Published online Aug 21, 2012. doi: 10.3748/wjg.v18.i31.4136
Revised: February 10, 2012
Accepted: April 9, 2012
Published online: August 21, 2012
AIM: To investigate the value of two-dimensional (2D) and three-dimensional (3D) double contrast-enhanced ultrasonography (DCUS) imaging for evaluation of gastric lesions.
METHODS: 2D and 3D DCUS imaging with both oral and intravenous administrations of contrast agents was used to assess gastroscopiclly-confirmed gastric lesions in 46 patients with benign and malignant diseases. Initially, liquid-based ultrasound contrast agent (Xinzhang®) was given orally at dose of 500-600 mL for conventional ultrasound examination of the gastric lesions, and then a microbubble-based contrast agent (SonoVue) was injected intravenously at dose of 1.2-2.4 mL in bolus fashion to assess the perfusion pattern of the lesions using contrast imaging modes. The parameters derived from time-intensity curves including the arrival time (AT), time to peak (TTP), peak intensity (PI) and enhanced intensity (EI) were measured on the 2D DCUS imaging. 3D DCUS of the lesions was acquired to demonstrate the value of this imaging mode.
RESULTS: There were 22 cases with benign lesions including chronic gastritis (n = 5), gastric ulcer (n = 9), gastric polyps (n = 3), gastric stromal tumors (n = 5), and 24 cases with malignant lesions including gastric cancer (n = 20), gastric cardia carcinoma (n = 3) and post-operative recurrent gastric cancer (n = 1) in the study. The oral contrast-enhanced ultrasonography (CEUS) imaging of the stomach clearly demonstrated the anatomy of the stomach and morphologic features of gastric lesions. With optimal scanning window and imaging display under oral CEUS, intravenous CEUS clearly showed the perfusion of gastric lesions with various characteristic manifestations. Both 2D and 3D DCUS images clearly demonstrated normal gastric wall as a three-layer structure, from the inside out, hyperechoic mucosa, hypoechoic muscularis and hyperechoic serosa, respectively. There were statistical significant differences of AT (8.68 ± 2.06 vs 10.43 ± 2.75, P = 0.017), PI (34.64 ± 6.63 vs 29.58 ± 8.22, P = 0.023) and EI (29.72 ± 6.69 vs 22.66 ± 7.01, P = 0.001) between malignant lesions and normal gastric wall. However, no differences of AT, PI and EI between benign lesions and normal gastric wall tissue were found. 3D DCUS could intuitively display morphological features and vascularities of the lesions with multiplanar and volume views. 3D DCUS imaging provided comprehensive information complementary to 2D imaging. The crater or wellhead appearances and feeding vessels as well as distorted nourishing vasculature of gastric carcinoma were better seen with 3D imaging than 2D imaging.
CONCLUSION: DCUS imaging can simultaneously display the anatomic and perfusion features of gastric lesions. 3D DCUS can provide additional information to 2D DCUS for evaluation of gastric lesions.
- Citation: Shi H, Yu XH, Guo XZ, Guo Y, Zhang H, Qian B, Wei ZR, Li L, Wang XC, Kong ZX. Double contrast-enhanced two-dimensional and three-dimensional ultrasonography for evaluation of gastric lesions. World J Gastroenterol 2012; 18(31): 4136-4144
- URL: https://www.wjgnet.com/1007-9327/full/v18/i31/4136.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i31.4136
The common methods for examination of the upper gastrointestinal (GI) tract are x-ray with oral barium-based contrast agent and endoscopy. Their shortcomings include the fact that they often cannot delineate submucosal mural structures of the GI tract. Limitations to the sonographic assessment of the upper GI tract and adjacent organs include patient body habitus and the presence of gas-filled bowel, which can produce shadowing artifacts[1,2]. Although ingestion of degassed water has been used to improve sonographic assessments of the GI tract and retroperitoneal structures, water simply displaces gas within the GI tract and can produce inconsistent results. Imaging water-filled stomach usually results in an increase in the wall through transmission, which may cause tissues of otherwise normal echogenicity to appear more echogenic than expected, creating a potential source of diagnostic error. Over the years, researchers attempted to develop oral contrast agents to improve the assessment of the GI tract and adjacent structures by absorbing and displacing bowel gas and provide an acoustic window for sonographic visualization of upper GI tract. One of such oral contrast agent Xinzhang® (Huqingyutang Pharmaceuticals Co., Hangzhou, China) has been developed and commercially available in China for ultrasound imaging of upper GI tract in clinical settings[3-5].
During the last two decades, contrast-enhanced ultrasound imaging (CEUS) with intravascular contrast agents has been investigated and has gradually emerged in clinical settings. The rapid development of contrast agents for sonography is precipitated by the performance limits of grayscale imaging and Doppler techniques. As US Imaging is used to study smaller and deeper structures in the abdomen, the spatial resolution of grayscale imaging and Doppler sensitivity becomes critical to the degree that it impacts the clinical utility of sonography. Contrast agents promise to improve the sensitivity and specificity of current sonographic diagnoses and have the potential to expand the already broad range of its applications.
