Published online May 14, 2013. doi: 10.3748/wjg.v19.i18.2799
Revised: February 1, 2013
Accepted: March 6, 2013
Published online: May 14, 2013
AIM: To examine the long-term therapeutic efficacies of endoscopic cauterization for gastric vascular ectasia, according to the type of lesion.
METHODS: Thirty-eight patients with hemorrhagic gastric vascular ectasia (VE) were treated by endoscopic cauterization: 13 by heater probe coagulationand 25 by argon plasma coagulation. Depending on the number of lesions, 14 and 24 patients were classified into localized VE (≤ 10; LVE) and extensive VE (> 10; EVE), respectively. The patients were followed-up by repeated endoscopic examinations after the therapy, and the incidences of VE recurrence and re-bleeding from the lesions were evaluated.
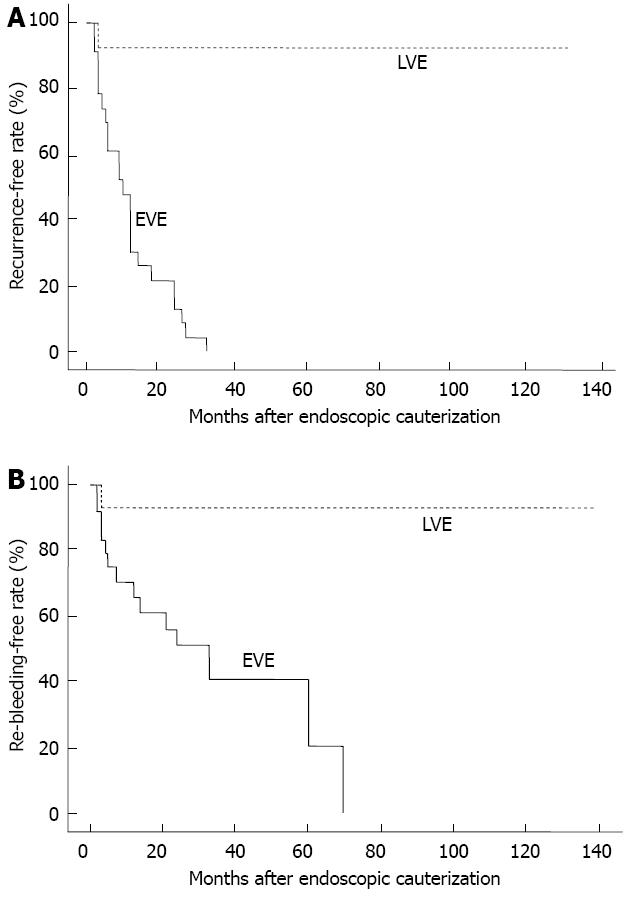
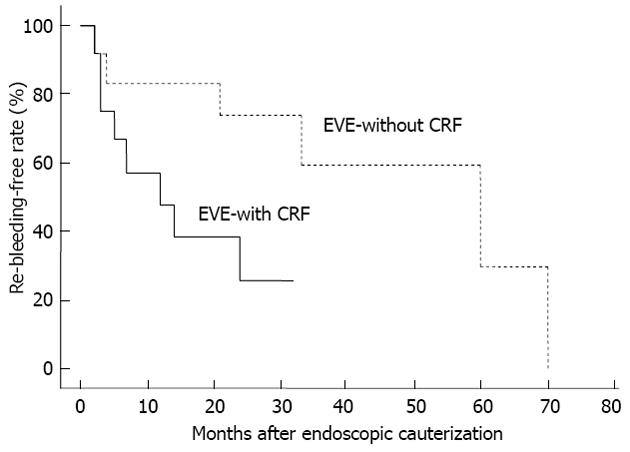
RESULTS: Although the VE lesions disappeared initially in all the patients after the therapy, the recurrence of VE developed in 25 patients (66%) over a mid-term observation period of 32 mo, and re-bleeding occurred in 15 patients (39%). The recurrence of VE was found in all patients with EVE, with re-bleeding occurring in 14 patients (58%). In contrast, only 1 patient (7%) with LVE showed recurrence of the lesions and complicating hemorrhage. Both the cumulative recurrence-free rates and cumulative re-bleeding-free rates were significantly lower in the EVE group than in the LVE group (P < 0.001 and P < 0.001, respectively). Moreover, the cumulative re-bleeding-free rate in the EVE group was 47.6% at 1 year and 25.4% at 2 years in patients with chronic renal failure, which were significantly lower than the rates in the patients without chronic renal failure (83.3% and 74.1%, respectively) (P < 0.05).
