| Chang et al[85] | 147/167 (28) | Median 36.3 mo (22-85) | p53 mutation associated with poor differentiation and advanced stage. Multivariate analysis shows p53 mutation most significant survival predictor, followed by CRC stage. No prognostic significance of p53 protein expression or anti-p53 |
| Shiota et al[112] | 18/71 (25) | Not stated | Anti-p53 associated with shorter overall survival (20 mo vs 56 mo) but highly significant association with metastases (M1). Cox regression showed prognostic significance with liver metastases, TNM stage, Dukes stage, Ca19-9 and anti-p53 (in that order) |
| Kressner et al[131] | 59/184 (32.1) | Median 6 years | Anti-p53 associated with decreased survival in univariate, but not multivariate analysis. Anti-p53 is independent prognostic indicator in Dukes’ A-C with curative surgery (i.e., when metastases excluded) |
| Houbiers et al[132] | 65/255 (25.5) | 36 mo | Anti-p53 associated with reduced overall (75% vs 88%) and disease-free survival (56% vs 64%) at 3 years in subgroup analysis of Dukes’ A and B1. No difference in overall survival (61% vs 68%) or disease-free survival (51% vs 58%) when all stages included |
Four studies report an adverse prognostic significance but in 3 of these, the prognostic significance was in selective univariate analysis where anti-p53 was associated with advanced stage, and prognostic significance was lost when stage was incorporated in multivariate analysis[112,131,132]. The fourth, and only study, to report anti-p53 auto-antibody as an independent prognostic indicator in multivariate analysis strongly associated anti-p53 auto-antibody with Dukes’ D to an extent that median survival of anti-p53 positive patients was extremely low (20 mo) compared to other studies reporting median survival up to 60 mo and 5-year survival > 50%[89,123,130]. Remarkably, anti-p53 auto-antibody prognostic significance was even weaker than CA19-9, a pancreatic tumour marker considered unsuitable for pancreatic cancer screening by ASCO[112,122]. The results of this study are hard to credit. As such, anti-p53 auto-antibody has no independent prognostic value.
The anti-p53 auto-antibody response is the end-point of a complex multi-factorial humoral response to the accumulation of p53 protein which is a product mainly of p53 gene mutation, but also mutation of p53 regulators and non-mutative pathways. Anti-p53 auto-antibody has low (13%-32%) sensitivity in CRC but is nearly 100% specific for malignancy. The auto-antibody frequency may increase with early local invasion or late nodal progression but is not sufficiently consistent to form a separate stage classification. There may be a promising future role of anti-p53 auto-antibody in screening and monitoring for disease recurrence. The characteristics of the immunoglobulin and the benefits of serum testing provide a promising role in guiding the radiological and endoscopic screening of high risk populations in conjunction with other current tumour markers. The most promising future focus of anti-p53 auto-antibody lies in being part of a bio-molecular panel of tumour markers to guide endoscopic and radiological screening in general population and high-risk population screening; and in post-operative cancer surveillance to guide earlier detection of cancer and cancer-recurrence; and finally with more significant impact on cost-efficacy and survival.
P- Reviewer Doll D S- Editor Wen LL L- Editor A E- Editor Zhang DN
| 1. | International Agency for Research on Cancer, World Health Organisation. IARC TP53 Database. Available from: http: //p53.iarc.fr/. [Cited in This Article: ] |
| 2. | Lutz W, Nowakowska-Swirta E. Gene p53 mutations, protein p53, and anti-p53 antibodies as biomarkers of cancer process. Int J Occup Med Environ Health. 2002;15:209-218. [PubMed] [Cited in This Article: ] |
| 3. | Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1212] [Cited by in F6Publishing: 1199] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 4. | Béroud C, Collod-Béroud G, Boileau C, Soussi T, Junien C. UMD (Universal mutation database): a generic software to build and analyze locus-specific databases. Hum Mutat. 2000;15:86-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Soussi T, Dehouche K, Béroud C. p53 website and analysis of p53 gene mutations in human cancer: forging a link between epidemiology and carcinogenesis. Hum Mutat. 2000;15:105-113. [PubMed] [Cited in This Article: ] |
| 6. | Levine AJ. The common mechanisms of transformation by the small DNA tumor viruses: The inactivation of tumor suppressor gene products: p53. Virology. 2009;384:285-293. [PubMed] [Cited in This Article: ] |
| 7. | Wiman KG. Strategies for therapeutic targeting of the p53 pathway in cancer. Cell Death Differ. 2006;13:921-926. [PubMed] [Cited in This Article: ] |
| 8. | Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278:261-263. [PubMed] [Cited in This Article: ] |
| 9. | Staples OD, Steele RJ, Lain S. p53 as a therapeutic target. Surgeon. 2008;6:240-243. [PubMed] [Cited in This Article: ] |
| 10. | Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57:1083-1093. [PubMed] [Cited in This Article: ] |