Recently, we have explored new technique which combines both oral and intravenous CEUS imaging methods, so called Double contrast-enhanced ultrasound (DCUS), for evaluation of gastric abnormalities. The purpose of this study was to investigate the value of DCUS imaging using both two-dimensional (2D) and three-dimensional (3D) modes for the evaluation of gastric lesions.
The study protocol was approved by our hospital ethical committee, and all patients gave informed consent and agreed to participate in the study. During a period from 2007 to 2011, 2D and 3D DCUS imaging with both oral and intravenous administrations of contrast agents was used to assess gastroscopiclly-detected gastric lesions in 46 patients with 22 begin cases and 24 malignant cases. All final diagnoses are confirmed by endoscopic biopsy or surgical pathological findings. There were 31 males and 15 females, aged from 23 years to 80 years with a mean age of 54.93 ± 12.49 years.
The DCUS was performed using full digital ultrasound scanners iU22 (Philips Medical Systems, Bothell, WA) with a C5-2 probe or Sequoia-512 (Siemens Medical Solutions, Mountain View, CA) with a 4C1 probe for 2D imaging. Philips C6-2 volume probe was used for acquiring 3D DCUS imaging. Conventional ultrasound imaging mode was used for oral contrast imaging while contrast imaging modes (Philips Pulse inversion harmonic imaging and Siemens contrast pulse sequencing techniques) were used for intravascular contrast imaging.
The commercially available oral contrast agent Xinzhang® (Huqingyutang Pharmaceuticals Co., Hangzhou, China) was supplied as powder which is derived form rice and soya. The 48 g per package was reconstituted by adding 500-600 mL of cooled boiling water and gently agitating by hand to form a homogeneous thin paste.
The intravenous contrast agent SonoVue® (Bracco SpA, Milan, Italy) was injected in bolus fashion at doses of 1.2-2.4 mL through brachial vein, followed by 5 mL normal saline flush.
DCUS exanimation was performed after patient’s fasting for at least 8 h on the day of the study. The stomach of all patients was scanned using real time gray-scale imaging when the patients swallowed the oral agent to expand the cavity of the stomach. Using contrast agent-filled gastric cavity with homogenous moderate echogenicity as an acoustic window, the location, shape and size of any possible lesions and the wall thickness of the stomach were carefully imaged and recorded under dynamic scanning with patients in the supine and both decubitus positions. The scanning parameters (e.g., the depth, focus, and gain) were adjusted to achieve optimal imaging display as conventional ultrasound examination.
After oral contrast imaging localization of the gastric lesion, vascular CEUS of was performed with a bolus injection of 1.2-2.4 mL of SonoVue via a 20-gauge peripheral intravenous catheter under contrast imaging mode with a low mechanical index (0.09-0.21). Initially, each subject underwent 2D imaging to observe the perfusion pattern and measure the time-intensity curve of the lesions and adjacent normal wall of stomach as control. The CEUS parameters of arrival time (AT), time to peak (TTP), infusion time (IT, IT = TTP - AT), baseline intensity (BI), peak intensity (PI) and enhanced intensity (EI, EI = PI - BI) was obtained and calculated from the time-intensity curve. Next, the regions of interest were selected based on the 2D contrast imaging and 3D images of the region of interest were acquired using a 3D probe with additional contrast agent injection during the arterial phase of enhancement. The 3D imaging volume files was stored digitally with both on-line and off-line imaging process and analysis.
SPSS 13.0 (SPSS Inc., Chicago, United States) was used for statistical analysis. The values of measurements were expressed as (mean ± SD). Two sample t-test was used to compare each parameter (AT, TTP, PI, EI and IT) between benign or malignant lesions and normal gastric walls. For all analyses, a P value of less than 0.05 was considered statistically significant.
A total of 46 pathologically-proved cases were enrolled in the study. There were 22 cases with begin lesions including chronic gastritis (n = 5), gastric ulcer (n = 9), gastric polyps (n = 3), gastric stromal tumors (n = 5), and 24 cases with malignant lesions including gastric cancer (n = 20), gastric cardia carcinoma (n = 3) and post-operative recurrent gastric cancer (n = 1). The 2D DCUS with oral and intravenous contrast enhancement was successfully performed in all 46 patients While 43 out of 46 patients underwent 3D contrast imaging studies. Both 2D and 3D DCUS images clearly demonstrated normal gastric wall as a three-layer structure, from the inside out, hyperechoic mucosa, hypoechoic muscularis and hyperechoic serosa, respectively (Figure 1). DCUS characteristic findings of gastric lesions were visualized as follows.