CONCLUSION: The recurrence of VE and re-bleeding from the lesions was more frequent in the patients with EVE, especially in those with complicating renal failure.
Core tip: The aim of this study was to examine the long-term therapeutic efficacies of endoscopic cauterization for gastric vascular ectasia (VE), according to the type of lesion. Depending on the number of lesions, 14 and 24 patients were classified into localized vascular ectasia (≤ 10) and extensive VE (> 10; EVE), respectively. The incidences of VE recurrence and re-bleeding from the lesions were evaluated. The recurrence of VE and re-bleeding from the lesions was more frequent in the patients with EVE, especially in those with complicating renal failure, even after the initial successful arrest of bleeding and disappearance of the lesions by the endoscopic therapy.
- Citation: Imai Y, Mizuno Y, Yoshino K, Watanabe K, Sugawara K, Motoya D, Oka M, Mochida S. Long-term efficacy of endoscopic coagulation for different types of gastric vascular ectasia. World J Gastroenterol 2013; 19(18): 2799-2805
- URL: https://www.wjgnet.com/1007-9327/full/v19/i18/2799.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i18.2799
Vascular ectasia (VE) of the stomach has been recognized as a rare cause of hemorrhage from the upper gastrointestinal tract[1-4]. Patients may present with either evidence of chronic occult bleeding, i.e., iron deficiency anemia, or with acute hematemesis or melena. VE is a term that encompasses a broad spectrum of lesions visualized on endoscopic examination, including angiodysplasia, watermelon stomach, diffuse antral VE (DAVE) and telangiectasis associated with Osler-Weber-Rendu disease[5]. In general, angiodysplasia is defined as VE with a limited number of red flat spots or reticulated vascular areas in the gastric mucosa on endoscopic examination. In contrast, gastric antral VE (GAVE) is diagnosed as watermelon stomach when the lesions are visualized as stripes radiating outwards from the pylorus and as DAVE in cases with diffuse red spots in the gastric antrum.
Various therapeutic procedures have been employed for hemorrhagic gastric VE. Surgical resection of the stomach is the most curative therapeutic procedure, but it is a highly invasive procedure that maybe associated with high mortality, especially in elderly patients with underlying diseases such as metabolic syndrome. Thus, endoscopic cauterization has generally been performed for the treatment of gastric VE. Several types of coagulation procedures may be employed for endoscopic cauterization, including heater probe coagulation[6], laser coagulation[7-10] and argon plasma coagulation[10-16], and these devices have been reported to be useful for the temporary arrest of bleeding from any type of gastric VE. However, the long-term prognosis of patients undergoing endoscopic cauterization has to be elucidated. In the present paper, we examined the long-term therapeutic efficacies of endoscopic cauterization, including the recurrence rate of the lesions and the rates of re-bleeding, in patients with hemorrhaging from gastric VE, according to the type of lesion.
The subjects included 42 patients with gastrointestinal bleeding from gastric VE lesions, which were diagnosed by gastrointestinal endoscopic examination between October 1996 and December 2007. Among them, 4 patients, who moved to a different institution for follow-up following discharge from our hospital after the successful arrest of bleeding from the VE, were excluded from the analysis. The remaining 38 patients consisted of 21 men and 17 women, with a median age of 69 years (range, 48-85 years). Twenty patients (53%) had underlying liver cirrhosis, and 15 patients (39%) were undergoing maintenance hemodialysis for chronic renal failure.