| 11. | Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007;26:1306-1316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 12. | Schwarzbraun T, Obenauf AC, Langmann A, Gruber-Sedlmayr U, Wagner K, Speicher MR, Kroisel PM. Predictive diagnosis of the cancer prone Li-Fraumeni syndrome by accident: new challenges through whole genome array testing. J Med Genet. 2009;46:341-344. [PubMed] [Cited in This Article: ] |
| 13. | Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215-221. [PubMed] [Cited in This Article: ] |
| 14. | Eliyahu D, Michalovitz D, Eliyahu S, Pinhasi-Kimhi O, Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci USA. 1989;86:8763-8767. [PubMed] [Cited in This Article: ] |
| 15. | Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3502] [Cited by in F6Publishing: 3451] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 16. | Bueter M, Gasser M, Lebedeva T, Benichou G, Waaga-Gasser AM. Influence of p53 on anti-tumor immunity (review). Int J Oncol. 2006;28:519-525. [PubMed] [Cited in This Article: ] |
| 17. | Gu B, Zhu WG. Surf the post-translational modification network of p53 regulation. Int J Biol Sci. 2012;8:672-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Pei D, Zhang Y, Zheng J. Regulation of p53: a collaboration between Mdm2 and Mdmx. Oncotarget. 2012;3:228-235. [PubMed] [Cited in This Article: ] |
| 19. | Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413-431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2178] [Cited by in F6Publishing: 2306] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 20. | Oren M, Damalas A, Gottlieb T, Michael D, Taplick J, Leal JF, Maya R, Moas M, Seger R, Taya Y. Regulation of p53: intricate loops and delicate balances. Ann N Y Acad Sci. 2002;973:374-383. [PubMed] [Cited in This Article: ] |
| 21. | Chekhonin VP, Shein SA, Korchagina AA, Gurina OI. VEGF in tumor progression and targeted therapy. Curr Cancer Drug Targets. 2013;13:423-443. [PubMed] [Cited in This Article: ] |
| 22. | Mitchell EP. Targeted therapy for metastatic colorectal cancer: role of aflibercept. Clin Colorectal Cancer. 2013;12:73-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2420] [Cited by in F6Publishing: 2380] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 24. | Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1198] [Cited by in F6Publishing: 1253] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 25. | Schlichtholz B, Legros Y, Gillet D, Gaillard C, Marty M, Lane D, Calvo F, Soussi T. The immune response to p53 in breast cancer patients is directed against immunodominant epitopes unrelated to the mutational hot spot. Cancer Res. 1992;52:6380-6384. [PubMed] [Cited in This Article: ] |
| 26. | Iacopetta B. TP53 mutation in colorectal cancer. Hum Mutat. 2003;21:271-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 27. | Saleh J, Kreissler-Haag D, Montenarh M. p53 autoantibodies from patients with colorectal cancer recognize common epitopes in the N- or C-terminus of p53. Int J Oncol. 2004;25:1149-1155. [PubMed] [Cited in This Article: ] |
| 28. | Lubin R, Schlichtholz B, Bengoufa D, Zalcman G, Trédaniel J, Hirsch A, Caron de Fromentel C, Preudhomme C, Fenaux P, Fournier G. Analysis of p53 antibodies in patients with various cancers define B-cell epitopes of human p53: distribution on primary structure and exposure on protein surface. Cancer Res. 1993;53:5872-5876. [PubMed] [Cited in This Article: ] |
| 29. | Moll UM, Petrenko O. The MDM2-p53 interaction. Mol Cancer Res. 2003;1:1001-1008. [PubMed] [Cited in This Article: ] |
| 30. | Iwakuma T, Lozano G. MDM2, an introduction. Mol Cancer Res. 2003;1:993-1000. [PubMed] [Cited in This Article: ] |
| 31. | Shirangi TR, Zaika A, Moll UM. Nuclear degradation of p53 occurs during down-regulation of the p53 response after DNA damage. FASEB J. 2002;16:420-422. [PubMed] [Cited in This Article: ] |
| 32. | Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296-299. [PubMed] [Cited in This Article: ] |
| 33. | Okamoto K, Taya Y, Nakagama H. Mdmx enhances p53 ubiquitination by altering the substrate preference of the Mdm2 ubiquitin ligase. FEBS Lett. 2009;583:2710-2714. [PubMed] [Cited in This Article: ] |
| 34. | Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J Biol Chem. 2000;275:8945-8951. [PubMed] [Cited in This Article: ] |
| 35. | Miyauchi Y, Yogosawa S, Honda R, Nishida T, Yasuda H. Sumoylation of Mdm2 by protein inhibitor of activated STAT (PIAS) and RanBP2 enzymes. J Biol Chem. 2002;277:50131-50136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Wang X, Taplick J, Geva N, Oren M. Inhibition of p53 degradation by Mdm2 acetylation. FEBS Lett. 2004;561:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Honda R, Yasuda H. Activity of MDM2, a ubiquitin ligase, toward p53 or itself is dependent on the RING finger domain of the ligase. Oncogene. 2000;19:1473-1476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 296] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Waning DL, Lehman JA, Batuello CN, Mayo LD. Controlling the Mdm2-Mdmx-p53 Circuit. Pharmaceuticals (Basel). 