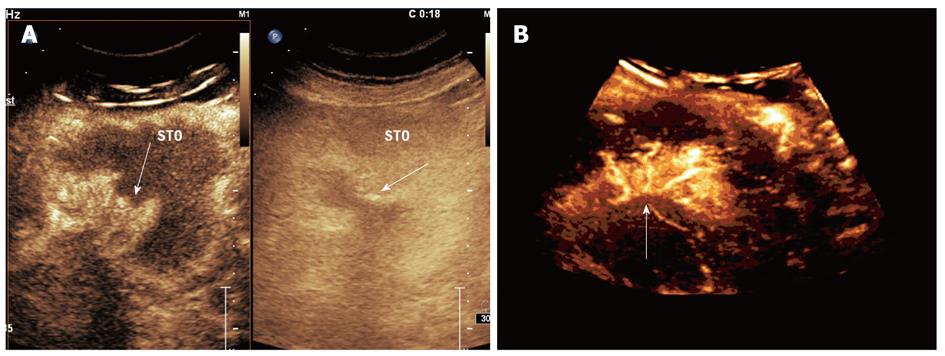
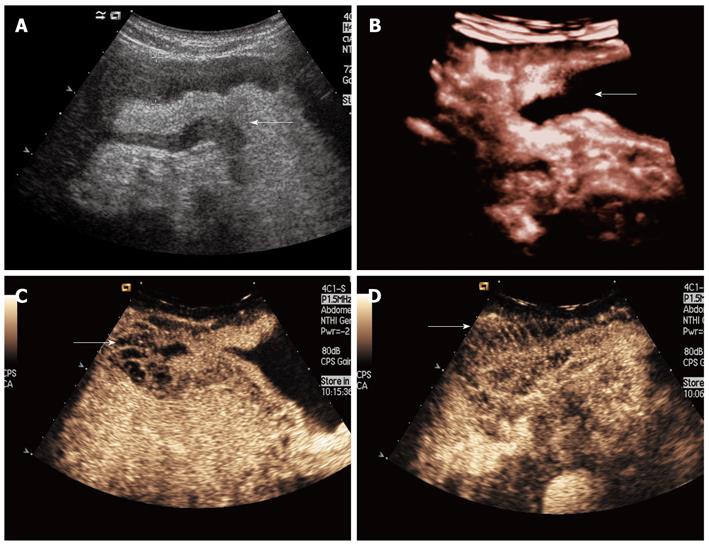
Gastric ulceration lesion: Gastric ulceration lesion appeared as a contrast agent-filled defect on the stomach wall with a spot-like mural hyperechogenic area in 9 cases. There was a lack of localized partial or prominent gastric wall thickening. Intravenous contrast 2D imaging shown uniformed enhancement of the gastric wall adjacent to the lesion and 3D DCUS imaging showed the gastric cavity and wall with a focal defect area consistent with an ulcer (Figure 2).
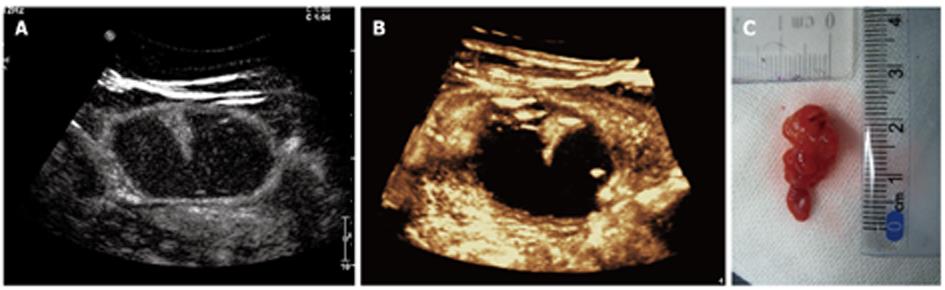
Gastric polyp: Gastric polyp appeared as a hyperechoic beansprout-shaped or a cone-shape mass projecting into the cavity of the stomach in 3 cases (Figure 3). Intravenous contrast 2D imaging shown simultaneous and equal enhancement of both lesions and normal gastric walls.