On endoscopic examination, bleeding from the gastric VE lesions was observed in 21 patients (55%). In the remaining 17 patients, other gastrointestinal lesions that could serve as the possible source of the bleeding were not detectable by the endoscopic examinations, including small intestinal endoscopy, despite the patient presenting with hematemesis or melena. The patients were classified into 2 groups: 14 patients with ≤ 10 VE lesions (localized VE, the LVE group) and 24 patients with > 10 VE lesions (extensive VE, the EVE group). The LVE group comprised 9 patients with a single lesion and 5 patients with multiple lesions (range, 3 to 10 lesions), including one patient with Osler-Weber-Rendu disease showing 3 lesions in the stomach. In contrast, the EVE group consisted of 5 patients with watermelon stomach and 19 patients with DAVE, including one patient with radiation-induced DAVE.
All patients were treated by endoscopic cauterization. Thereafter, follow-up endoscopic examinations were performed at intervals ranging from 1 to 6 mo, depending on the clinical features of each patient, and the rate of the recurrence of VE with or without re-bleeding from the lesions was evaluated. The recurrence of VE was defined as the presence of > 10 lesions in the EVE group, and of at least one lesion in the LVE group. The diagnosis of re-bleeding was conducted when the patients showed hematemesis or melena and/or hemorrhagic features of the gastric VE lesions on endoscopic examination irrespective of the number of lesions. Fifteen and twenty patients received treatment with a proton pump inhibitor and H2-receptor antagonist, respectively, during the observation period. Written informed consent for the endoscopic procedures was obtained from all the patients. This study was retrospectively performed with the approval of the Institutional Review Board of the Hospital.
Thirteen patients seen between October 1996 and March 2000 were treated by heater probe coagulation, and twenty-five patients seen after April 2000 were treated by argon plasma coagulation. The therapies were repeated until the VE lesions completely disappeared from the stomach following the arrest of hemorrhaging. Heater probe coagulation was performed using a heater probe unit (Olympus Co., Tokyo, Japan) at 15 J for patients with bleeding VE and/or those with VE lesions with a diameter of > 3 mm and at 10 J for those with non-bleeding VE lesions with a diameter of ≤ 3 mm. Argon plasma coagulation was performed using a high-frequency generator (Erbotorm ICC200; ERBE, Tubingen, Germany) with an automatically regulated argon source (APC300; ERBE) and a flexible APC probe (ERBE) with a high-frequency output at 60 W. Argon gas was delivered at a flow rate of between 1.0 and 2.0 L/min.
The differences in the characteristics between the 2 groups were analyzed by Mann-Whitney’s U test, Fisher’s exact test and the χ2 test. The cumulative recurrence-free and rebleeding-free rates were analyzed by the Kaplan-Meier method. Factors associated with the type of VE and the treatment methods were compared by the log-rank test. P values of less than 0.05 were considered to be statistically significant.
As shown in Table 1, there were no significant differences in the sex distribution or age of the patients between the LVE and EVE groups. Hemorrhaging from the VE lesions was observed in 43% (6/14) and 63% (15/24) of the patients in the LVE and EVE groups, respectively. Additionally, 21% (3/14) and 36% (5/14) of the patients in the LVE group and 50% (12/24) and 63% (15/24) of the patients in the EVE group had underlying liver cirrhosis and chronic renal failure, respectively, but the prevalences of the 2 underlying diseases were not significantly different between the two groups.