2010;3:1576-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Winter M, Milne D, Dias S, Kulikov R, Knippschild U, Blattner C, Meek D. Protein kinase CK1delta phosphorylates key sites in the acidic domain of murine double-minute clone 2 protein (MDM2) that regulate p53 turnover. Biochemistry. 2004;43:16356-16364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 1999;18:1660-1672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 538] [Cited by in F6Publishing: 552] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 41. | Zhang Y, Xiong Y. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science. 2001;292:1910-1915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 42. | Galluzzi L, Morselli E, Kepp O, Maiuri MC, Kroemer G. Defective autophagy control by the p53 rheostat in cancer. Cell Cycle. 2010;9:250-255. [PubMed] [Cited in This Article: ] |
| 43. | Sui X, Jin L, Huang X, Geng S, He C, Hu X. p53 signaling and autophagy in cancer: a revolutionary strategy could be developed for cancer treatment. Autophagy. 2011;7:565-571. [PubMed] [Cited in This Article: ] |
| 44. | Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D’Amelio M, Criollo A, Morselli E, Zhu C, Harper F. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10:676-687. [PubMed] [Cited in This Article: ] |
| 45. | Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127-1130. [PubMed] [Cited in This Article: ] |
| 46. | Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G. Autophagy regulation by p53. Curr Opin Cell Biol. 2010;22:181-185. [PubMed] [Cited in This Article: ] |
| 47. | Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043-3053. [PubMed] [Cited in This Article: ] |
| 48. | Lorin S, Pierron G, Ryan KM, Codogno P, Djavaheri-Mergny M. Evidence for the interplay between JNK and p53-DRAM signalling pathways in the regulation of autophagy. Autophagy. 2010;6:153-154. [PubMed] [Cited in This Article: ] |
| 49. | Yee KS, Wilkinson S, James J, Ryan KM, Vousden KH. PUMA- and Bax-induced autophagy contributes to apoptosis. Cell Death Differ. 2009;16:1135-1145. [PubMed] [Cited in This Article: ] |
| 50. | Alvarez S, Drané P, Meiller A, Bras M, Deguin-Chambon V, Bouvard V, May E. A comprehensive study of p53 transcriptional activity in thymus and spleen of gamma irradiated mouse: high sensitivity of genes involved in the two main apoptotic pathways. Int J Radiat Biol. 2006;82:761-770. [PubMed] [Cited in This Article: ] |
| 51. | Zhang Y, Ma WY, Kaji A, Bode AM, Dong Z. Requirement of ATM in UVA-induced signaling and apoptosis. J Biol Chem. 2002;277:3124-3131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Midgley CA, Owens B, Briscoe CV, Thomas DB, Lane DP, Hall PA. Coupling between gamma irradiation, p53 induction and the apoptotic response depends upon cell type in vivo. J Cell Sci. 1995;108:1843-1848. [PubMed] [Cited in This Article: ] |
| 53. | Braithwaite AW, Royds JA, Jackson P. The p53 story: layers of complexity. Carcinogenesis. 2005;26:1161-1169. [PubMed] [Cited in This Article: ] |
| 54. | Ljungman M. Dial 9-1-1 for p53: mechanisms of p53 activation by cellular stress. Neoplasia. 2000;2:208-225. [PubMed] [Cited in This Article: ] |
| 55. | Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453-456. [PubMed] [Cited in This Article: ] |
| 56. | Soussi T. TP53 mutations and cancer. Available from: http: //p53.fr/index.html. [Cited in This Article: ] |
| 57. | Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157-2165. [PubMed] [Cited in This Article: ] |
| 58. | Sherr CJ. Divorcing ARF and p53: an unsettled case. Nat Rev Cancer. 2006;6:663-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 421] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 59. | Crook T, Tidy JA, Vousden KH. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell. 1991;67:547-556. [PubMed] [Cited in This Article: ] |
| 60. | Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129-1136. [PubMed] [Cited in This Article: ] |
| 61. | Dong X, Hamilton KJ, Satoh M, Wang J, Reeves WH. Initiation of autoimmunity to the p53 tumor suppressor protein by complexes of p53 and SV40 large T antigen. J Exp Med. 1994;179:1243-1252. [PubMed] [Cited in This Article: ] |
| 62. | Chène P. Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat Rev Cancer. 2003;3:102-109. [PubMed] [Cited in This Article: ] |
| 63. | Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237-1245. [PubMed] [Cited in This Article: ] |
| 64. | Leach FS, Tokino T, Meltzer P, Burrell M, Oliner JD, Smith S, Hill DE, Sidransky D, Kinzler KW, Vogelstein B. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993;53:2231-2234. [PubMed] [Cited in This Article: ] |
| 65. | Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1398] [Cited by in F6Publishing: 1424] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 66. | Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843-21850. [PubMed] [Cited in This Article: ] |
| 67. | Gottlieb TM, Leal JF, Seger R, Taya Y, Oren M. Cross-talk between Akt, p53 and Mdm2: possible implications for the regulation of apoptosis. Oncogene. 2002;21:1299-1303. [PubMed] [Cited in This Article: ] |
| 68. | Harlozińska A, Bar J, Montenarh M. Analysis of the immunoreactivity of three anti-p53 antibodies and estimation of the relations between p53 status and MDM2 protein expression in ovarian carcinomas. Anticancer Res. 2000;20:1049-1056. [PubMed] [Cited in This Article: ] |
| 69. | Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27:462-467. [PubMed] [Cited in This Article: ] |
| 70. | Choi HJ, Chung TW, Kang SK, Lee YC, Ko JH, Kim JG, Kim CH. Ganglioside GM3 modulates tumor suppressor PTEN-mediated cell cycle progression--transcriptional induction of p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway. Glycobiology. 2006;16:573-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Moll UM, Riou G, Levine AJ. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc Natl Acad Sci USA. 1992;89:7262-7266. [PubMed] [Cited in This Article: ] |
| 72. | Bosari S, Viale G, Roncalli M, Graziani D, Borsani G, Lee AK, Coggi G. p53 gene mutations, p53 protein accumulation and compartmentalization in colorectal adenocarcinoma. Am J Pathol. 1995;147:790-798. [PubMed] [Cited in This Article: ] |
| 73. | Moll UM, LaQuaglia M, Bénard J, Riou G. Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors. Proc Natl Acad Sci USA. 1995;92:4407-4411. [PubMed] [Cited in This Article: ] |
| 74. | Weiss J, Heine M, Körner B, Pilch H, Jung EG. Expression of p53 protein in malignant melanoma: clinicopathological and prognostic implications. Br J Dermatol. 1995;133:23-31. [PubMed] [Cited in This Article: ] |
| 75. | Olivier M, Petitjean A, Marcel V, Pétré A, Mounawar M, Plymoth A, de Fromentel CC, Hainaut P. Recent advances in p53 research: an interdisciplinary perspective. Cancer Gene Ther. 2009;16:1-12. [PubMed] [Cited in This Article: ] |
| 76. | Sigal A, Rotter V. Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res. 2000;60:6788-6793. [PubMed] [Cited in This Article: ] |
| 77. | Strano S, Munarriz E, Rossi M, Cristofanelli B, Shaul Y, Castagnoli L, Levine AJ, Sacchi A, Cesareni G, Oren M. Physical and functional interaction between p53 mutants and different isoforms of p73. J Biol Chem. 2000;275:29503-29512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 78. | Crawford LV, Pim DC, Bulbrook RD. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982;30:403-408. [PubMed] [Cited in This Article: ] |
| 79. | Bargonetti J, Manfredi JJ. Multiple roles of the tumor suppressor p53. Curr Opin Oncol. 2002;14:86-91. [PubMed] [Cited in This Article: ] |
| 80. | Schlichtholz B, Trédaniel J, Lubin R, Zalcman G, Hirsch A, Soussi T. Analyses of p53 antibodies in sera of patients with lung carcinoma define immunodominant regions in the p53 protein. Br J Cancer. 1994;69:809-816. [PubMed] [Cited in This Article: ] |
| 81. | Angelopoulou K, Stratis M, Diamandis EP. Humoral immune response against p53 protein in patients with colorectal carcinoma. Int J Cancer. 1997;70:46-51. [PubMed] [Cited in This Article: ] |
| 82. | Shimada H, Ochiai T, Nomura F. Titration of serum p53 antibodies in 1,085 patients with various types of malignant tumors: a multiinstitutional analysis by the Japan p53 Antibody Research Group. Cancer. 2003;97:682-689. [PubMed] [Cited in This Article: ] |
| 83. | Russo A, Bazan V, Iacopetta B, Kerr D, Soussi T, Gebbia N. The TP53 colorectal cancer international collaborative study on the prognostic and predictive significance of p53 mutation: influence of tumor site, type of mutation, and adjuvant treatment. J Clin Oncol. 2005;23:7518-7528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 84. | Forslund A, Kressner U, Lindmark G, Inganäs M, Lundholm K. Serum anti-p53 in relation to mutations across the entire translated p53 gene in colorectal carcinomas. Int J Oncol. 2001;19:501-506. [PubMed] [Cited in This Article: ] |
| 85. | Chang SC, Lin JK, Lin TC, Liang WY. Genetic alteration of p53, but not overexpression of intratumoral p53 protein, or serum p53 antibody is a prognostic factor in sporadic colorectal adenocarcinoma. Int J Oncol. 2005;26:65-75. [PubMed] [Cited in This Article: ] |
| 86. | Rohayem J, Conrad K, Zimmermann T, Frank KH. Comparison of the diagnostic accuracy of three commercially available enzyme immunoassays for anti-p53 antibodies. Clin Chem. 1999;45:2014-2016. [PubMed] [Cited in This Article: ] |
| 87. | Metcalfe S, Wheeler TK, Picken S, Negus S. P53 autoantibodies in 1006 patients followed up for breast cancer. Breast Cancer Res. 2000;2:438-443. [PubMed] [Cited in This Article: ] |
| 88. | Lechpammer M, Lukac J, Lechpammer S, Kovacević D, Loda M, Kusić Z. Humoral immune response to p53 correlates with clinical course in colorectal cancer patients during adjuvant chemotherapy. Int J Colorectal Dis. 2004;19:114-120. [PubMed] [Cited in This Article: ] |
| 89. | Tang R, Ko MC, Wang JY, Changchien CR, Chen HH, Chen JS, Hsu KC, Chiang JM, Hsieh LL. Humoral response to p53 in human colorectal tumors: a prospective study of 1,209 patients. Int J Cancer. 2001;94:859-863. [PubMed] [Cited in This Article: ] |
| 90. | Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777-1788. [PubMed] [Cited in This Article: ] |
| 91. | Tavassoli M, Brunel N, Maher R, Johnson NW, Soussi T. p53 antibodies in the saliva of patients with squamous cell carcinoma of the oral cavity. Int J Cancer. 1998;78:390-391. [PubMed] [Cited in This Article: ] |
| 92. | Munker R, Stötzer O, Darsow M, Classen S, Lebeau A, Wilmanns W. Autoantibodies against p53 are not increased in human ascites and pleural effusions. Cancer Immunol Immunother. 1996;42:200-201. [PubMed] [Cited in This Article: ] |
| 93. | Wollenberg B, Jan NV, Pitzke P, Reiter W, Stieber P. Anti-p53 antibodies in serum of smokers and head and neck cancer patients. Anticancer Res. 1997;17:413-418. [PubMed] [Cited in This Article: ] |
| 94. | Weller M, Bornemann A, Ständer M, Schabet M, Dichgans J, Meyermann R. Humoral immune response to p53 in malignant glioma. J Neurol. 1998;245:169-172. [PubMed] [Cited in This Article: ] |
| 95. | Fonseca RF, Kawamura MT, Oliveira JA, Teixeira A, Alves G, Carvalho Mda G. Anti-P53 antibodies in Brazilian brain tumor patients. Genet Mol Res. 2003;2:185-190. [PubMed] [Cited in This Article: ] |
| 96. | Shibata Y, Kotanagi H, Andoh H, Koyama K, Itoh H, Kudo S. Detection of circulating anti-p53 antibodies in patients with colorectal carcinoma and the antibody’s relation to clinical factors. Dis Colon Rectum. 1996;39:1269-1274. [PubMed] [Cited in This Article: ] |
| 97. | Nozoe T, Yasuda M, Honda M, Inutsuka S, Korenaga D. Clinicopathologic significance in serum presence of anti-p53 antibody in patients with colorectal carcinoma. Hepatogastroenterology. 2007;54:1422-1425. [PubMed] [Cited in This Article: ] |
| 98. | Takeda A, Shimada H, Nakajima K, Yoshimura S, Suzuki T, Asano T, Ochiai T, Isono K. Serum p53 antibody as a useful marker for monitoring of treatment of superficial colorectal adenocarcinoma after endoscopic resection. Int J Clin Oncol. 2001;6:45-49. [PubMed] [Cited in This Article: ] |
| 99. | Shimada H, Takeda A, Arima M, Okazumi S, Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T. Serum p53 antibody is a useful tumor marker in superficial esophageal squamous cell carcinoma. Cancer. 2000;89:1677-1683. [PubMed] [Cited in This Article: ] |
| 100. | Bowles CJ, Leicester R, Romaya C, Swarbrick E, Williams CB, Epstein O. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut. 2004;53:277-283. [PubMed] [Cited in This Article: ] |
| 101. | Nelson DB, McQuaid KR, Bond JH, Lieberman DA, Weiss DG, Johnston TK. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc. 2002;55:307-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 276] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 102. | Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624-1633. [PubMed] [Cited in This Article: ] |
| 103. | Hilsden R, Rostom A. Colorectal cancer screening using flexible sigmoidoscopy: United Kingdom study demonstrates significant incidence and mortality benefit. Can J Gastroenterol. 2010;24:479-480. [PubMed] [Cited in This Article: ] |
| 104. | Hoff G, Grotmol T, Skovlund E, Bretthauer M. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338:b1846. [PubMed] [Cited in This Article: ] |
| 105. | Littlejohn C, Hilton S, Macfarlane GJ, Phull P. Systematic review and meta-analysis of the evidence for flexible sigmoidoscopy as a screening method for the prevention of colorectal cancer. Br J Surg. 2012;99:1488-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Wild N, Andres H, Rollinger W, Krause F, Dilba P, Tacke M, Karl J. A combination of serum markers for the early detection of colorectal cancer. Clin Cancer Res. 2010;16:6111-6121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 107. | Park Y, Kim Y, Lee JH, Lee EY, Kim HS. Usefulness of serum anti-p53 antibody assay for lung cancer diagnosis. Arch Pathol Lab Med. 2011;135:1570-1575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Sharp L, Tilson L, Whyte S, O’Ceilleachair A, Walsh C, Usher C, Tappenden P, Chilcott J, Staines A, Barry M. Cost-effectiveness of population-based screening for colorectal cancer: a comparison of guaiac-based faecal occult blood testing, faecal immunochemical testing and flexible sigmoidoscopy. Br J Cancer. 2012;106:805-816. [PubMed] [Cited in This Article: ] |
| 109. | Hanly P, Skally M, Fenlon H, Sharp L. Cost-effectiveness of computed tomography colonography in colorectal cancer screening: a systematic review. Int J Technol Assess Health Care. 2012;28:415-423. [PubMed] [Cited in This Article: ] |
| 110. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [PubMed] [Cited in This Article: ] |
| 111. | Bielicki D, Karbowniczek M, Sulzyc-Bielicka V, Kładny J, Boer C, Marlicz K, Domagała W. Clinico-pathological characteristics of colorectal cancer and serum anti-p53 antibodies. Pol J Pathol. 1999;50:77-81. [PubMed] [Cited in This Article: ] |
| 112. | Shiota G, Ishida M, Noguchi N, Oyama K, Takano Y, Okubo M, Katayama S, Tomie Y, Harada K, Hori K. Circulating p53 antibody in patients with colorectal cancer: relation to clinicopathologic features and survival. Dig Dis Sci. 2000;45:122-128. [PubMed] [Cited in This Article: ] |
| 113. | Thomas P, Toth CA, Saini KS, Jessup JM, Steele G. The structure, metabolism and function of the carcinoembryonic antigen gene family. Biochim Biophys Acta. 1990;1032:177-189. [PubMed] [Cited in This Article: ] |
| 114. | Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Ann Surg Oncol. 2009;16:3087-3093. [PubMed] [Cited in This Article: ] |
| 115. | Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23:338-351. [PubMed] [Cited in This Article: ] |
| 116. | Begent RH. The value of carcinoembryonic antigen measurement in clinical practice. Ann Clin Biochem. 1984;21:231-238. [PubMed] [Cited in This Article: ] |
| 117. | Mourtzikou A, Stamouli M, Kroupis C, Christodoulou S, Skondra M, Kastania A, Pectasides D, Athanasas G, Dimas C. Evaluation of carcinoembryonic antigen (CEA), epidermal growth factor receptor (EGFR), epithelial cell adhesion molecule EpCAM (GA733-2), and carbohydrate antigen 19-9 (CA 19-9) levels in colorectal cancer patients and correlation with clinicopathological characteristics. Clin Lab. 2012;58:441-448. [PubMed] [Cited in This Article: ] |
| 118. | Wilson AP, Van Dalen A, Sibley PE, Kasper LA, Durham AP, el Shami AS. Multicentre tumour marker reference range study. Anticancer Res. 1999;19:2749-2752. [PubMed] [Cited in This Article: ] |
| 119. | Duffy MJ. Carcinoembryonic antigen as a marker for colorectal cancer: is it clinically useful? Clin Chem. 2001;47:624-630. [PubMed] [Cited in This Article: ] |
| 120. | Park IJ, Kim HC, Yu CS, Yoo JH, Kim JC. Cutoff values of preoperative s-CEA levels for predicting survivals after curative resection of colorectal cancer. J Korean Med Sci. 2005;20:624-627. [PubMed] [Cited in This Article: ] |
| 121. | Tan E, Gouvas N, Nicholls RJ, Ziprin P, Xynos E, Tekkis PP. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg Oncol. 2009;18:15-24. [PubMed] [Cited in This Article: ] |
| 122. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [PubMed] [Cited in This Article: ] |
| 123. | Müller M, Meyer M, Schilling T, Ulsperger E, Lehnert T, Zentgraf H, Stremmel W, Volkmann M, Galle PR. Testing for anti-p53 antibodies increases the diagnostic sensitivity of conventional tumor markers. Int J Oncol. 2006;29:973-980. [PubMed] [Cited in This Article: ] |
| 124. | Nicolini A, Ferrari P, Duffy MJ, Antonelli A, Rossi G, Metelli MR, Fulceri F, Anselmi L, Conte M, Berti P. Intensive risk-adjusted follow-up with the CEA, TPA, CA19.9, and CA72.4 tumor marker panel and abdominal ultrasonography to diagnose operable colorectal cancer recurrences: effect on survival. Arch Surg. 2010;145:1177-1183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 125. | Cai HY, Wang XH, Tian Y, Gao LY, Zhang LJ, Zhang ZY. Changes of serum p53 antibodies and clinical significance of radiotherapy for esophageal squamous cell carcinoma. World J Gastroenterol. 2008;14:4082-4086. [PubMed] [Cited in This Article: ] |
| 126. | Zalcman G, Trédaniel J, Schlichtholz B, Urban T, Milleron B, Lubin R, Meignin V, Couderc LJ, Hirsch A, Soussi T. Prognostic significance of serum p53 antibodies in patients with limited-stage small cell lung cancer. Int J Cancer. 2000;89:81-86. [PubMed] [Cited in This Article: ] |
| 127. | Porrini R, Vercellino V, Rocchetti V, Renò F, Giorda E, Pomato E, Cannas M, Sabbatini M. Serum anti-p53 antibodies as a diagnostic tumor marker: observations in patients with malignant and premalignant oral cavity lesions. Minerva Stomatol. 2010;59:233-239, 233-239. [PubMed] [Cited in This Article: ] |
| 128. | Polge A, Bourgaux JF, Bancel E, Pignodel C, Boyer JC, Poirey S, de Bornier BM, Balmes JL, Bali JP. p53 and follow-up of colorectal adenocarcinomas. Dig Dis Sci. 1998;43:1434-1442. [PubMed] [Cited in This Article: ] |
| 129. | Soong R, Powell B, Elsaleh H, Gnanasampanthan G, Smith DR, Goh HS, Joseph D, Iacopetta B. Prognostic significance of TP53 gene mutation in 995 cases of colorectal carcinoma. Influence of tumour site, stage, adjuvant chemotherapy and type of mutation. Eur J Cancer. 2000;36:2053-2060. [PubMed] [Cited in This Article: ] |
| 130. | Suppiah A, Alabi A, Madden L, Hartley JE, Monson JR, Greenman J. Anti-p53 autoantibody in colorectal cancer: prognostic significance in long-term follow-up. Int J Colorectal Dis. 2008;23:595-600. [PubMed] [Cited in This Article: ] |
| 131. | Kressner U, Glimelius B, Bergström R, Påhlman L, Larsson A, Lindmark G. Increased serum p53 antibody levels indicate poor prognosis in patients with colorectal cancer. Br J Cancer. 1998;77:1848-1851. [PubMed] [Cited in This Article: ] |
| 132. | Houbiers JG, van der Burg SH, van de Watering LM, Tollenaar RA, Brand A, van de Velde CJ, Melief CJ. Antibodies against p53 are associated with poor prognosis of colorectal cancer. Br J Cancer. 1995;72:637-641. [PubMed] [Cited in This Article: ] |
| 133. | Wu M, Mao C, Chen Q, Cu XW, Zhang WS. Serum p53 protein and anti-p53 antibodies are associated with increased cancer risk: a case-control study of 569 patients and 879 healthy controls. Mol Biol Rep. 2010;37:339-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 134. | Kulić A, Sirotković-Skerlev M, Jelisavac-Cosić S, Herceg D, Kovac Z, Vrbanec D. Anti-p53 antibodies in serum: relationship to tumor biology and prognosis of breast cancer patients. Med Oncol. 2010;27:887-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | Atta MM, el-Masry SA, Abdel-Hameed M, Baiomy HA, Ramadan NE. Value of serum anti-p53 antibodies as a prognostic factor in Egyptian patients with hepatocellular carcinoma. Clin Biochem. 2008;41:1131-1139. [PubMed] [Cited in This Article: ] |