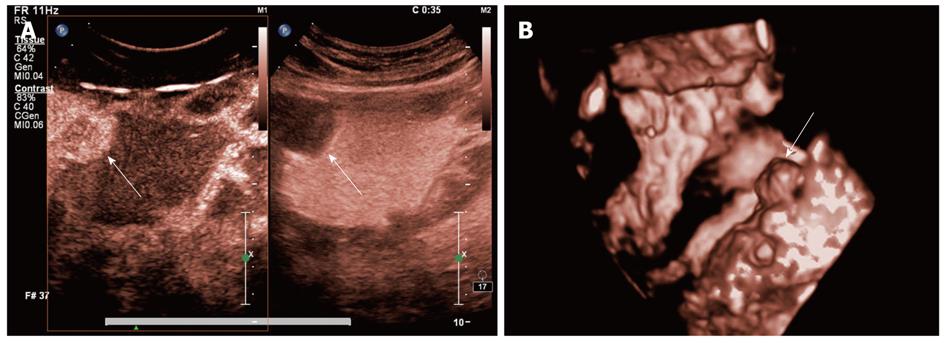
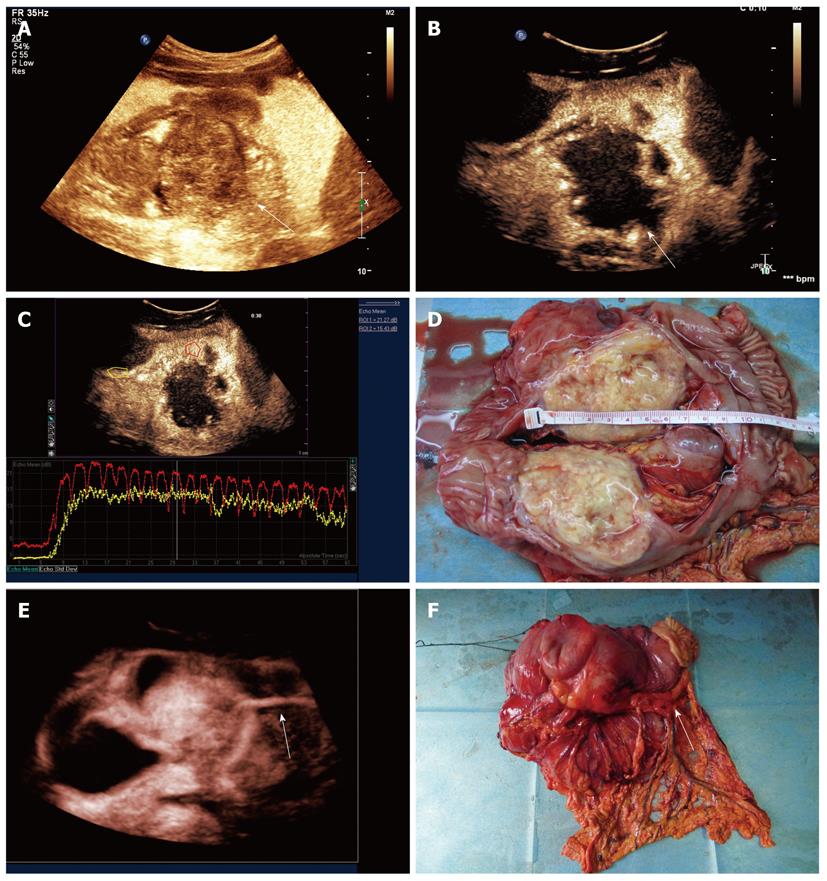
Gastric stromal tumor: Gastric stromal tumor shown as a hypoechoic or nearly anechoic mass within gastric wall under oral contrast imaging in 5 cases (Figure 4). These lesions had clear demarcation, regular around shape, and homogeneous echotexture. Larger ones protruded into the stomach cavity (n = 3) and have inhomogeneous echotexture (n = 2). Intravenous contrast 2D imaging demonstrated simultaneous or delayed enhancement of stromal tumors with homogeneous iso- or hypo-enhancement when compared with adjacent normal gastric wall. A ring enhancement appear in hypo-enhancement lesions (n = 2).
Gastric inflammatory lesion: Inflammatory thickening of the gastric wall was clearly seen under oral contrast displays in 5 chronic gastritis cases. The focal gastric inflammatory lesion appeared as homogeneous hypoechoic thickening associated with mild elevation of smooth surface of the wall. There was no remarkable change in the layers of the gastric wall. Intravenous contrast 2D imaging of the thickening wall shown uniform, simultaneous and iso- or hyper-enhancement compared to the normal gastric wall.
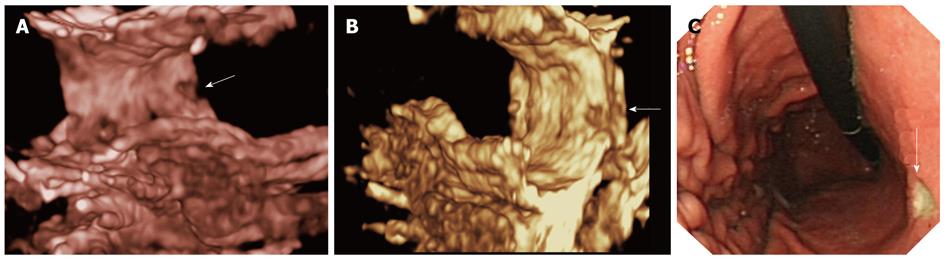
The characteristics of gastric carcinoma were demonstrated with oral contrast imaging in 24 cases. The features of malignant lesions included irregular shape, heterogeneous echotexture and disrupted layers of the gastric wall on the 2D oral contrast imaging. The mass-like lesion shown as a solid mass protruding into the cavity while the diffuse lesion appeared as a localized wall thickening and irregular surface of the lesions. The lesions with ulceration in 6 cases shown as filling defects within the lesions (Figure 5). Extensive infiltrative lesions in 9 cases shown diffuse heterogeneous thickening of the gastric wall which resulted in gastric cavity narrowing. The passage of oral contrast agent through the narrow gastric cavity can be seen with slow and stiff gastric peristalsis in real-time imaging. In 3 patients with carcinoma of gastric cardia, oral contrast imaging shown hypoechoic wall thickening of the distal esophageal and gastric cardia. Echogenic contrast agents passing through the narrow lumen of gastroesophageal junction was seen during the swallowing of contrast agent. In 6 patients with gastric carcinoma, enlarged lymph nodes adjacent to gastric wall were identified with hypoechogenic and round-shape features.
Combining with oral contrast imaging, intravenous 2D contrast imaging demonstrated variable patterns of enhancement of the lesions. When compared to adjacent normal gastric wall, there were iso-enhancement in 2 lesions, hypo-enhancement in 1 lesions and hyper-enhancement in 21 lesions. The feeding vessels and distorted tumor vasculature was clearly identified with 2D DCUS imaging (Figure 6). All lesions appeared as earlier enhancement in wash-in phase than the normal gastric wall. Under DCUS imaging condition, the enhancement parameters of time-intensity curves in both begin and malignant lesions shown in Table 1.
| Benign lesions | Normal gastric wall | P value | Malignant lesions | Normal gastric wall | P value | |
| AT (s) | 9.43 ± 2.25 | 9.22 ± 2.37 | 0.753 | 8.68 ± 2.06 | 10.43 ± 2.75 | 0.017 |
| TTP (s) | 16.24 ± 3.67 | 16.43 ± 3.32 | 0.862 | 15.86 ± 3.80 | 17.86 ± 4.19 | 0.089 |
| IT (s) | 6.85 ± 2.56 | 7.22 ± 2.57 | 0.643 | 7.17 ± 2.45 | 7.44 ± 3.03 | 0.344 |
| BI (dB) | 5.07 ± 3.49 | 5.29 ± 4.16 | 0.846 | 4.93 ± 3.25 | 6.92 ± 4.59 | 0.09 |
| PI (dB) | 31.36 ± 8.55 | 32.96 ± 8.58 | 0.538 | 34.64 ± 6.63 | 29.58 ± 8.22 | 0.023 |
| EI (dB) | 26.28 ± 9.90 | 27.64 ± 9.59 | 0.648 | 29.72 ± 6.69 | 22.66 ± 7.01 | 0.001 |
Reconstructed 3D imaging demonstrated global rendering of DCUS imaging with different prospective of morphology for both normal structures and gastric lesions. 3D imaging displayed intuitive pictures of lesions and the gastric layers from multiple imaging angles and views, which corresponded well with surgical specimens (Figures 2, 3 and 7).
In cases with thickened wall bulging unevenly or a mass protruding into (or outward) the gastric cavity, 3D DCUS imaging provided comprehensive information to complementary to 2D imaging. The crater or wellhead appearances and feeding vessels as well as distorted nourishing vasculature of gastric carcinoma were better seen with 3D imaging than 2D imaging (Figures 5, 7).
Ultrasound imaging is a convenient and noninvasive diagnostic tool for evaluation of abdominal organs. However, its use in diagnosing gastric abnormalities is limited by the interference of the gas in the GI tract. In 1978, Warren first used hydrophilic methyl cellulose oral suspension in ultrasound examination to image retroperitoneal organs such as stomach, duodenum or pancreas[6]. Since then, researchers have done many studies in oral contrast agent for gastric ultrasound imaging[7-12]. Early-developed oral contrast agents have short emptying, large required quantity, and an unpalatable taste. The oral contrast agent Xinzhang® used in this study is vegetable-based with main components being beans and starch[13], which is a uniform thin paste with pleasant taste and slow emptying feature without side effects, and thus is easily accepted by patients especially children and the elderly. The thin paste-based agent produces uniform moderate echogenic reflection within well-filled stomach, which clearly shows all normal layers of gastric wall, gastric lesions and surrounding structures under optimal contrast imaging. The gastric lesions revealed by oral contrast ultrasound in this study included mild thickening of the gastric wall, polypoid lesions and other stomach masses. Small lesions such as 0.5 cm diameter ulcer and 1.0 cm diameter stromal tumors can be identified. In addition, oral contrast gastric ultrasound imaging can be carried out using conventional ultrasound systems, which is easily applied in the clinical practice.
Although oral contrast imaging can show normal anatomy of the stomach and the location, shape and size of gastric lesions, its ability to determine internal structures and blood perfusion status of the lesion is limited. For example, gray-scale imaging cannot determine a very hypoechoic mass (such as gastric stromal tumor) whether it is cystic or solid[14-16]. Also, color Doppler ultrasound has a poor sensitivity in revealing small blood flow of the gastric wall or lesions. In previous study, intravenous contrast imaging has been used for the evaluation of gastric tumor in canine to determine the blood perfusion status of the tumors[17-19]. However, without appropriated gray-scale imaging of the stomach, Intravenous contrast imaging cannot achieve useful information of blood perfusion for assessment of tumor vascularity and surrounding structures. Therefore, DCUS imaging is necessary for evaluation of gastric lesions in order to obtain comprehensive information.