| Total | Groups2 | P value | ||
| LVE | EVE | |||
| No. of patients | 38 | 14 | 24 | |
| Sex: male/female1 | 21 / 17 | 9/5 | 12/12 | NS |
| Age, yr (medium) | 69 | 67.5 | 72 | NS |
| Bleeding from lesions at examination1 | 21 | 6 | 15 | NS |
| Cauterization3 | ||||
| Method : HPC/APC1 | 13 / 25 | 6/8 | 7/17 | NS |
| No. of treatment sessions (mean ± SD) | 2.3 ± 1.6 | 1.1 ± 0.3 | 3.0 ± 1.6 | < 0.0001 |
| Observation period, mo (medium) | 32 | 68.5 | 29.5 | < 0.01 |
| Medication during observation1 | ||||
| Proton pump inhibitors | 15 | 4 | 11 | NS |
| H2-receptor antagonists | 20 | 9 | 11 | |
| None | 3 | 1 | 2 | |
| Underlying diseases1 | ||||
| Chronic renal failure | 15 | 3 | 12 | NS |
| Liver cirrhosis | 20 | 5 | 15 | NS |
Among the 21 patients in whom active bleeding from the VE lesions was observed, heater probe coagulation was performed in 3 LVE and 4 EVE patients, and argon plasma coagulation in 3 LVE and 11 EVE patients. Hemostasis was obtained in all patients after either endoscopic coagulation procedure. Although the VE lesions diminished after the procedures in all patients, the number of treatment sessions required for the complete disappearance of the lesions in the stomach differed between the LVE and EVE groups (Table 1). A single treatment session was sufficient to achieve the complete disappearance of the VE lesions in all the patients of the LVE group, except one, who required 2 sessions of heater probe coagulation. In contrast, the patients in the EVE group needed a mean of 3.0 treatment sessions (range, 2-7 treatment sessions) for the complete disappearance of the lesions. No complications of the endoscopic coagulation treatments were encountered in either treatment group.
In all patients, the recurrence of VE and of re-bleeding was examined by repeated follow-up endoscopic examinations for more than 6 mo after the final session of endoscopic cauterization therapy; the median observation period was 32 mo (range, 6-139 mo). As shown in Table 1, however, the observation periods were significantly longer in the LVE group than in the EVE group (P < 0.01). The medications prescribed for the patients during the observation period were similar between the two groups. The endoscopic examination revealed recurrence of the VE in 25 of 38 patients (66%), and re-bleeding from the recurrent gastric VE lesions in 15 of these patients (39%) over a median observation period of 5 mo (range, 2-70 mo) after the final session of the coagulation therapy. Particularly in the EVE group, the VE recurred in all the patients, and re-bleeding from the recurrent lesions developed in 14 of these patients (58%) (Table 2). No patients in the EVE group developed re-bleeding with the re-appearance of ≤ 10 VE lesions. All of the patients showing re-bleeding in the EVE group had underlying diseases: 8 with chronic renal failure, 5 with liver cirrhosis and 1 with radiation-induced mucosal damage of the gastrointestinal tract. In contrast, in the LVE group, the recurrence of the gastric VE was found in only 1 patient (7%), who had a single lesion that was found before the first session of argon plasma coagulation therapy. This patient also had underlying renal failure and was under long-term maintenance hemodialysis. Consequently, re-bleeding from the recurrent VE lesions developed in 60% (9/15) of the patients with chronic renal failure, which was significantly higher than the percentage in the patients without chronic renal failure (26%; 6/23) (P = 0.036).
| Groups | Recurrence rate | Re-bleeding rate |
| LVE | 1 (7.1) | 1 (7.1) |
| Without CRF | 0 (0.0) | 0 (0.0) |
| With CRF | 1 (33.3) | 1 (33.3) |
| EVE | 24 (100.0) | 14 (58.3) |
| Without CRF | 12 (100.0) | 6 (50.0) |
| With CRF | 12 (100.0) | 8 (66.7) |
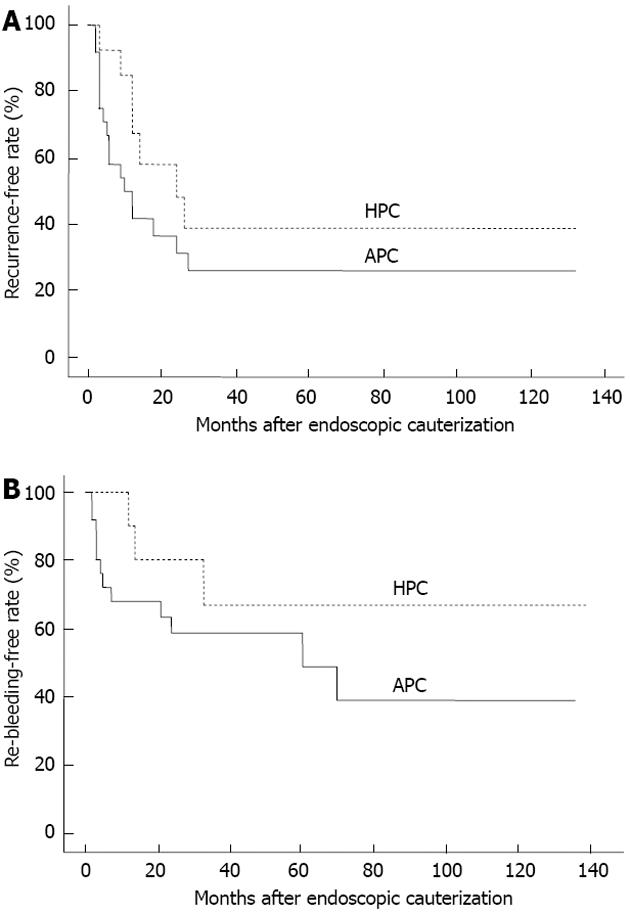
Kaplan-Meier analysis revealed that the cumulative recurrence-free rates of the patients with VE were 29.2% and 0% at 1 and 3 years, respectively, after the endoscopic coagulation therapy in the EVE group (Figure 1A), and the cumulative re-bleeding-free rates in these patients were 65.9%, 40.8% and 20.4% at 1, 3 and 5 years, respectively, after the therapy (Figure 1B). Both the recurrence-free rates and re-bleeding-free rates were significantly lower in the EVE group than in the LVE group. Moreover, the cumulative re-bleeding-free rate in the EVE group was 47.6% at 1 year and 25.4% at 2 years after the therapy in patients with chronic renal failure, which were significantly lower than the rates in the patients without chronic renal failure (83.3% and 74.1%, respectively) (Figure 2). There were no significant differences in either the recurrence-free rates or re-bleeding-free rates between the patients treated by heater probe coagulation and those treated by argon plasma coagulation, as shown in Figure 3.
In the present study, we examined the long-term prognosis of 38 patients with hemorrhagic gastric VE treated by endoscopic coagulation procedures, with a medium observation period of 32 mo after the therapies. Although both heater probe coagulation and argon plasma coagulation were useful to achieve the arrest of bleeding and disappearance of the VE lesions, the recurrence of VE and re-bleeding from the recurrent lesions were noted in 66% and 39% of the patients, respectively. Additionally, we found that the recurrence of VE and/or re-bleeding from the recurrent lesions were more frequent in patients with EVE, especially those with underlying chronic renal failure undergoing maintenance hemodialysis, than in those with LVE. We also found that neither the lesion recurrence rate nor the re-bleeding rate differed significantly between the patients treated by heater probe coagulation and those treated by argon plasma coagulation, although the number of patients evaluated in the study was relatively small.
In Japan, endoscopic laser therapy has not been widely employed for the treatment of VE in the gastrointestinal tract because of the high risk of severe complications, such as perforation[8]. Thus, endoscopic cauterization either with heater probe coagulation or argon plasma coagulation is the standard therapeutic strategy used for gastric VE with or without bleeding. However, few studies have been performed to determine the long-term outcomes of such therapies. Olmos et al[13] reported that argon plasma coagulation was effective for the prevention of recurrent bleeding from VE in the gastrointestinal tract. Although they showed that re-bleeding did not occur from recurrent lesions in 83% of the patients over a median observation periods of 18 mo, only 10 patients with hemorrhaging gastric VE were included in their study after the exclusion of patients with chronic renal failure, liver cirrhosis and GAVE, including patients with the lesions caused by radiation. Moreover, Zushi et al[14] examined the outcomes in 16 patients with liver cirrhosis with hemorrhagic GAVE treated by endoscopic cauterization and reported that 25% of the patients showed re-bleeding from recurrent lesions. In their study, however, the mean observation period was only 10 mo, which is too short to evaluate the long-term outcomes of therapy in these patients. In contrast, Nakamura et al[15] evaluated the long-term efficacy of argon plasma coagulation in 22 patients with GAVE and reported that the cumulative re-bleeding rates at 1, 2 and 3 years after the therapy were 50.3%, 64.5% and 64.5%, respectively, over a mean observation period of 23.5 mo. Herrera et al[17] examined the therapeutic efficacy of argon plasma coagulation in patients with VE depending on the type of lesion, and reported that there were no cases of re-bleeding from recurrent lesions among the patients with LVE. Our results regarding the EVE group were in line with those reported by Nakamura et al[15] but not with those by Herrera et al[17], in which re-bleeding after the therapies developed only in 1 of 8 patients with GAVE. In our study, re-bleeding from recurrent VE was especially frequent in patients with EVE and in those with chronic renal failure undergoing maintenance hemodialysis, whereas underlying diseases such as chronic renal failure were found only in 3 patients with GAVE in the study by Herrera et al[17]. These differences in the clinical features of the patients may have produced the discrepancies in the results by Herrera et al[17], those by Nakamura et al[15] and those in the present study.
Both LVE and EVE have been reported to develop at a high frequency in association with chronic renal failure[18]. Clouse et al[3] reported that 18 of 30 patients (60%) with hemorrhagic angiodysplasia had underlying renal failure, with 10 of these patients (33%) under long-term maintenance hemodialysis and/or who underwent renal transplantation. The present study demonstrated that endoscopic cauterization did not provide satisfactory long-term outcomes in patients with EVE complicated with chronic renal failure, even after the successful initial arrest of bleeding had been achieved by the endoscopic cauterization therapy. Notably, the VE developed again after the therapies in all of the patients with EVE, even in the absence of underlying chronic renal failure, followed by re-bleeding from the recurrent lesions in half of these patients, with a cumulative re-bleeding rate of approximately 40% at 3 years. These observations prompted us to postulate that the etiology and the clinical characteristics may be different between the LVE and EVE groups, and also between VE patients with and without chronic renal failure. Based on the findings, careful endoscopic follow-up after the initial successful cauterization therapy is required for patients with EVE, regardless of the presence/absence of underlying diseases, including chronic renal failure.
With respect to the endoscopic cauterization procedures available, argon plasma coagulation has been employed more frequently compared with heater probe coagulation for the treatment of gastric VE in Japan. Similarly, at our institution, almost all patients seen after the year 2000 have been treated by argon plasma coagulation. As shown in Figure 3, both the cumulative recurrence and re-bleeding rates were equivalent between patients with gastric VE treated by argon plasma coagulation and those treated by heater probe coagulation. The limitation of our evaluation is that it is a retrospective and single cohort study. A randomized controlled study would be required to confirm our results. Additionally, the safety, including the frequency of complications, convenience of instrument handling, and number of sessions required for the treatment, has been reported to not be significantly different between the patients treated by the two procedures[19-22]. Thus, the criteria for the selection of either procedure for patients with gastric ectasia need to be established in the future. Recently, endoscopic band ligation was shown to be useful for the treatment of GAVE[23-27]. Wells et al[25] reported that endoscopic band ligation was superior to thermal therapies, including argon plasma coagulation, in terms of the therapeutic efficacy to arrest bleeding, the volume of blood transfusion needed after the procedure and the duration of hospitalization in 22 patients with GAVE. Sato et al[26] also reported the superiority of endoscopic band ligationcompared with argon plasma coagulation for GAVE associated with liver diseases. Moreover, a novel endoscopic ablation method, the HALO90 system, has been reported to be useful for the treatment of GAVE in a few patients. A large-scale study would be required to compare the efficacy and safety of these novel procedures with those of argon plasma coagulation and heater probe coagulation.
In conclusion, the recurrence of VE and re-bleeding from the lesions was frequent in patients with EVE, especially in those with underlying chronic renal failure after the initial successful control of the bleeding and disappearance of the lesions by endoscopic cauterization. Therefore, careful observation by endoscopy is important for these patients even after initial successful therapy.
Vascular ectasia (VE) of the stomach has been recognized as a rare cause of hemorrhage from the upper gastrointestinal tract. Endoscopic coagulation has been reported to be useful for the temporary arrest of bleeding from any type of gastric VE. However, the long-term prognosis of patients undergoing endoscopic coagulation has not yet been elucidated.
The recurrence of VE and re-bleeding from the lesions was frequent in patients with extensive VE, especially in those with underlying chronic renal failure, after the initial successful control of the bleeding and disappearance of the lesions by endoscopic cauterization.
The findings of this study may help establish the treatment and follow-up strategy for the patients with bleeding gastric VE.
VE is a term encompassing a broad spectrum of lesions visualized on endoscopic examination, including angiodysplasia, watermelon stomach and diffuse antral VE. In this study, VE lesions were classified into 2 subtypes: localized VE with ≤ 10 VE lesions and extensive VE with > 10 VE lesions.
This is a paper on the treatment of VE of the stomach with some novel findings. The cohort is large and well described. It is worth publishing to demonstrate the novel finding of worse outcomes in those patients with chronic renal failure.
P- Reviewer Selinger CP S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Wheeler MH, Smith PM, Cotton PB, Evans DM, Lawrie BW. Abnormal blood vessels in the gastric antrum: a cause of upper-gastrointestinal bleeding. Dig Dis Sci. 1979;24:155-158. [PubMed] [Cited in This Article: ] |
| 2. | Jabbari M, Cherry R, Lough JO, Daly DS, Kinnear DG, Goresky CA. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology. 1984;87:1165-1170. [PubMed] [Cited in This Article: ] |
| 3. | Clouse RE, Costigan DJ, Mills BA, Zuckerman GR. Angiodysplasia as a cause of upper gastrointestinal bleeding. Arch Intern Med. 1985;145:458-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Jensen DM. Current diagnosis and treatment of severe obscure GI hemorrhage. Gastrointest Endosc. 2003;58:256-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Clouse RE. Vascular lesions: ectasias, tumours and malformations. Textbook of Gastroenterology. 3rd ed. Philadelphia: Lippincott Williams and Wilkins 1999; 2564-2582. [Cited in This Article: ] |
| 6. | Petrini JL, Johnston JH. Heat probe treatment for antral vascular ectasia. Gastrointest Endosc. 1989;35:324-328. [PubMed] [Cited in This Article: ] |
| 7. | Gostout CJ, Ahlquist DA, Radford CM, Viggiano TR, Bowyer BA, Balm RK. Endoscopic laser therapy for watermelon stomach. Gastroenterology. 1989;96:1462-1465. [PubMed] [Cited in This Article: ] |
| 8. | Naveau S, Aubert A, Poynard T, Chaput JC. Long-term results of treatment of vascular malformations of the gastrointestinal tract by neodymium YAG laser photocoagulation. Dig Dis Sci. 1990;35:821-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Sargeant IR, Loizou LA, Rampton D, Tulloch M, Bown SG. Laser ablation of upper gastrointestinal vascular ectasias: long term results. Gut. 1993;34:470-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Pavey DA, Craig PI. Endoscopic therapy for upper-GI vascular ectasias. Gastrointest Endosc. 2004;59:233-238. [PubMed] [Cited in This Article: ] |
| 11. | Wahab PJ, Mulder CJ, den Hartog G, Thies JE. Argon plasma coagulation in flexible gastrointestinal endoscopy: pilot experiences. Endoscopy. 1997;29:176-181. [PubMed] [Cited in This Article: ] |
| 12. | Yusoff I, Brennan F, Ormonde D, Laurence B. Argon plasma coagulation for treatment of watermelon stomach. Endoscopy. 2002;34:407-410. [PubMed] [Cited in This Article: ] |
| 13. | Olmos JA, Marcolongo M, Pogorelsky V, Varela E, Dávolos JR. Argon plasma coagulation for prevention of recurrent bleeding from GI angiodysplasias. Gastrointest Endosc. 2004;60:881-886. [PubMed] [Cited in This Article: ] |
| 14. | Zushi S, Imai Y, Fukuda K, Yabuta T, Tsujino S, Yamada T, Kurokawa M. Endoscopic coagulation therapy is useful for improving encephalopathy in cirrhotic patients with gastric antral vascular ectasia. Digest Endosc. 2005;17:32-35. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Nakamura S, Mitsunaga A, Konishi H, Oi I, Shiratori K, Suzuki S. Long-term follow up of gastric antral vascular ectasia treated by argon plasma coagulation. Digest Endosc. 2006;18:128-133. [DOI] [Cited in This Article: ] |
| 16. | Lecleire S, Ben-Soussan E, Antonietti M, Goria O, Riachi G, Lerebours E, Ducrotté P. Bleeding gastric vascular ectasia treated by argon plasma coagulation: a comparison between patients with and without cirrhosis. Gastrointest Endosc. 2008;67:219-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Herrera S, Bordas JM, Llach J, Ginès A, Pellisé M, Fernández-Esparrach G, Mondelo F, Mata A, Cárdenas A, Castells A. The beneficial effects of argon plasma coagulation in the management of different types of gastric vascular ectasia lesions in patients admitted for GI hemorrhage. Gastrointest Endosc. 2008;68:440-446. [PubMed] [Cited in This Article: ] |
| 18. | Cunningham JT. Gastric telangiectasias in chronic hemodialysis patients: a report of six cases. Gastroenterology. 1981;81:1131-1133. [PubMed] [Cited in This Article: ] |
| 19. | Protell RL, Rubin CE, Auth DC, Silverstein FE, Terou F, Dennis M, Piercey JR. The heater probe: a new endoscopic method for stopping massive gastrointestinal bleeding. Gastroenterology. 1978;74:257-262. [PubMed] [Cited in This Article: ] |
| 20. | Imai Y, Kinoshita M, Asakura Y, Kakinuma T, Arai S, Shimoji K, Sasaki K, Yabe S, Ota S, Fujiwara K. Usefulness of heater probe therapy for non-ulcer lesion. Prog Dig Endosc. 1999;54:40-42. [Cited in This Article: ] |
| 21. | Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol. 1994;2:42-46. [PubMed] [Cited in This Article: ] |
| 22. | Asakura Y, Imai Y, Arai S, Kinoshita M, Kakinuma T, Kakoi K, Rai F, Eguchi Y, Fujiwara K, Ota S. Efficacy of argon plasma coagulation for bleeding gastroduodenal ulcers. Digest Endosc. 2002;14:99-102. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Sinha SK, Udawat HP, Varma S, Lal A, Rana SS, Bhasin DK. Watermelon stomach treated with endoscopic band ligation. Gastrointest Endosc. 2006;64:1028-1031. [PubMed] [Cited in This Article: ] |
| 24. | Kumar R, Mohindra S, Pruthi HS. Endoscopic band ligation: a novel therapy for bleeding gastric antral vascular ectasia. Endoscopy. 2007;39 Suppl 1:E56-E57. [PubMed] [Cited in This Article: ] |
| 25. | Wells CD, Harrison ME, Gurudu SR, Crowell MD, Byrne TJ, Depetris G, Sharma VK. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc. 2008;68:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Sato T, Yamazaki K, Akaike J. Endoscopic band ligation versus argon plasma coagulation for gastric antral vascular ectasia associated with liver diseases. Dig Endosc. 2012;24:237-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Gross SA, Al-Haddad M, Gill KR, Schore AN, Wallace MB. Endoscopic mucosal ablation for the treatment of gastric antral vascular ectasia with the HALO90 system: a pilot study. Gastrointest Endosc. 2008;67:324-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |