Published online Jun 21, 2013. doi: 10.3748/wjg.v19.i23.3555
Revised: March 15, 2013
Accepted: April 10, 2013
Published online: June 21, 2013
Ischemia/reperfusion (I/R) injury of the gut is a significant problem in a variety of clinical settings and is associated with a high morbidity and mortality. Although the mechanisms involved in the pathogenesis of gut I/R injury have not been fully elucidated, it is generally believed that oxidative stress with subsequent inflammatory injury plays an important role. Heme oxygenase (HO) is the rate-limiting enzyme in the catabolism of heme, followed by production of CO, biliverdin, and free iron. The HO system is believed to confer cytoprotection by inhibiting inflammation, oxidation, and apoptosis, and maintaining microcirculation. HO-1, an inducible form of HO, serves a vital metabolic function as the rate-limiting step in the heme degradation pathway, and affords protection in models of intestinal I/R injury. HO-1 system is an important player in intestinal I/R injury condition, and may offer new targets for the management of this condition.
Core tip: In this review, we focused on the heme oxygenase (HO)-1 system and its possible roles and mechanisms in gut ischemia/reperfusion (I/R) injury studied to date. This review, for the first time, reviews in detail the relationship between HO-1 and gut I/R injury.
- Citation: Liao YF, Zhu W, Li DP, Zhu X. Heme oxygenase-1 and gut ischemia/reperfusion injury: A short review. World J Gastroenterol 2013; 19(23): 3555-3561
- URL: https://www.wjgnet.com/1007-9327/full/v19/i23/3555.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i23.3555
Ischemia/reperfusion (I/R) injury of the gut occurs frequently in a variety of clinical settings, including abdominal aortic aneurysm surgery, mesenteric artery occlusion, small bowel transplantation, cardiopulmonary bypass, strangulated hernias, trauma, and shock[1]; the exact mechanisms involved in the pathogenesis of which have not been fully elucidated. Gut I/R injury is associated with a substantial morbidity and mortality[2].
Heme oxygenase (HO) is the rate-limiting enzyme in heme degradation, resulting in the formation of CO, biliverdin, and free iron[3]. There are three distinct HO isoforms (HO-1, HO-2, and HO-3) identified to date. HO-1 is the inducible form of the enzyme, and is expressed in relatively low amounts in most tissues[3]. HO-2 is the constitutive isoform, expressed mainly in brain and testis. HO-3 is identified only in rats, and its physiological role remains unclear[4].
HO-1, as an inducible form, also belongs to a member of the heat shock protein family and is highly inducible by a vast array of stimuli[3]. Many studies indicated that the induction of HO-1 plays a significant protective role against inflammatory processes and oxidative tissue injury[5-7]. In this review, we focus on the current understanding of the cytoprotective effects observed with the HO system during gut I/R injury. The implications for possible therapeutic manipulation of HO in gut I/R injury are elucidated.
Interruption of blood supply results in ischemic injury which rapidly damages metabolically active tissues. Paradoxically, reintroduction of blood flow obtained following ischemia initiates a cascade of events that can potentially worsen the original injury. This effect is known as reperfusion injury[8]. The intestine is composed of labile cells that are very susceptible to I/R injury[9]. Multiple factors have been shown to be involved in the process of intestinal I/R injury. The primary pathophysiological events of this injury involve microcirculatory flow disturbances caused by the production of reactive oxygen species (ROS). Tissue ischemia and oxidative stress activate families of protein kinases that converge on specific transcriptional factors that regulate the expression of inflammatory genes. The resulting gene products include enzymes [e.g., inducible nitric oxide synthase (iNOS); phospholipase A2, and cyclooxygenase-2 (COX-2)], cytokines [e.g., tumor necrosis factor α (TNF-α); interleukin-1 (IL-1); interleukin-6 (IL-6)], prostaglandins (e.g., PGE-2), and adhesion molecules [e.g., intracellular adhesion molecule (ICAM-1); E-selectin][10-14]. These initiate local inflammation, which is further amplified by the recruitment of circulating leukocytes[12], which appear to be key effector cells in causing tissue injury. Furthermore, I/R injury induces widespread endothelial cell apoptosis and the loss of endothelial cells in the vessels serving the organ results in thrombosis[15,16] directly in the intestine[16]. This injury observed during I/R is believed to trigger a systemic inflammatory response leading to multiple organ failure[17,18], which frequently involves the lungs[19,20] and liver[21]. Intestinal I/R injury is a complex, multifactorial pathophysiological process, dependent upon an understanding of which the optimal therapeutic approach is aimed at ameliorating I/R injury (Table 1). HO-1 system might be one of the most promising approaches among the potential therapeutic options.
| Treatment | I/R model | Ref. | |
| HO-1 | |||
| Glutamine | Warm ischemia | [30] | |
| Ischemic preconditioning | Resuscitation after shock | [37] | |
| Doxorubicin | Warm ischemia | [20] | |
| Hypothermia | Warm ischemia | [32] | |
| IL-2 | Warm ischemia | [34] | |
| Hemin | Warm ischemia | [29] | |
| Hypertonic saline | Warm ischemia | [31] | |
| Pyrrolidine dithiocarbamate | Warm ischemia | [28] | |
| Hyperthermia | Warm ischemia | [33] | |
| Ischemic preconditioning | Warm ischemia | [36] | |
| Cobalt-protoporphyrin | Warm ischemia | [27] | |
| Ischemic preconditioning | Endotoxic shock | [18] | |
| Radix Paeoniae Rubra | Warm ischemia | [38] | |
| AICAR preconditioning | Warm ischemia | [35] | |
| CO | |||
| Gas inhalation | Intestinal transplants | [11] | |
| Gas inhalation | Intestinal transplants | [13] | |
| Gas inhalation | Intestinal transplants | [14] | |
| Gas inhalation | Intestinal transplants | [47] | |
| CO solution | Intestinal transplants | [49] | |
| CORM preconditioning | Warm ischemia | [48] | |
| CORM preconditioning | Warm ischemia | [12] | |
| Biliverdin/bilirubin | |||
| Bilirubin | Warm ischemia | [60] | |
| Bilirubin | Warm ischemia | [61] | |
| Biliverdin | Intestinal transplants | [47] | |
HO-1 is expressed constitutively in normal gastric, intestinal, and colonic mucosa[22,23], and is up-regulated in their inflamed tissue[23]. Many studies showed that HO-1 is involved in a variety of regulatory and protective cellular mechanisms as a stress-responsive protein[5,6]. The normal expression and up-regulation of HO-1 suggest that activation of HO-1 could act as a natural defensive mechanism to alleviate inflammation and tissue injury in the gastrointestinal tract[24,25]. HO has been shown to have potent cytoprotective effects on intestinal I/R injury as well[26]. For example, induction of HO-1 by cobalt-protoporphyrin administration before intestinal I/R resulted in a significant reduction of intestinal tissue injury[27]. Another enhancer (pyrrolidine dithiocarbamate) of HO production improves intestinal microvascular perfusion and attenuates I/R injury of the intestine, possibly via HO production[28]. Similarly, administration with a HO inducer (hemin) results in lessened mucosal injury and improved intestinal transit following gut I/R[29]. Glutamine protects the intestine from warm ischemic injury, which was considered to be associated with inducible HO-1 expression through the interaction with cellular antioxidative activity and the inhibition of cytokines[30]. Several studies demonstrated that intraischemic hypothermia, hypertonic saline resuscitation, and whole-body hyperthermia decrease inflammation and protect against intestinal injury in a model of gut I/R[31-33]. Administration of IL-2, an immunoregulatory cytokine, resulted in clinical improvement of the study animals after intestinal I/R[34]. These protective interventions were associated with the induction of HO-1. Postischemic leukocyte-endothelial cell adhesive interactions are prevented by 5-aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside preconditioning 24 h prior to I/R in the small intestine by HO-dependent mechanisms[35]. Furthermore, ischemic preconditioning of the intestine might prove to be an effective strategy for the amelioration of I/R injury, in which HO is involved[18,36,37]. Pretreatment with Radix Paeoniae Rubra[38], or the anticancer drug doxorubicin[20], can attenuate acute lung injury resulting from intestinal I/R. These results demonstrate that HO-1 is implicated in cytoprotection and may be an effective agent for the treatment of gut I/R.
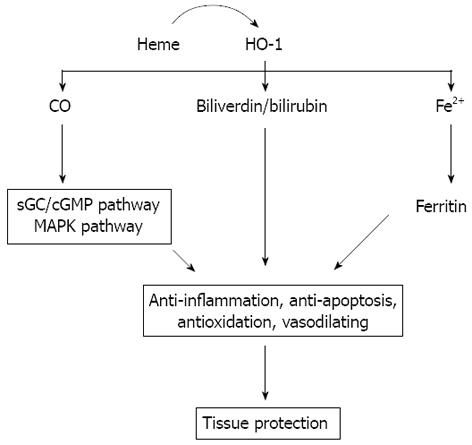
There is increasing evidence that HO-1 plays an important protective role in gut I/R injury. There are four factors that could be responsible for the protection of HO-1 in intestinal I/R, including: (1) removal of free heme; (2) CO; (3) biliverdin/bilirubin; and (4) Fe2+.
Heme, an essential iron chelate, is a potentially damaging species that not only provides a lipophilic form of iron, but also can directly attack and impair a multiplicity of intracellular targets[39]; production increases under conditions of oxidant stress, especially in I/R injury[39,40]. HO-1 is the key enzyme in heme degradation and plays a key role in regulating the intracellular heme level. HO-1 activity leads to rapid removal of free heme. Thus, in order to prevent heme from both extracellular and intracellular sources reacting and producing ROS, the heme degradation step is an important consideration in the cytoprotection afforded by the HO system[40].
CO is one of the three products of heme degradation by HO-1 and has profound effects as a signaling molecule that culminates in anti-inflammatory, antiapoptotic, and vasodilating effects[41,42]. A number of studies have revealed that CO mediates potent cytoprotective and anti-inflammatory effects in models of I/R injury of the heart, lung, kidney, and liver[43-46]. Some studies demonstrate that the efficacy of CO gas inhalation for the prevention of cold intestinal I/R injury using a small intestinal transplantation model, in which CO is able to effectively inhibit an early up-regulation of proinflammatory mediators such as IL-6, IL-1, TNF-α, ICAM-1, iNOS, and COX-2[11,13,14,47]. It has been reported that pre-treatment with CO-releasing molecules also markedly reduced intestinal inflammation induced by surgical manipulation of the small intestine[48] or by occluding the superior mesenteric artery[12]. Similarly, one study showed that cold storage in a preservation solution that was bubbled with 5% CO significantly reduced I/R injury associated with intestinal transplantation, which reduced inflammatory mediator up-regulation and improved graft blood flow[49]. Moreover, CO-treated animals showed early up-regulation of the anti-apoptotic molecule Bcl-2, and down-regulation of the proapoptotic signal Bax, and reduced in vivo apoptosis of both vascular endothelial cells and intestinal epithelial cells[11]. The protective effects of CO are arbitrated by activating one or both of the two key signaling pathways. One of the pathways is soluble guanylate cyclase/cyclic guanosine monophosphate and the other one is the p38 mitogen-activated protein kinase pathway which transduces oxidative stress and inflammatory signaling[11,48-53], through which CO exerts significant cytoprotection due to its anti-inflammatory, vasodilating, and anti-apoptotic properties in gut I/R injury.
HO degrades heme into equimolar quantity of biliverdin. Biliverdin is, in turn, very rapidly converted to bilirubin by the enzyme biliverdin reductase[3]. Biliverdin and its reduced product, bilirubin, scavenge various ROS and are hence considered potent antioxidants[54,55], which have been shown to confer cytoprotection against oxidative stress conditions in various cell types[56]. Several studies have also demonstrated that the administration of biliverdin and/or bilirubin is potently cytoprotective in I/R injury of the liver and heart, and in organ transplantation[57-59]. Evidence from an experimental small intestinal I/R injury model in rats describes a protective effect for bilirubin[60], in which the bilirubin is infused via the jugular vein. Similarly, another study showed that increased serum bilirubin ameliorates the extent of intestinal IR injury[61]. Recent studies have suggested that biliverdin, in addition to its antioxidant properties, may have anti-inflammatory action. For example, treatment with biliverdin can significantly decrease mRNA expression of iNOS, COX-2, and ICAM-1, as well as the inflammatory cytokines IL-6 and IL-1, and decreased neutrophil infiltration into the jejunal muscularis in rat syngeneic small intestinal transplants[47]. These results suggest that bilirubin possesses complex immune-modulatory and antioxidant effects.
Though HO activity is generally associated with cellular protection, Fe2+, the third product of heme decomposition, participates in the Fenton reaction to promote the generation of ROS and is believed to have potential deleterious effects. Increased iron levels, on the one hand, can upregulate an iron-transporter pump that removes intracellular Fe2+ from the cell[62]. On the other hand, iron release from HO activity induces the expression of ferritin (an iron storing protein)[63,64]. Expression of ferritin was originally reported to protect endothelial cells against oxidant damage in vitro[64]. In addition, over-expression of H-ferritin (heavy chain ferritin) has also been shown to protect cultured endothelial cells from undergoing apoptosis and protects the liver from transplant-associated I/R injury[65]. Thus, ferritin seems to confer cytoprotection against oxidative challenge. There is no information about the roles of iron and ferritin in gut I/R injury, but in such a mechanism they could still be operative.
As we mentioned above, the HO-1 system plays an important role in the cytoprotective process, up-regulation of which seems to be a potential therapeutic option for gut I/R injury. As far as we know, there have been no definitive trials designed to evaluate the efficacy of chemical HO-1 inducers in the clinical setting. Hemin, as an inducer of HO-1, has been used extensively in experimental studies, but has only been used by physicians experienced in the management of porphyrias clinically. However, there are increasing reports showing that hemin-induced HO-1 activity is a host defense mechanism in different animal models, such as the thrombosis vascular model[66], in liver I/R injury[67], acute pancreatitis with multi-organ failure[68,69], human immunodeficiency virus-1 infection[70], and spontaneously hypertension[71]. Such disease states share part or common physiopathological process with gut I/R injury, which suggests that hemin could offer a therapeutic benefit for gut I/R injury. A richer understanding of the cytoprotective mechanisms of hemin therapy will be necessary, which will also pave the way for clinical application in the treatment of gut I/R injury.
Intestinal I/R injury is a complex, multifactorial pathophysiological process. Despite its complexity, the HO-1 system, owing to its antioxidative, anti-inflammatory, anti-apoptosis, and potent cytoprotective properties (Figure 1), may serve as promising potential therapeutic options for intestinal me/R injury.
The modulation of HO-1 expression using genetic or pharmacological strategies may offer therapeutic strategies for intestinal I/R injury. Furthermore, HO-1-related molecules, including CO and biliverdin/bilirubin, might be employed as drugs in the management of intestinal I/R injury. More importantly, regulating the HO-1 system with different agents has already been demonstrated as important for attenuating I/R injury in other organs including the brain, liver, and kidney[40,72-74]. It is reasonable to assume that such a mechanism could also be operative in intestinal I/R injury. Research focused on the underlying mechanisms for the observed effects of HO-1 and its products will be necessary before their use can be evaluated in clinical applications for the prevention and/or treatment of human diseases such as intestinal I/R injury.
P- Reviewers Camara CR, Kondo T, Wiley JW S- Editor Gou SX L- Editor Rutherford A E- Editor Zhang DN
| 1. | Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 427] [Cited by in F6Publishing: 444] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Grootjans J, Lenaerts K, Derikx JP, Matthijsen RA, de Bruïne AP, van Bijnen AA, van Dam RM, Dejong CH, Buurman WA. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176:2283-2291. [PubMed] [Cited in This Article: ] |
| 3. | Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1997] [Cited by in F6Publishing: 1943] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 4. | McCoubrey WK, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 644] [Cited by in F6Publishing: 610] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 5. | Fan W, Huang F, Zhu X, Li D, Fu S, He H. The heme oxygenase system and oral diseases. Oral Dis. 2011;17:252-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Zhu X, Fan WG, Li DP, Kung H, Lin MC. Heme oxygenase-1 system and gastrointestinal inflammation: a short review. World J Gastroenterol. 2011;17:4283-4288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 53] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Bae GS, Kim MS, Park KC, Koo BS, Jo IJ, Choi SB, Lee DS, Kim YC, Kim TH, Seo SW. Effect of biologically active fraction of Nardostachys jatamansi on cerulein-induced acute pancreatitis. World J Gastroenterol. 2012;18:3223-3234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 8. | Stallion A, Kou TD, Miller KA, Dahms BB, Dudgeon DL, Levine AD. IL-10 is not protective in intestinal ischemia reperfusion injury. J Surg Res. 2002;105:145-152. [PubMed] [Cited in This Article: ] |
| 9. | Yamamoto S, Tanabe M, Wakabayashi G, Shimazu M, Matsumoto K, Kitajima M. The role of tumor necrosis factor-α and interleukin-1beta in ischemia-reperfusion injury of the rat small intestine. J Surg Res. 2001;99:134-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Scott JR, Gray DK, Bihari A, Badhwar A, Zhang X, Shan P, Lee PJ, Chakrabarti S, Harris KA, Potter RF. Heme oxygenase modulates small intestine leukocyte adhesion following hindlimb ischemia/reperfusion by regulating the expression of intercellular adhesion molecule-1. Crit Care Med. 2005;33:2563-2570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA, Otterbein LE. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol. 2003;163:1587-1598. [PubMed] [Cited in This Article: ] |
| 12. | Katada K, Bihari A, Mizuguchi S, Yoshida N, Yoshikawa T, Fraser DD, Potter RF, Cepinskas G. Carbon monoxide liberated from CO-releasing molecule (CORM-2) attenuates ischemia/reperfusion (I/R)-induced inflammation in the small intestine. Inflammation. 2010;33:92-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Nakao A, Moore BA, Murase N, Liu F, Zuckerbraun BS, Bach FH, Choi AM, Nalesnik MA, Otterbein LE, Bauer AJ. Immunomodulatory effects of inhaled carbon monoxide on rat syngeneic small bowel graft motility. Gut. 2003;52:1278-1285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Nakao A, Kimizuka K, Stolz DB, Seda Neto J, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Bauer AJ, Nalesnik MA. Protective effect of carbon monoxide inhalation for cold-preserved small intestinal grafts. Surgery. 2003;134:285-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Ryter SW, Otterbein LE. Carbon monoxide in biology and medicine. Bioessays. 2004;26:270-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Shah KA, Shurey S, Green CJ. Apoptosis after intestinal ischemia-reperfusion injury: a morphological study. Transplantation. 1997;64:1393-1397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Ceppa EP, Fuh KC, Bulkley GB. Mesenteric hemodynamic response to circulatory shock. Curr Opin Crit Care. 2003;9:127-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Tamion F, Richard V, Renet S, Thuillez C. Intestinal preconditioning prevents inflammatory response by modulating heme oxygenase-1 expression in endotoxic shock model. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1308-G1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Park YY. Ischemia/reperfusion Lung Injury Increases Serum Ferritin and Heme Oxygenase-1 in Rats. Korean J Physiol Pharmacol. 2009;13:181-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Ito K, Ozasa H, Kojima N, Miura M, Iwa T, Senoo H, Horikawa S. Pharmacological preconditioning protects lung injury induced by intestinal ischemia/reperfusion in rat. Shock. 2003;19:462-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Zhao HD, Zhang F, Shen G, Li YB, Li YH, Jing HR, Ma LF, Yao JH, Tian XF. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J Gastroenterol. 2010;16:3002-3010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 79] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 22. | Coëffier M, Le Pessot F, Leplingard A, Marion R, Lerebours E, Ducrotté P, Déchelotte P. Acute enteral glutamine infusion enhances heme oxygenase-1 expression in human duodenal mucosa. J Nutr. 2002;132:2570-2573. [PubMed] [Cited in This Article: ] |
| 23. | Barton SG, Rampton DS, Winrow VR, Domizio P, Feakins RM. Expression of heat shock protein 32 (hemoxygenase-1) in the normal and inflamed human stomach and colon: an immunohistochemical study. Cell Stress Chaperones. 2003;8:329-334. [PubMed] [Cited in This Article: ] |
| 24. | Guo X, Shin VY, Cho CH. Modulation of heme oxygenase in tissue injury and its implication in protection against gastrointestinal diseases. Life Sci. 2001;69:3113-3119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Yun KJ, Choi SC, Oh JM. [Expression of heme oxygenase-1 in ischemic colitis]. Korean J Gastroenterol. 2005;45:335-339. [PubMed] [Cited in This Article: ] |
| 26. | Nakao A, Kaczorowski DJ, Sugimoto R, Billiar TR, McCurry KR. Application of heme oxygenase-1, carbon monoxide and biliverdin for the prevention of intestinal ischemia/reperfusion injury. J Clin Biochem Nutr. 2008;42:78-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Wasserberg N, Pileggi A, Salgar SK, Ruiz P, Ricordi C, Inverardi L, Tzakis AG. Heme oxygenase-1 upregulation protects against intestinal ischemia/reperfusion injury: a laboratory based study. Int J Surg. 2007;5:216-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Mallick IH, Yang WX, Winslet MC, Seifalian AM. Pyrrolidine dithiocarbamate reduces ischemia-reperfusion injury of the small intestine. World J Gastroenterol. 2005;11:7308-7313. [PubMed] [Cited in This Article: ] |
| 29. | Attuwaybi BO, Kozar RA, Moore-Olufemi SD, Sato N, Hassoun HT, Weisbrodt NW, Moore FA. Heme oxygenase-1 induction by hemin protects against gut ischemia/reperfusion injury. J Surg Res. 2004;118:53-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Tamaki T, Konoeda Y, Yasuhara M, Tanaka M, Yokota N, Hayashi T, Katori M, Uchida Y, Kawamura A. Glutamine-induced heme oxygenase-1 protects intestines and hearts from warm ischemic injury. Transplant Proc. 1999;31:1018-1019. [PubMed] [Cited in This Article: ] |
| 31. | Attuwaybi B, Kozar RA, Gates KS, Moore-Olufemi S, Sato N, Weisbrodt NW, Moore FA. Hypertonic saline prevents inflammation, injury, and impaired intestinal transit after gut ischemia/reperfusion by inducing heme oxygenase 1 enzyme. J Trauma. 2004;56:749-758; discussion 758-759. [PubMed] [Cited in This Article: ] |
| 32. | Attuwaybi BO, Hassoun HT, Zou L, Kozar RA, Kone BC, Weisbrodt NW, Moore FA. Hypothermia protects against gut ischemia/reperfusion-induced impaired intestinal transit by inducing heme oxygenase-1. J Surg Res. 2003;115:48-55. [PubMed] [Cited in This Article: ] |
| 33. | Sakamoto N, Kokura S, Okuda T, Hattori T, Katada K, Isozaki Y, Nakabe N, Handa O, Takagi T, Ishikawa T. Heme oxygenase-1 (Hsp32) is involved in the protection of small intestine by whole body mild hyperthermia from ischemia/reperfusion injury in rat. Int J Hyperthermia. 2005;21:603-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Nüssler NC, Müller AR, Weidenbach H, Vergopoulos A, Platz KP, Volk HD, Neuhaus P, Nussler AK. IL-10 increases tissue injury after selective intestinal ischemia/reperfusion. Ann Surg. 2003;238:49-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Gaskin FS, Kamada K, Yusof M, Durante W, Gross G, Korthuis RJ. AICAR preconditioning prevents postischemic leukocyte rolling and adhesion: role of K(ATP) channels and heme oxygenase. Microcirculation. 2009;16:167-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Mallick IH, Yang W, Winslet MC, Seifalian AM. Protective effects of ischemic preconditioning on the intestinal mucosal microcirculation following ischemia-reperfusion of the intestine. Microcirculation. 2005;12:615-625. [PubMed] [Cited in This Article: ] |
| 37. | Tamion F, Richard V, Lacoume Y, Thuillez C. Intestinal preconditioning prevents systemic inflammatory response in hemorrhagic shock. Role of HO-1. Am J Physiol Gastrointest Liver Physiol. 2002;283:G408-G414. [PubMed] [Cited in This Article: ] |
| 38. | Chen C, Zhang F, Xia ZY, Lin H, Mo AS. Protective effects of pretreatment with Radix Paeoniae Rubra on acute lung injury induced by intestinal ischemia/reperfusion in rats. Chin J Traumatol. 2008;11:37-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289-309. [PubMed] [Cited in This Article: ] |
| 40. | Katori M, Anselmo DM, Busuttil RW, Kupiec-Weglinski JW. A novel strategy against ischemia and reperfusion injury: cytoprotection with heme oxygenase system. Transpl Immunol. 2002;9:227-233. [PubMed] [Cited in This Article: ] |
| 41. | Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583-650. [PubMed] [Cited in This Article: ] |
| 42. | Takagi T, Naito Y, Uchiyama K, Suzuki T, Hirata I, Mizushima K, Tsuboi H, Hayashi N, Handa O, Ishikawa T. Carbon monoxide liberated from carbon monoxide-releasing molecule exerts an anti-inflammatory effect on dextran sulfate sodium-induced colitis in mice. Dig Dis Sci. 2011;56:1663-1671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Kohmoto J, Nakao A, Sugimoto R, Wang Y, Zhan J, Ueda H, McCurry KR. Carbon monoxide-saturated preservation solution protects lung grafts from ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2008;136:1067-1075. [PubMed] [Cited in This Article: ] |
| 44. | Lavitrano M, Smolenski RT, Musumeci A, Maccherini M, Slominska E, Di Florio E, Bracco A, Mancini A, Stassi G, Patti M. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004;18:1093-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Caumartin Y, Stephen J, Deng JP, Lian D, Lan Z, Liu W, Garcia B, Jevnikar AM, Wang H, Cepinskas G. Carbon monoxide-releasing molecules protect against ischemia-reperfusion injury during kidney transplantation. Kidney Int. 2011;79:1080-1089. [PubMed] [Cited in This Article: ] |
| 46. | Lee LY, Kaizu T, Toyokawa H, Zhang M, Ross M, Stolz DB, Huang C, Gandhi C, Geller DA, Murase N. Carbon monoxide induces hypothermia tolerance in Kupffer cells and attenuates liver ischemia/reperfusion injury in rats. Liver Transpl. 2011;17:1457-1466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Nakao A, Otterbein LE, Overhaus M, Sarady JK, Tsung A, Kimizuka K, Nalesnik MA, Kaizu T, Uchiyama T, Liu F. Biliverdin protects the functional integrity of a transplanted syngeneic small bowel. Gastroenterology. 2004;127:595-606. [PubMed] [Cited in This Article: ] |
| 48. | De Backer O, Elinck E, Blanckaert B, Leybaert L, Motterlini R, Lefebvre RA. Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut. 2009;58:347-356. [PubMed] [Cited in This Article: ] |
| 49. | Nakao A, Toyokawa H, Tsung A, Nalesnik MA, Stolz DB, Kohmoto J, Ikeda A, Tomiyama K, Harada T, Takahashi T. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am J Transplant. 2006;6:2243-2255. [PubMed] [Cited in This Article: ] |
| 50. | Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015-1026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 793] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 51. | Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-α-induced apoptosis in cultured fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2000;278:L312-L319. [PubMed] [Cited in This Article: ] |
| 52. | Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1667] [Cited by in F6Publishing: 1663] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 53. | Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 820] [Cited by in F6Publishing: 836] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 54. | Jansen T, Daiber A. Direct Antioxidant Properties of Bilirubin and Biliverdin. Is there a Role for Biliverdin Reductase? Front Pharmacol. 2012;3:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 55. | Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2595] [Cited by in F6Publishing: 2556] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 56. | Foresti R, Green CJ, Motterlini R. Generation of bile pigments by haem oxygenase: a refined cellular strategy in response to stressful insults. Biochem Soc Symp. 2004;177-192. [PubMed] [Cited in This Article: ] |
| 57. | Fondevila C, Shen XD, Tsuchiyashi S, Yamashita K, Csizmadia E, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Bach FH. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology. 2004;40:1333-1341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643-H651. [PubMed] [Cited in This Article: ] |
| 59. | Ollinger R, Wang H, Yamashita K, Wegiel B, Thomas M, Margreiter R, Bach FH. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid Redox Signal. 2007;9:2175-2185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Ceran C, Sönmez K, Türkyllmaz Z, Demirogullarl B, Dursun A, Düzgün E, Başaklar AC, Kale N. Effect of bilirubin in ischemia/reperfusion injury on rat small intestine. J Pediatr Surg. 2001;36:1764-1767. [PubMed] [Cited in This Article: ] |
| 61. | Hammerman C, Goldschmidt D, Caplan MS, Kaplan M, Bromiker R, Eidelman AI, Gartner LM, Hochman A. Protective effect of bilirubin in ischemia-reperfusion injury in the rat intestine. J Pediatr Gastroenterol Nutr. 2002;35:344-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Barañano DE, Doré S. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152-157. [PubMed] [Cited in This Article: ] |
| 63. | Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA. 1994;91:2607-2610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 401] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 64. | Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148-18153. [PubMed] [Cited in This Article: ] |
| 65. | Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, Ke B, Shen X, Busuttil RW, Yamashita K, Csizmadia E. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 2003;17:1724-1726. [PubMed] [Cited in This Article: ] |
| 66. | Desbuards N, Rochefort GY, Schlecht D, Machet MC, Halimi JM, Eder V, Hyvelin JM, Antier D. Heme oxygenase-1 inducer hemin prevents vascular thrombosis. Thromb Haemost. 2007;98:614-620. [PubMed] [Cited in This Article: ] |
| 67. | Fang J, Qin H, Seki T, Nakamura H, Tsukigawa K, Shin T, Maeda H. Therapeutic potential of pegylated hemin for reactive oxygen species-related diseases via induction of heme oxygenase-1: results from a rat hepatic ischemia/reperfusion injury model. J Pharmacol Exp Ther. 2011;339:779-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Habtezion A, Kwan R, Yang AL, Morgan ME, Akhtar E, Wanaski SP, Collins SD, Butcher EC, Kamal A, Omary MB. Heme oxygenase-1 is induced in peripheral blood mononuclear cells of patients with acute pancreatitis: a potential therapeutic target. Am J Physiol Gastrointest Liver Physiol. 2011;300:G12-G20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Habtezion A, Kwan R, Akhtar E, Wanaski SP, Collins SD, Wong RJ, Stevenson DK, Butcher EC, Omary MB. Panhematin provides a therapeutic benefit in experimental pancreatitis. Gut. 2011;60:671-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Devadas K, Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol. 2006;176:4252-4257. [PubMed] [Cited in This Article: ] |
| 71. | Shamloul R, Wang R. Monitoring circulatory heme level in hemin therapy for lowering blood pressure in rats. Cell Mol Biol (Noisy-le-grand). 2005;51:507-512. [PubMed] [Cited in This Article: ] |
| 72. | Hoetzel A, Schmidt R. Regulatory role of anesthetics on heme oxygenase-1. Curr Drug Targets. 2010;11:1495-1503. [PubMed] [Cited in This Article: ] |
| 73. | Gueler F, Park JK, Rong S, Kirsch T, Lindschau C, Zheng W, Elger M, Fiebeler A, Fliser D, Luft FC. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am J Pathol. 2007;170:1192-1199. [PubMed] [Cited in This Article: ] |
| 74. | Tsuchihashi S, Fondevila C, Kupiec-Weglinski JW. Heme oxygenase system in ischemia and reperfusion injury. Ann Transplant. 2004;9:84-87. [PubMed] [Cited in This Article: ] |