Micro-bubble-based SonoVue is a second-generation intravenous ultrasound contrast agent. It has phospholipids as capsule, containing sulfur hexafluoride gas. Its diameter is similar to that of red blood cells, which enables it to reach microvessels of all peripheral organs through intravenous injection. This agent has an average half-life of 12 min in vivo. It is removed by lungs through respiration in 15 min and poses no obvious toxic effect to the liver and kidney. In term of the difference from CT contrast agents such as lipiodol, microbubble-based contrast agent does not penetrate vessel wall and leak into interstitial space. Its distribution in the lesion represents the distribution of the microvessels, and the intensity of the lesion enhancement represents the density of those vessels[20-28]. Therefore, DCUS can be used to evaluate both the morphology and vascularity of gastric lesions.
In this study, we demonstrated that oral contrast imaging can provide excellent acoustic window for evaluation of a variety of gastric lesions by conventional gray-scale imaging. Furthermore, oral contrast imaging severs as important platform for assessment of blood perfusion of the lesions by intravascular contrast imaging. Thus, DCUS is able to demonstrate both morphologic appearances and perfusion status of both normal and abnormal structures, which improves the ability of differential diagnosis. For example, oral contrast imaging revealed 4 gastric stromal tumors as anechoic lesions which was difficult to decide whether they are cystic or solid lesions. However, the use of intravenous contrast imaging demonstrated internal blood perfusion of these tumor lesions, which confirmed they are solid lesions instead of cystic lesions. More important, DCUS can show the relationship of the lesion’s vasculature and the gastric wall as well as their contours outlined by contrast imaging, which accentuates pathological features. Indeed, DCUS images can clearly show the pathological features of the lesions, which made sonographic diagnosis to fit with internationally wide adopted Borrmann’s classification of advanced gastric cancer, i.e., polypoid lesion, ulcerated lesion, ulcerating infiltrative lesion, and infiltrative lesion.
Comparing malignant lesions with surrounding normal gastric tissue using contrast-enhanced time-intensity curve, values of AT, PI and EI parameters except TTP and IT were statistically significant (P < 0.05). Whereas comparing benign lesions with surrounding normal gastric tissue, all parameters including AT, TTP, IT, PI and EI are not statistically significant (P > 0.05). Thus, early AT and increased PI, EI, can be used as potential indexes and indicators for evaluating gastric begin and malignant lesions[29]. Since EI eliminated baseline intensity factor, it should reflects the actual intensity of the enchantment. Previous study have shown that, in gastric cancer, EI correlates well to the pathological microvessel density (r = 0.921, P < 0.001)[30], which may reflect the density of microvessels with the lesion, and could be a new parameter for evaluating biological behavior and angiogenesis of gastric cancer.
3D DCUS imaging provides comprehensive and observational perspective for gastric wall and lesion morphology. It can show the intuitive appearance of the lesion relevant to the gastric wall which closely correlates to pathological specimen and increases the confidence of clinical diagnosis. In malignant lesions, 3D imaging shown the dense, tortuous distorted and heavily cluttered vasculatures of the lesions, which is similar to the “tumor vascularity” seen in the liver and other organ malignancies[31-35]. 3D DCUS imaging can supplement 2D imaging and provide more evidence for the diagnosis of benign and malignant gastric lesions.
It should be point it out that this is preliminary observation of DCUS imaging applications. The limitations of this study include small number cases with various gastric abnormalities and no blinded comparison with endoscopic examinations. Prospective study design with larger clinical trial is needed for further investigations.
In conclusion, DCUS imaging is able to simultaneously display the sonographic features of various gastric lesions and its vasculatures as well as perfusion patterns. The parameters of AT, PI and EI could serve as potential indicators for differentiating benign and malignant gastric lesions. 3D DCUS could provide additional information to 2D DCUS for evaluation of gastric lesions.
The common methods for examination of the upper gastrointestinal (GI) tract are x-ray with oral barium-based contrast agent and endoscopy. Their shortcomings include the fact that they often cannot delineate submucosal mural structures of the GI tract. Over the years, oral contrast agents to improve the assessment of the GI tract and adjacent structures by absorbing and displacing bowel gas and provide an acoustic window for sonographic visualization of upper GI tract have been developed and commercially available in China, one of such oral contrast agent was Xinzhang®. Recently, the authors have explored new technique which combines both oral and intravenous contrast-enhanced ultrasonography (CEUS) imaging methods, called Double contrast-enhanced ultrasound, for evaluation both morphologic appearances and perfusion status of gastric abnormalities.
Two-dimensional (2D) double contrast-enhanced ultrasound (DCUS) could clearly demonstrate normal gastric wall and differentiate normal gastric wall from benign and malignant lesions. Three-dimensional (3D) DCUS could intuitively display morphological features and vascularities of the lesions with multiplanar and volume views.
The oral contrast agent Xinzhang® used in this study is vegetable-based with main components being beans and starch, which is a uniform thin paste with pleasant taste and slow emptying feature without side effects, and thus is easily accepted by patients especially children and the elderly. DCUS is able to demonstrate both morphologic appearances and perfusion status of both normal and abnormal structures, which improves the ability of differential diagnosis. In the study, small lesions such as 0.5 cm diameter ulcer and 1.0 cm diameter stromal tumors can be identified.
The study results suggest that 2D and 3D double DCUS was safe, highly sensitive and specific and could be applied for evaluation of gastric lesions.
This is an interesting and well written study aimed at assessing the role of contrast enhanced 2D and 3D US in the evaluation of gastric lesions. Even if it is a preliminary study and further prospective validation is need, the manuscript is very clear and very well documented by images.
Peer reviewer: Ji-Bin Liu, Professor, Department of Radiology, Thomas Jefferson University Hospital, 132 S. 10th Street, 7th Main Building, Philadelphia, PA 19107, United States
S- Editor Lv S L- Editor A E- Editor Zheng XM
| 1. | Gritzmann N, Hollerweger A, Macheiner P, Rettenbacher T. Transabdominal sonography of the gastrointestinal tract. Eur Radiol. 2002;12:1748-1761. [PubMed] [Cited in This Article: ] |
| 2. | Badea R, Ciobanu L, Gomotirceanu A, Hagiu C, Socaciu M. Contrast ultrasonography of the digestive tract lumen. Review of the literature and personal experience. Med Ultrason. 2010;12:52-61. [PubMed] [Cited in This Article: ] |
| 3. | Guo XZ, Yao GC. [Examination of stomach and duodenum with oral ultrasonic contrast agent]. Zhongguo Yixue Yingxiang Jishu. 1995;1:56-57. [Cited in This Article: ] |
| 4. | Guo XZ, Zhang W. A New Type of Acoustic Contrast for Visualizing the Upper Digestive Tract Walls and Pancreas. The 3rd Congress of Asian Federation of Societies for Ultrasound in Medicine and Biology. 1992;. [Cited in This Article: ] |
| 5. | Wang XC, Shi H, Yu XH, Zhang H, Li L, Xu AF, Wei ZR, Kong ZX, Yao C, Xu JP. [Comparison of contrast-enhanced ultrasound and gastroscopy in the diagnosis of gastric stromal tumor]. Zhonghua Yixue Chaosheng Zazhi (Electronic Edition). 2011;8:1033-1038 http: //chaosheng.cma-cme.com.cn, E-mail: csbjb@vip.sohu.com. [Cited in This Article: ] |
| 6. | Warren PS, Garrett WJ, Kossoff G. The liquid-filled stomach--an ultrasonic window to the upper abdomen. J Clin Ultrasound. 1978;6:315-320. [PubMed] [Cited in This Article: ] |
| 7. | Weighall SL, Wolfman NT, Watson N. The fluid-filled stomach: a new sonic window. J Clin Ultrasound. 1979;7:353-356. [PubMed] [Cited in This Article: ] |
| 8. | Sisler WJ, Tilcock C. Effect of cellulose concentration on the efficacy of a cellulose-based oral contrast agent for gastrointestinal ultrasonography. J Ultrasound Med. 1995;14:267-272. [PubMed] [Cited in This Article: ] |
| 9. | Muradali D, Burns PN, Pron G, Hope-Simpson D, Wilson S. Improved retroperitoneal and gastrointestinal sonography using oral contrast agents in a porcine model. AJR Am J Roentgenol. 1998;171:475-481. [PubMed] [Cited in This Article: ] |
| 10. | Lund PJ, Fritz TA, Unger EC, Hunt RK, Fuller E. Cellulose as a gastrointestinal US contrast agent. Radiology. 1992;185:783-788. [PubMed] [Cited in This Article: ] |
| 11. | Liao SR, Dai Y, Huo L, Yan K, Zhang L, Zhang H, Gao W, Chen MH. Transabdominal ultrasonography in preoperative staging of gastric cancer. World J Gastroenterol. 2004;10:3399-3404. [PubMed] [Cited in This Article: ] |
| 12. | Chaubal N, Dighe M, Shah M, Chaubal J. Sonography of the gastrointestinal tract. J Ultrasound Med. 2006;25:87-97. [PubMed] [Cited in This Article: ] |
| 13. | Guo XZ, Zhang W. [Clinical application of oral contrast-enhanced gastrointestinal ultrasound]. Zhonghua Yixue Chaosheng Zazhi (Electronic Edition). 2010;7:4-8 http://chaosheng.cma-cme.com.cn. [Cited in This Article: ] |
| 14. | Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293-1301. [PubMed] [Cited in This Article: ] |
| 15. | Fukuta N, Kitano M, Maekawa K, Chikugo T, Kudo M. Estimation of the malignant potential of gastrointestinal stromal tumors: the value of contrast-enhanced coded phase-inversion harmonics US. J Gastroenterol. 2005;40:247-255. [PubMed] [Cited in This Article: ] |
| 16. | Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705-712. [PubMed] [Cited in This Article: ] |
| 17. | Kamino D, Hata J, Haruma K, Manabe N, Tanaka S, Chayama K. Real-time visualization and quantitation of canine gastric mucosal blood flow by contrast-enhanced ultrasonography. Scand J Gastroenterol. 2006;41:856-861. [PubMed] [Cited in This Article: ] |
| 18. | Stock K, Hann von Weyhern C, Slotta-Huspenina J, Burian M, Clevert DA, Meining A, Prinz C, Pachmann C, Holzapfel K, Schmid RM. Microcirculation of subepithelial gastric tumors using contrast-enhanced ultrasound. Clin Hemorheol Microcirc. 2010;45:225-232. [PubMed] [Cited in This Article: ] |
| 19. | Piscaglia F, Corradi F, Mancini M, Giangregorio F, Tamberi S, Ugolini G, Cola B, Bazzocchi A, Righini R, Pini P. Real time contrast enhanced ultrasonography in detection of liver metastases from gastrointestinal cancer. BMC Cancer. 2007;7:171. [PubMed] [Cited in This Article: ] |
| 20. | Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775-1785. [PubMed] [Cited in This Article: ] |
| 21. | Sedelaar JP, van Leenders GJ, Hulsbergen-van de Kaa CA, van der Poel HG, van der Laak JA, Debruyne FM, Wijkstra H, de la Rosette JJ. Microvessel density: correlation between contrast ultrasonography and histology of prostate cancer. Eur Urol. 2001;40:285-293. [PubMed] [Cited in This Article: ] |
| 22. | Ng IO, Poon RT, Lee JM, Fan ST, Ng M, Tso WK. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma. Am J Clin Pathol. 2001;116:838-845. [PubMed] [Cited in This Article: ] |
| 23. | Imao T, Egawa M, Takashima H, Koshida K, Namiki M. Inverse correlation of microvessel density with metastasis and prognosis in renal cell carcinoma. Int J Urol. 2004;11:948-953. [PubMed] [Cited in This Article: ] |
| 24. | Yao DF, Wu XH, Zhu Y, Shi GS, Dong ZZ, Yao DB, Wu W, Qiu LW, Meng XY. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:220-226. [PubMed] [Cited in This Article: ] |
| 25. | Lim M, Cheshier S, Steinberg GK. New vessel formation in the central nervous system during tumor growth, vascular malformations, and Moyamoya. Curr Neurovasc Res. 2006;3:237-245. [PubMed] [Cited in This Article: ] |
| 26. | Zhao HC, Qin R, Chen XX, Sheng X, Wu JF, Wang DB, Chen GH. Microvessel density is a prognostic marker of human gastric cancer. World J Gastroenterol. 2006;12:7598-7603. [PubMed] [Cited in This Article: ] |
| 27. | Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823-1832. [PubMed] [Cited in This Article: ] |
| 28. | Du JR, Jiang Y, Zhang YM, Fu H. Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol. 2003;9:1604-1606. [PubMed] [Cited in This Article: ] |
| 29. | Wang GX, Xu S, Wang W, Li T, Luo YY. [Clinical application of contrast-enhanced ultrasound in differential diagnosis of malignant and benign gastrointestinal neoplasms]. Zhonghua Yixue Chaosheng Zazhi. 2007;4:35-37. [Cited in This Article: ] |
| 30. | Shiyan L, Pintong H, Zongmin W, Fuguang H, Zhiqiang Z, Yan Y, Cosgrove D. The relationship between enhanced intensity and microvessel density of gastric carcinoma using double contrast-enhanced ultrasonography. Ultrasound Med Biol. 2009;35:1086-1091. [PubMed] [Cited in This Article: ] |
| 31. | Ohto M, Kato H, Tsujii H, Maruyama H, Matsutani S, Yamagata H. Vascular flow patterns of hepatic tumors in contrast-enhanced 3-dimensional fusion ultrasonography using plane shift and opacity control modes. J Ultrasound Med. 2005;24:49-57. [PubMed] [Cited in This Article: ] |
| 32. | Yukisawa S, Ohto M, Masuya Y, Okabe S, Fukuda H, Yoshikawa M, Ebara M, Saisho H, Ohtsuka M, Miyazaki M. Contrast-enhanced three-dimensional fusion sonography of small liver metastases with pathologic correlation. J Clin Ultrasound. 2007;35:1-8. [PubMed] [Cited in This Article: ] |
| 33. | Coakley FV, Schwartz LH. Imaging of hepatocellular carcinoma: a practical approach. Semin Oncol. 2001;28:460-473. [PubMed] [Cited in This Article: ] |
| 34. | Kudo M. Imaging blood flow characteristics of hepatocellular carcinoma. Oncology. 2002;62 Suppl 1:48-56. [PubMed] [Cited in This Article: ] |
| 35. | Shi H, Yu XH, Zhang H, Li L. [Application of three-dimensional contrast-enhanced ultrasound in the vascular characteristics on display for abdominal tumors]. Zhongguo Yixue Yingxiang Jishu. 2008;8:1227-1230. [Cited in This Article: ] |