| 136. | Mattioni M, Soddu S, Porrello A, D’Alessandro R, Spila A, Guadagni F. Serum anti-p53 antibodies as a useful marker for prognosis of gastric carcinoma. Int J Biol Markers. 2007;22:302-306. [PubMed] [Cited in This Article: ] |
| 137. | Akere A, Otegbayo JA. Evaluation of the pattern and prognostic implications of anti-p53 in hepatocellular carcinoma. Singapore Med J. 2007;48:41-44. [PubMed] [Cited in This Article: ] |
| 138. | Neri M, Betta P, Marroni P, Filiberti R, Cafferata M, Mereu C, Ivaldi G, Montanaro F, Puntoni R, Paganuzzi M. Serum anti-p53 autoantibodies in pleural malignant mesothelioma, lung cancer and non-neoplastic lung diseases. Lung Cancer. 2003;39:165-172. [PubMed] [Cited in This Article: ] |
| 139. | Numa F, Umayahara K, Suehiro Y, Hirakawa H, Nawata S, Suminami Y, Oga A, Ito T, Sasaki K, Kato H. Serum anti-p53 antibodies in uterine and ovarian cancer: association with dna sequence copy number abnormalities. Tumour Biol. 2001;22:162-168. [PubMed] [Cited in This Article: ] |
| 140. | Mack U, Ukena D, Montenarh M, Sybrecht GW. Serum anti-p53 antibodies in patients with lung cancer. Oncol Rep. 2000;7:669-674. [PubMed] [Cited in This Article: ] |
| 141. | Chow V, Yuen AP, Lam KY, Ho WK, Wei WI. Prognostic significance of serum p53 protein and p53 antibody in patients with surgical treatment for head and neck squamous cell carcinoma. Head Neck. 2001;23:286-291. [PubMed] [Cited in This Article: ] |
| 142. | Moch C, Moysan A, Lubin R, de la Salmonière P, Soufir N, Galisson F, Vilmer C, Venutolo E, Le Pelletier F, Janin A. Divergence between the high rate of p53 mutations in skin carcinomas and the low prevalence of anti-p53 antibodies. Br J Cancer. 2001;85:1883-1886. [PubMed] [Cited in This Article: ] |
| 143. | Hofele C, Schwager-Schmitt M, Volkmann M. [Prognostic value of antibodies against p53 in patients with oral squamous cell carcinoma--five years survival rate]. Laryngorhinootologie. 2002;81:342-345. [PubMed] [Cited in This Article: ] |
| 144. | Hagiwara N, Onda M, Miyashita M, Sasajima K. Detection of circulating anti-p53 antibodies in esophageal cancer patients. J Nippon Med Sch. 2000;67:110-117. [PubMed] [Cited in This Article: ] |
| 145. | Ralhan R, Arora S, Chattopadhyay TK, Shukla NK, Mathur M. Circulating p53 antibodies, p53 gene mutational profile and product accumulation in esophageal squamous-cell carcinoma in India. Int J Cancer. 2000;85:791-795. [PubMed] [Cited in This Article: ] |
| 146. | Blanchard P, Quero L, Pacault V, Schlageter MH, Baruch-Hennequin V, Hennequin C. Prognostic significance of anti-p53 and anti-KRas circulating antibodies in esophageal cancer patients treated with chemoradiotherapy. BMC Cancer. 2012;12:119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 147. | Bergström S, Dreilich M, Wagenius G, Brattström D, Larsson A, Hesselius P, Bergqvist M. The presence of anti-p53 antibodies in sera from patients with oesophageal carcinoma: correlation to treatment, tumour volume and survival. In Vivo. 2004;18:615-620. [PubMed] [Cited in This Article: ] |
| 148. | Kozłowski M, Kovalchuk O, Nikliński J, Chyczewski L, Starosławska E, Ciechański A, Dabrowski A, Niklińska W, Dziegielewski P, Lapuć G. Circulating anti-p53 antibodies in esophageal cancer patients. Folia Histochem Cytobiol. 2001;39 Suppl 2:173-174. [PubMed] [Cited in This Article: ] |
| 149. | Castelli M, Cianfriglia F, Manieri A, Palma L, Pezzuto RW, Falasca G, Delpino A. Anti-p53 and anti-heat shock proteins antibodies in patients with malignant or pre-malignant lesions of the oral cavity. Anticancer Res. 2001;21:753-758. [PubMed] [Cited in This Article: ] |
| 150. | Qiu T, Yang Q, Li XR, Yang H, Qiu LL, Wang L. Detection of serum anti-p53 antibodies from patients with ovarian cancer in China: correlation to clinical parameters. Cancer Invest. 2007;25:563-568. [PubMed] [Cited in This Article: ] |
| 151. | Abendstein B, Marth C, Müller-Holzner E, Widschwendter M, Daxenbichler G, Zeimet AG. Clinical significance of serum and ascitic p53 autoantibodies in epithelial ovarian carcinoma. Cancer. 2000;88:1432-1437. [PubMed] [Cited in This Article: ] |
| 152. | Broll R, Duchrow M, Oevermann E, Wellm C, Schwandner O, Schimmelpenning H, Roblick UJ, Bruch HP, Windhövel U. p53 autoantibodies in sera of patients with a colorectal cancer and their association to p53 protein concentration and p53 immunohistochemistry in tumor tissue. Int J Colorectal Dis. 2001;16:22-27. [PubMed] [Cited in This Article: ] |
| 153. | Charuruks N, Tangkijvanich P, Voravud N, Chatsantikul R, Theamboonlers A, Poovorawan Y. Clinical significance of p53 antigen and anti-p53 antibodies in the sera of hepatocellular carcinoma patients. J Gastroenterol. 2001;36:830-836. [PubMed] [Cited in This Article: ] |
| 154. | Tangkijvanich P, Janchai A, Charuruks N, Kullavanijaya P, Theamboonlers A, Hirsch P, Poovorawan Y. Clinical associations and prognostic significance of serum anti-p53 antibodies in Thai patients with hepatocellular carcinoma. Asian Pac J Allergy Immunol. 2000;18:237-243. [PubMed] [Cited in This Article: ] |
| 155. | Sitruk V, Vaysse J, Chevret S, Ganne-Carrie N, Christidis C, Trinchet J, Beaugrand M. [Prevalence and prognostic value of serum anti-p53 antibodies in hepatocellular carcinoma. A study of 159 patients]. Gastroenterol Clin Biol. 2000;24:1159-1163. [PubMed] [Cited in This Article: ] |
| 156. | Watanabe J, Nishiyama H, Kawanishi H, Ito M, Kamoto T, Ogawa O. Clinical evaluation of serum p53 antibodies in patients with upper urinary tract tumors. J Urol. 2005;174:73-75. [PubMed] [Cited in This Article: ] |
| 157. | Gumus E, Demirel G, Tanriverdi O, Horasanli K, Ozmen G, Miroglu C. Importance of serum p53 antibodies during follow-up after treatment of invasive bladder tumors. Urol Int. 2004;72:292-298. [PubMed] [Cited in This Article: ] |
| 158. | Gumus E, Erdamar S, Demirel G, Horasanli K, Kendirci M, Miroglu C. Association of positive serum anti-p53 antibodies with poor prognosis in bladder cancer patients. Int J Urol. 2004;11:1070-1077. [PubMed] [Cited in This Article: ] |
| 159. | Morita T, Tachikawa N, Kumamaru T, Nukui A, Ikeda H, Suzuki K, Tokue A. Serum anti-p53 antibodies and p53 protein status in the sera and tumors from bladder cancer patients. Eur Urol. 2000;37:79-84. [PubMed] [Cited in This Article: ] |
| 160. | Wunderlich H, Hindermann W, Hüller M, Reichelt O, Werner W, Schubert J, Kosmehl H. The correlation of p53 protein overexpression and p53 antibodies in serum of patients with transitional cell carcinoma of the urinary bladder. Urol Int. 2000;64:13-17. [PubMed] [Cited in This Article: ] |
| 161. | Bergqvist M, Brattström D, Larsson A, Hesselius P, Brodin O, Wagenius G. The role of circulating anti-p53 antibodies in patients with advanced non-small cell lung cancer and their correlation to clinical parameters and survival. BMC Cancer. 2004;4:66. [PubMed] [Cited in This Article: ] |
| 162. | Bergqvist M, Brattström D, Lamberg K, Hesselius P, Wernlund J, Larsson A, Wagenius G. The presence of anti-p53 antibodies in sera prior to thoracic surgery in non small cell lung cancer patients: its implications on tumor volume, nodal involvement, and survival. Neoplasia. 2003;5:283-287. [PubMed] [Cited in This Article: ] |
| 163. | Cioffi M, Vietri MT, Gazzerro P, Magnetta R, D’Auria A, Durante A, Nola E, Puca GA, Molinari AM. Serum anti-p53 antibodies in lung cancer: comparison with established tumor markers. Lung Cancer. 2001;33:163-169. [PubMed] [Cited in This Article: ] |
| 164. | Lawniczak M, Bielicki D, Sulzyc-Bielicka V, Marlicz K, Starzyńska T. [Serum anti-p53 antibodies in gastric cancer patients]. Pol Merkur Lekarski. 2007;23:192-195. [PubMed] [Cited in This Article: ] |
| 165. | Nakajima K, Suzuki T, Shimada H, Hayashi H, Takeda A, Ochiai T. Detection of preoperative serum anti-p53 antibodies in gastric cancer. Tumour Biol. 1999;20:147-152. [PubMed] [Cited in This Article: ] |
| 166. | Maehara Y, Kakeji Y, Watanabe A, Baba H, Kusumoto H, Kohnoe S, Sugimachi K. Clinical implications of serum anti-p53 antibodies for patients with gastric carcinoma. Cancer. 1999;85:302-308. [PubMed] [Cited in This Article: ] |
| 167. | Nozoe T, Mori E, Kono M, Iguchi T, Maeda T, Matsukuma A, Ezaki T. Serum appearance of anti-p53 antibody in triple negative breast cancer. Breast Cancer. 2012;19:11-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 168. | Gao RJ, Bao HZ, Yang Q, Cong Q, Song JN, Wang L. The presence of serum anti-p53 antibodies from patients with invasive ductal carcinoma of breast: correlation to other clinical and biological parameters. Breast Cancer Res Treat. 2005;93:111-115. [PubMed] [Cited in This Article: ] |
| 169. | Volkmann M, Sinn HP, Gaugel D, Frey M, Hajjar Y, Ludwig J, Hänsel S, Bastert G, Wallwiener D, Fiehn W. Anti-p53 in breast cancer: concordance of different assay procedures and association with p53 antigen expression. Oncology. 2002;63:297-305. [PubMed] [Cited in This Article: ] |
| 170. | Ohshio G, Suwa H, Imamura M. Clinical implication of anti-p53 antibodies and p53-protein in pancreatic disease. Int J Gastrointest Cancer. 2002;31:129-135. [PubMed] [Cited in This Article: ] |
| 171. | Messmer BT, Nour-Omid TS, Ghia E, Sanchez AB, Kipps TJ. Autoantibodies against p53 are associated with chromosome 17p deletions in chronic lymphocytic leukemia. Leuk Res. 2011;35:965-967. [PubMed] [Cited in This Article: ] |
| 172. | Limpaiboon T, Tapdara S, Jearanaikoon P, Sripa B, Bhudhisawasdi V. Prognostic significance of microsatellite alterations at 1p36 in cholangiocarcinoma. World J Gastroenterol. 2006;12:4377-4382. [PubMed] [Cited in This Article: ] |
| 173. | Tangkijvanich P, Kasemsupatana K, Janchai A, Kullavanijaya P, Theamboonlers A, Poovorawan Y. Prevalence and clinical relevance of serum anti-p53 antibodies in patients with cholangiocarcinoma. Asian Pac J Allergy Immunol. 2000;18:173-176. [PubMed] [Cited in This Article: ] |
| 174. | Takeda A, Shimada H, Nakajima K, Imaseki H, Suzuki T, Asano T, Ochiai T, Isono K. Monitoring of p53 autoantibodies after resection of colorectal cancer: relationship to operative curability. Eur J Surg. 2001;167:50-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |