Published online Jun 7, 2012. doi: 10.3748/wjg.v18.i21.2609
Revised: January 31, 2012
Accepted: April 21, 2012
Published online: June 7, 2012
Gut flora and bacterial translocation (BT) play important roles in the pathogenesis of chronic liver disease, including cirrhosis and its complications. Intestinal bacterial overgrowth and increased bacterial translocation of gut flora from the intestinal lumen predispose patients to bacterial infections, major complications and also play a role in the pathogenesis of chronic liver disorders. Levels of bacterial lipopolysaccharide, a component of gram-negative bacteria, are increased in the portal and/or systemic circulation in several types of chronic liver disease. Impaired gut epithelial integrity due to alterations in tight junction proteins may be the pathological mechanism underlying bacterial translocation. Preclinical and clinical studies over the last decade have suggested a role for BT in the pathogenesis of nonalcoholic steatohepatitis (NASH). Bacterial overgrowth, immune dysfunction, alteration of the luminal factors, and altered intestinal permeability are all involved in the pathogenesis of NASH and its complications. A better understanding of the cell-specific recognition and intracellular signaling events involved in sensing gut-derived microbes will help in the development of means to achieve an optimal balance in the gut-liver axis and ameliorate liver diseases. These may suggest new targets for potential therapeutic interventions for the treatment of NASH. Here, we review some of the mechanisms connecting BT and NASH and potential therapeutic developments.
- Citation: Ilan Y. Leaky gut and the liver: A role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol 2012; 18(21): 2609-2618
- URL: https://www.wjgnet.com/1007-9327/full/v18/i21/2609.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i21.2609
Bacterial translocation (BT) and the derangement of gut flora are of substantial clinical relevance to patients with chronic liver disease and cirrhosis[1,2]. Intestinal bacterial overgrowth and increased bacterial translocation of gut flora from the intestinal lumen predispose patients to bacterial infections and major complications[3,4]. Furthermore, levels of bacterial lipopolysaccharide (LPS), a component of gram-negative bacteria, are increased in the portal and/or systemic circulation in several types of chronic liver disease. Bauer et al[5-7] have demonstrated this phenomenon in cirrhosis. Impaired gut epithelial integrity due to alterations in tight junction proteins may be the pathological mechanism underlying bacterial translocation. Over the last decade, increased gut permeability and increased LPS levels have been described in patients with alcoholic and nonalcoholic steatohepatitis (NASH)[8,9]. Increased serum LPS levels and activation of proinflammatory signaling cascades have been suggested to be important for disease progression in these settings[10]. Some potential mechanisms to explain the association between BT and liver disease associated with lipid accumulation and the development of NASH are reviewed here. These mechanisms may suggest new targets for potential therapeutic interventions for the treatment of NASH.
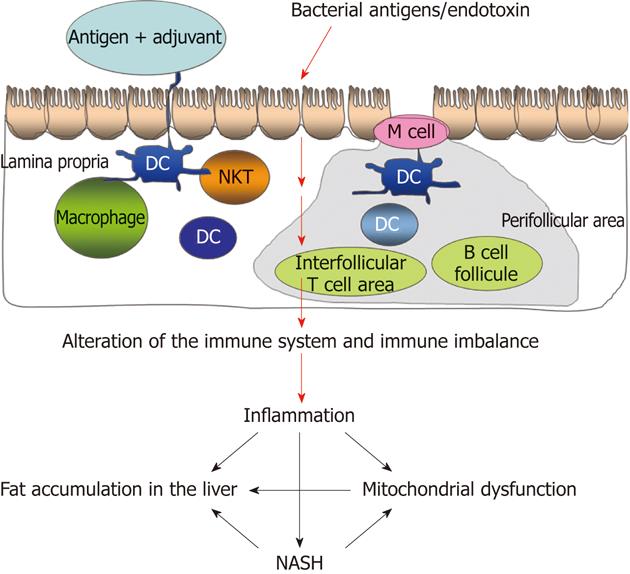
Inflammation is involved in the pathogenesis of chronic liver diseases and plays a role in the development of progressive hepatic damage and fibrosis[11]. Liver inflammation and chronic damage are mediated by innate immune responses that are regulated by toll-like receptors (TLRs)[12]. Innate immune cells can both initiate and maintain inflammation in the liver. Bacteria translocated from the gut activate lymphocytes after interacting at the mesenteric lymph nodes (MLNs)[13].
Innate immune cells, particularly dendritic cells, play a pivotal role in sensing pathogens and initiating adaptive immune responses through the activation and regulation of T lymphocyte responses[11]. The immune system is abnormally activated in patients and experimental models with cirrhosis and ascites[14-16]. In an animal model of cirrhosis, systemic activation of the immune system occurs before ascites develop and is driven by the recirculation of cells activated in hepatic lymph nodes (HLNs)[13]. In compensated cirrhosis, bacterial DNA fragments reach the MLNs, where they elicit a local inflammatory response. Bacterial DNA fragments were present in the MLNs of 54% of rats with cirrhosis, indicating their potential role in systemic inflammation[13]. BT initiates a Th1 immune response in MLNs, leading to T-helper 1 (Th1) polarization and the production of tumor necrosis factor (TNF)-α by monocytes. The recirculation of these activated effector immune cells into the blood promotes systemic inflammation[16]. A systemic inflammatory state with increased circulating TNF-α has been linked to increased susceptibility to bacterial infections and hemodynamic dysfunctionin patients with cirrhosis[15-18].
The liver provides a tolerogenic immune environment for antigen-specific T cells. The liver is a source for activated immune cells present in the blood. The activation of Kupffer cells, recruited macrophages, and inflammatory cells results in the production of cytokines and chemokines that lead to prolonged inflammation and hepatocyte damage[11]. A direct correlation between activated cells in the blood and HLNs, but not in MLNs, supports this concept, as does the fact that the changes in activated cells in the MLNs, but not in the blood or HLNs, can be reversed by gut decontamination with antibiotics[13]. Th1 cells and monocytes were expanded and activated to produce intracellular interferon (IFN)-γ and TNF-α in the MLNs of cirrhotic rats[18]. Abrogation of bacterial translocation by bowel decontamination reduced the number of activated Th1 cells and monocytes and normalized IFN-γ and TNF-α production by monocytes in the MLNs and blood[16,19].
TLRs and TLR ligands play roles in the pathophysiology of liver fibrosis[20,21] and cirrhosis, viral hepatitis, ALD[22,23], nonalcoholic fatty liver disease (NAFLD) and hepatocellular carcinoma[24]. TLRs recognize pathogen-associated molecular patterns (PAMPs) to detect the presence of pathogens[24]. TLRs are expressed on immune cells, Kupffer cells, endothelial cells, dendritic cells, biliary epithelial cells, HSCs, and hepatocytes. TLR signaling induces potent innate immune responses in these cells[25]. The liver is constantly exposed to PAMPs, such as LPS and bacterial DNA, through bacterial translocation via the portal vein system connecting it to the intestine[25].
TLRs also play a role in the regulation of inflammation based on their ability to recognize endogenous TLR ligands, termed damage-associated molecular patterns (DAMPs)[24]. The liver not only represents a major target of bacterial PAMPs in many disease states but also upregulates several DAMPs following injury[11,24]. The activation of inflammatory cells, including Kupffer cells, is a crucial step in the activation of hepatic stellate cells (HSCs)[25]. Intestinal bacterial microflora and functional TLR4, but not TLR2, are required for hepatic fibrogenesis[20]. Crosstalk between TLR4 signaling and transforming growth factor beta (TGF-β) signaling in HSCs has been reported[25]. Quiescent HSCs have been shown to be the target through which TLR4 ligands promote fibrogenesis. TGF-β signaling through the TLR4-MyD88-NFkappaB axis provides a novel link between proinflammatory and profibrogenic signals[20].
The activation of innate immune responses involving TLR4 and complement play important roles in initiating alcoholic steatohepatitis and fibrosis[26]. Activation of the TLR4-mediated myeloid differentiation factor 88 (MyD88)-independent [TRIF/interferon regulatory factor (IRF)-3] signaling pathway in Kupffer cells contributes to alcoholic steatohepatitis, whereas activation of TLR4 signaling in HSCs promotes liver fibrosis[26].
Activation of the innate immune system and increased release of proinflammatory cytokines and other mediators play an important role in the development of alcoholic liver disease (ALD)[27,28]. Alcohol-induced hepatocellular damage may occur as a result of bacterial or endotoxin translocation due to a reduction in reticuloendothelial system function in ALD. The recognition of gut-derived endotoxin by TLR4 contributes to the development of ALD through the activation of TLR-induced intracellular signaling pathways, cytokine production, and ROS[29]. TLR-dependent, ethanol-induced oxidative stress is important for the regulation of NFkappaB activation and cytokine production by Kupffer cells[28]. Kupffer cells are stimulated by gut-derived endotoxin via mechanisms dependent on increased gut permeability and alcohol-induced liver injury[28].
TLR9 and TLR2 mediate Propionibacterium acnes-induced sensitization to LPS-triggered acute liver injury in mice[12]. Ligand-specific activation of TLR2 and TLR9 is dependent on the common TLR adaptor MyD88. MyD88 in immune cells, but not in liver parenchymal cells, plays important roles in inflammatory cell recruitment and liver injury[12].
Activation of TLR9 induces type I interferons via IRF-7. Type I IFNs were upregulated during TLR9-associated liver injury in WT mice. Type I IFN signaling is therefore required for protection from immune-mediated liver injury[30]. Type I IFNs have anti-inflammatory effects mediated by endogenous interleukin (IL)-1ra, which regulates the extent of TLR9-induced liver damage[30,31]. These data support the notion that bacterial translocation, the innate immune system and TLRs play an important role in the pathogenesis of liver damage.
Several mechanisms have been proposed to explain the association between fat accumulation in the liver and bacterial translocation. A link between inflammation and hepatic steatosis was shown both in alcoholic and nonALD[32,33]. The consumption of refined carbohydrates in soft drinks has been postulated to be a key factor in the development of NAFLD.
Results of several studies have shown that an increased consumption of fructose may result in an increased lipid accumulation in the liver which was accompanied by insulin resistance and elevated plasma triglycerides (Ackerman, 2005 No. 260; Jurgens, 2005 No. 261; Faeh, 2005 No. 262; Lewis, 2004 No. 264). Consumption of high levels of fructose lead to liver damage through overfeeding and also may induce a proinflammatory response by increasing intestinal translocation of endotoxin[34]. In a mouse model, hepatic lipid accumulation was higher in mice consuming fructose; these mice also showed high endotoxin levels in portal blood, lipid peroxidation and TNF-α expression[34].
Macrophages facilitate the clearance of cholesterol from the body via reverse cholesterol transport[35]. LPS has been shown to suppress PPARgamma1 and its downstream target genes in macrophages, inducing foam cell formation. This was proposed as a mechanism underlying the development of bacterial infection-induced atherosclerosis[35]. LPS induces the expression of adipocyte enhancer-binding protein 1 (AEBP1) during monocyte differentiation. LPS-induced down-regulation of pivotal macrophage cholesterol efflux mediators, leading to foam cell formation, is mediated by AEBP1. AEBP1-independent pathways contribute to the delayed effects of LPS on macrophage cholesterol efflux and the development of foam cells[35].
The published data support the hypothesis that bacterial translocation may underlie some of the mechanisms associated with fat accumulation in the liver.
Mitochondrial dysfunction is a pathogenic feature of NASH[29,36] and there is evidence that mitochondrial damage contributes to apoptotic/necrotic cellular damage in NASH[37]. NASH is associated with an increase in reactive oxygen species (ROS) production in Kupffer cells and hepatocytes[38]. The greater the decrease in cytochrome and oxidase activity seen, the more significant is the increase in ROS production. Mitochondrial dysfunction and overproduction of ROS play key roles in the progression of chronic hepatitis C and ethanol-induced liver injury. Ethanol also causes bacterial translocation in the intestine, and the resulting LPS activates Kupffer cells to produce pro-inflammatory cytokines[38]. It has been suggested that NASH may also result from increased ROS production in Kupffer cells and hepatocytes that may be dependent on bacterial translocation[38]. Therefore, in addition to its effects which are directly or indirectly associated with fat delivery, BT may also be associated with mitochondrial dysfunction that further contributes to fat accumulation in NAFLD.
Evidence supporting a role for the liver-gut axis in the pathogenesis of NAFLD has been slowly accumulating over the past 7 years[39-41]. Both preclinical and clinical data suggest an association between BT, small intestinal bacterial overgrowth (SIBO) and NASH[42,43]. Recently, the presence of SIBO has been associated with the severity of liver steatosis[44]. Exposure to bacterial products of intestinal origin, most notably endotoxin, including LPS, leads to liver inflammation, hepatocyte injury and hepatic fibrosis[43].
TLR4[45] and its coreceptor, myeloid differentiation factor-2 (MD-2), recognize LPS and activate proinflammatory signaling pathways. TLR4 can specifically recognize LPS as a danger signal and induce activation of inflammation-associated genes[46,47]. A 4-wk high-fat diet increased plasma LPS concentration two to three times[48] and the LPS recognition complex (TLR4 and MD-2) activates NADPH in liver steatosis and induces fibrosis in a NASH model in mice. These data support the role of these receptors in the development of steatosis, inflammation and fibrosis in NASH[49].
The suppression of inflammation and immune tolerance are known to occur in normal livers. Suppressed inflammation has been shown despite bacterial colonization in normal human livers maintaining liver immune homeostasis[50]. In spite of increased bacterial colonization of liver tissues, lower levels of TLR2/4 mRNA and TLR4 and pIKKalpha, a marker for nuclear factor-kappa B (NFkappaB) activation, proteins were found in liver tissues from healthy subjects compared with samples from patients with primary biliary cirrhosis and NASH. Although these data raise the question of whether BT initiates the inflammatory process in the liver or whether it is instead a result of the inflammatory process, these results further support a role for BT in the pathogenesis of the disease.
SIBO also plays a role in NASH via interactions with TLR-4 and the induction of IL-8[43]. SIBO has been reported to coexist with NASH[51-56]. Patients with NASH show higher levels of expression of TLR-4/MD-2 on CD14-positive cells[43]. Serum levels of the proinflammatory cytokine IL-8 were higher in samples from NASH patients than in those from control subjects and were correlated positively with TLR-4 expression.
Leptin is a proinflammatory cytokine associated with the progression of NASH. Leptin enhanced TNF-α production and caused a dose-dependent increase in MAPK activity in LPS-stimulated KCs[57]. KCs isolated from the leptin receptor-deficient Zucker rat (fa/fa) showed reduced production of TNF-α upon stimulation with LPS[57]. Furthermore, treatment of normal rats with leptin increased LPS-induced hepatic TNF-α production in vivo, and leptin receptor-deficient Zucker rats showed reduced hepatic TNF-α production upon addition of LPS in vivo[57].
Following BT, LPS activates inflammasomes[58-60]. Inflammasomes respond to endogenous and exogenous danger signals by inducing the processing of pre-IL-1ss into secreted IL-1ss[36]. In the methionine-choline-deficient (MCD) or high fat diet-induced models, saturated fatty palmitic acid activates the inflammasome and sensitizes hepatocytes to LPS-induced IL-1ss release. Hepatocytes exposed to saturated fatty acid release danger signals that trigger inflammasome activation in immune cells[36]. LPS treatment significantly increased hepatic TNF-α production in MCD mice. LPS also induced a significant increase in TUNEL-positive cells[61]. This increase in apoptosis was inhibited by treatment with a neutralizing anti-mouse TNF receptor antibody or pentoxifylline.
In humans, dietary fructose intake has been associated with increased intestinal permeability and translocation of bacterial endotoxin; plasminogen activator inhibitor (PAI-1) may also contribute to the development of NAFLD in humans[62]. Plasma concentrations of endotoxin and PAI-1 and hepatic mRNA expression levels of TLR4 and PAI-1 were higher in NAFLD patients than in control subjects. Serum levels of LPS-binding protein (LBP) were increased in obese patients with NAFLD[63]. Plasma levels of LBP were further increased in patients with steatohepatitis when compared with patients with simple steatosis[63]. TNF-α mRNA expression in liver tissue was significantly higher in patients with NASH than in control subjects and was correlated with the increase in plasma levels of LBP.
BT is also involved in nitric oxide synthase (NOS) upregulation through the activation of both endothelial NOS and inducible NOS[64-66]. The prevention of intestinal gram-negative bacterial translocation by norfloxacin corrects circulatory changes by decreasing nitric oxide (NO) production in cirrhosis. Norfloxacin administration significantly decreased the incidence of gram-negative bacterial translocation and production of proinflammatory TNF-α, IFN-γ and IL-6[66,67].
The published data support the hypothesis that BT is associated with the development and maintenance of continued lipid accumulation, inflammation and fibrosis in patients with NASH (Figure 1).
BT was shown to affect the development of chronic liver disease and the associated complications. It is also associated with an impaired prognosis[68]. Bacterial DNA is a marker of bacterial translocation and can be detected in uninfected patients with cirrhosis and ascites[69,70]. It is associated with a marked inflammatory response and with the activation of the inducible form of NOS and the release of NO. A similar effect is observed in patients with SBP[68].
The induction of cirrhosis in rats by CCl4 led to prolonged oxidative stress in the intestine, accompanied by increased sugar content in both the intestinal brush border and the surfactant layers[71]. This was accompanied by changes in bacterial flora in the gut, and these bacteria showed increased hydrophobicity and adherence to the mucosa. These data support the notion that oxidative stress in the intestine during cirrhosis alters mucosal glycosylation and increases the hydrophobicity of the luminal bacteria, enabling increased bacterial adherence to epithelial cells, facilitating BT[71].
In a human trial, the presence of bacterial DNA was associated with aggravation of peripheral vasodilation and with a worsening of intrahepatic endothelial dysfunction[68]. Patients exposed to bacterial DNA had a significantly lower mean arterial pressure and systemic vascular resistance. In response to increased blood flow caused by postprandial hyperemia, these patients had greater increases in hepatic vein pressure gradient and impaired hepatic vasorelaxation[68]. In contrast, a prospective trial of 151 patients with cirrhosis and ascites found no evidence that the detection of bacterial DNA in the ascites of cirrhotics is of clinical or diagnostic relevance to the detection of SBP[72]. This discrepancy in the published data remains to be resolved.
Increased intestinal permeability and abnormal motility were frequently observed in cirrhotics without ascites, even in the absence of evidence of BT. It has been suggested that these factors facilitate BT and thus precede it[73]. Systemic reactivity to microbial components as measured by the development of antibodies was suggested to reflect the compromised mucosal immunity in cirrhotic patients[74]. The presence of bacterial DNA in blood and ascites correlates with BT and is frequent in patients with advanced cirrhosis without overt infection; BT can also precede the occurrence of overt bacterial infection in patients with cirrhosis[73]. Altered permeability of the mucosa and deficiencies in host immune defenses that allow bacterial translocation from the intestine due to intestinal bacterial overgrowth have been implicated in the development of SBP[71]. Altered intestinal permeability was observed in 45% of patients with cirrhosis and was associated with Child-Pugh status, with the presence of ascites, and with a history of SBP[75,76]. SIBO is much more frequent in patients with cirrhosis and was highly correlated with BT, especially in ascitic patients[77].
Higher levels of Enterobacteriaceae were identified in cirrhotic rats than in healthy rats, and Bifidobacteria treatment resulted in lower levels of Enterobacteriaceae[61]. These results suggest the existence of an imbalance in the gut flora in cirrhotic rats, which may further result in BT and altered liver function[61].
BT to MLNs in cirrhosis has been linked to impaired host defense in these patients[78]. BT and endotoxemia are contributing factors in the expansion of specific subsets of lymphocyte populations[79]. In a clinical trial of 40 cirrhotics, the percentage of activated monocytes and T lymphocytes was increased in patients, and the proportions of effector cells and of those expressing CD95+ were higher. LBP modulates the biologic activity of circulating endotoxin, and its levels have been shown to rise in response to LPS[80-82]. Patients with elevated levels of LBP showed higher frequencies of regulatory T cells (CD4+CD25+FoxP3+) than those with normal levels of LBP[79]. In a rat model, BT was associated with an increase in the phagocytic capacity of polymorphonuclear leukocytes[78]. Both TNF-α and IL-6 were increased in patients with translocation of bacterial DNA from gram-positive microorganisms regardless of endotoxin and LBP levels[83].
These data suggest that BT is of clinical relevance in patients with chronic liver disease and may be a contributing factor in the development of liver disease and the degree of severity of the associated complications.
Detoxification of gut-derived toxins and microbial products from gut-derived microbes is one function of the liver. Levels of bacterial LPS are increased in the portal and/or systemic circulation in several types of chronic liver diseases. Increased gut permeability and LPS also play roles in several liver disorders. NASH is associated with increased serum LPS levels and the activation of proinflammatory signaling[8,11], both of which suggest BT as a potential therapeutic target in these disorders.
Probiotics have been suggested as a treatment for different types of chronic liver damage because of their abilities to augment intestinal barrier function and to prevent BT[84,85]. The administration of probiotics reduced BT in a rat model[86]. Both viable and heat-killed yeast cells prevented BT. This effect was suggested to be the result of an immune modulatory effect and the maintenance of gut barrier integrity[87,88]. Oral treatment with viable or heat-killed Saccharomyces cerevisiae strain UFMG 905 prevented BT in a murine model of intestinal obstruction. Treatment with either viable or heat-killed yeast reduced intestinal permeability and increased IL-10 levels. Orally administered probiotics, nonpathogenic Escherichia coli, and gentamicin decreased BT and attenuated liver damage, decreasing levels of TNF-α, IL-6, IL-10 and IL-12[89]. An enteral diet supplemented with Chlorella sp. microalgae had significant protective effects on the intestinal mucosal barrier in a rat model of obstructive jaundice and reduced BT[90].
Oral treatment with resveratrol, curcumin or simvastatin ameliorated small intestinal inflammation by maintaining gut barrier function, preventing BT, and decreasing Th1-type immune responses[91]. Oral administration of these compounds increased regulatory T cell numbers and augmented intestinal epithelial cell regeneration in the ileum. Levels of the anti-inflammatory cytokine IL-10 in the ileum, MLNs and spleen were increased, whereas the proinflammatory cytokines IL-23p19, IFN-γ and TNF-α were decreased[91]. Treated animals displayed fewer proinflammatory enterobacteria and enterococci and higher anti-inflammatory lactobacilli and Bifidobacteria loads.
Glutamine decreased intestinal permeability and BT to physiologic levels in treated animals and preserved intestinal barrier integrity[92]. Arginine supplementation reduced intestinal permeability and BT, leading to mucosal ileum preservation[93]. A specific nutritional combination rich in protein, L-leucine, fish oil and specific oligosaccharides resulted in reduced BT along with reduced production of proinflammatory cytokines[94], and supplementation with honey in the presence of obstructive jaundice ameliorated BT[95].
Treatment with an anti-TNF-α mAb in a model of CCl4-induced cirrhosis decreased the incidence of BT in a TNF-α- and TNF-α receptor-independent manner without increasing the risk of systemic infection[96].
Desferrioxamine attenuated mucosal injury from post-hepatectomy liver dysfunction, and this was associated with decreased BT in the portal circulation, decreased portal endotoxin levels, and decreased systemic endotoxin levels[97]. Low concentrations of histamine inhibited bacteria from entering epithelial cells and inhibited intestinal BT[98]. In histamine-treated rats, the average numbers of bacteria in the liver and lymph nodes were much lower than those in control rats.
The sympathetic nervous system is activated in advanced cirrhosis, particularly in the splanchnic circulation, and exerts potent immunosuppressive actions. Splanchnic sympathectomy reduced bacterial translocation to MLNs in cirrhotic rats[99].
In light of the potential role of BT in the development of steatosis, steatohepatitis and fibrosis, several studies have evaluated the potential effects of treatments aimed at BT.
Probiotics exhibit immunoregulatory and anti-inflammatory activity. Administration of the probiotic VSL#3 modulated liver fibrosis via the modulation of collagen expression and impairment of TGF-β signaling in a NASH model[100]; however, this treatment did not prevent inflammation and steatosis.
Oxidative stress contributes to the development of NASH, suggesting that antioxidants, which decrease oxidative stress, may ameliorate the disease. Increasing hepatic α- or γ-tocopherol protected against LPS-induced NASH by decreasing liver damage, lipid peroxidation, and inflammation without affecting body mass or hepatic steatosis[101]. Resveratrol decreased NAFLD severity in rats via TNF-α inhibition and antioxidant activity[102]. Specifically, it decreased fat deposition, increased levels of activity of superoxide dismutase, glutathione peroxidase and catalase and decreased NOS in the liver.
LPS-induced liver injury was prevented by 8A8, a synthetic triglyceride containing an arachidonic acid branch, through the inhibition of TNF-α and NO production by hepatic macrophages[103]. Stimulation of peripheral blood mononuclear cells from NASH patients with LPS resulted in a strong increase in TNF-α production. Pentoxifylline caused a dose-dependent suppression of TNF-α secretion, suggesting that it may be able to serve as a potential treatment for NASH[104].
In an open-label trial, subjects with biopsy-proven NASH and insulin resistance were orally treated for 30 days with an IgG-enhanced fraction of enterotoxigenic Escherichia coli colostrum[105] (Imm-124©, Immuron, Australia). An alleviation of insulin resistance was detected by a decrease in fasting glucose levels, an elevation in the early peak of insulin secretion following glucose administration, improved OGTT, improved insulin secretion during the OGTT, and improvements in the HOMA score and HBA1C levels. Treated patients showed a decrease in serum levels of triglycerides, total cholesterol, and LDL cholesterol. A decrease in liver enzymes was noted in most treated patients. These effects were associated with increased serum levels of GLP-1 and adiponectin. An increase in CD25+ and CD4+CD25+Foxp3+ regulatory T cells was also noted. An anti-LPS effect along with the promotion of regulatory T cells suggests that a combined mechanism is responsible for these effects.
Alterations to intestinal microbiota seem to play important roles in the induction and promotion of liver damage progression and in the development and severity of NASH. Bacterial overgrowth, immune dysfunction, alteration of the luminal factors, and altered intestinal permeability are all involved in the pathogenesis of NASH and its complications, including progression to cirrhosis, infections, hepatic encephalopathy, SBP and renal failure[84]. A better understanding of the cell-specific recognition and intracellular signaling events involved in sensing gut-derived microbes will help in the development of means to achieve an optimal balance in the gut-liver axis and ameliorate liver diseases[36]. The data described here support the notion that BT may serve as a new therapeutic target for NASH.
BT induces an immune imbalance leading to a state of chronic inflammation, fat accumulation in the liver, mitochondrial dysfunction and NASH.
Peer reviewers: Stephan Johannes Ott, PD, MD, Clinic for Internal Medicine I, University-Hospital Schleswig-Holstein, Campus Kiel, Arnold-Heller-Street 3, 24105 Kiel, Germany; Bernardo Frider, MD, Professor, Department of Medicine-Hepatology, Argerich Hospital, University of Buenos Aires, Maimonides University, Salguero 2601, 1425 Buenos Aires, Argentina; Yuichi Yoshida, Assistant Professor, Department of Gastroenterology and Hepatology, Osaka University, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Almeida J, Galhenage S, Yu J, Kurtovic J, Riordan SM. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol. 2006;12:1493-1502. [PubMed] [Cited in This Article: ] |
| 2. | Floch MH, Katz J, Conn HO. Qualitative and quantitative relationships of the fecal flora in cirrhotic patients with portal systemic encephalopathy and following portacaval anastomosis. Gastroenterology. 1970;59:70-75. [PubMed] [Cited in This Article: ] |
| 3. | Pardo A, Bartolí R, Lorenzo-Zúñiga V, Planas R, Viñado B, Riba J, Cabré E, Santos J, Luque T, Ausina V. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31:858-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 492] [Cited by in F6Publishing: 484] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 5. | Bauer TM, Schwacha H, Steinbrückner B, Brinkmann FE, Ditzen AK, Aponte JJ, Pelz K, Berger D, Kist M, Blum HE. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002;97:2364-2370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Bauer TM, Steinbrückner B, Brinkmann FE, Ditzen AK, Schwacha H, Aponte JJ, Pelz K, Kist M, Blum HE. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol. 2001;96:2962-2967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Bauer TM, Schwacha H, Steinbrückner B, Brinkmann FE, Ditzen AK, Kist M, Blum HE. Diagnosis of small intestinal bacterial overgrowth in patients with cirrhosis of the liver: poor performance of the glucose breath hydrogen test. J Hepatol. 2000;33:382-386. [PubMed] [Cited in This Article: ] |
| 8. | Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis. 2010;28:737-744. [PubMed] [Cited in This Article: ] |
| 9. | Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304-1313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 162] [Cited by in F6Publishing: 171] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 10. | Szabo G, Mandrekar P, Dolganiuc A, Catalano D, Kodys K. Reduced alloreactive T-cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL-10, IL-13, and decreased IFNgamma levels. Alcohol Clin Exp Res. 2001;25:1766-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Szabo G, Mandrekar P, Dolganiuc A. Innate immune response and hepatic inflammation. Semin Liver Dis. 2007;27:339-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Hritz I, Velayudham A, Dolganiuc A, Kodys K, Mandrekar P, Kurt-Jones E, Szabo G. Bone marrow-derived immune cells mediate sensitization to liver injury in a myeloid differentiation factor 88-dependent fashion. Hepatology. 2008;48:1342-1347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Úbeda M, Muñoz L, Borrero MJ, Díaz D, Francés R, Monserrat J, Lario M, Lledó L, Such J, Álvarez-Mon M. Critical role of the liver in the induction of systemic inflammation in rats with preascitic cirrhosis. Hepatology. 2010;52:2086-2095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 15. | Albillos A, Hera Ad Ade L, Reyes E, Monserrat J, Muñoz L, Nieto M, Prieto A, Sanz E, Alvarez-Mon M. Tumour necrosis factor-α expression by activated monocytes and altered T-cell homeostasis in ascitic alcoholic cirrhosis: amelioration with norfloxacin. J Hepatol. 2004;40:624-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Muñoz L, Albillos A, Nieto M, Reyes E, Lledó L, Monserrat J, Sanz E, de la Hera A, Alvarez-Mon M. Mesenteric Th1 polarization and monocyte TNF-α production: first steps to systemic inflammation in rats with cirrhosis. Hepatology. 2005;42:411-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Chedid A, Mendenhall CL, Moritz TE, French SW, Chen TS, Morgan TR. Expression of the beta 1 chain (CD29) of integrins and CD45 in alcoholic liver disease. The VA Cooperative Study Group No. 275. Am J Gastroenterol. 1993;88:1920-1927. [PubMed] [Cited in This Article: ] |
| 18. | Girón JA, Alvarez-Mon M, Menéndez-Caro JL, Abreu L, Albillos A, Manzano L, Durántez A. Increased spontaneous and lymphokine-conditioned IgA and IgG synthesis by B cells from alcoholic cirrhotic patients. Hepatology. 1992;16:664-670. [PubMed] [Cited in This Article: ] |
| 19. | Heumann D, Barras C, Severin A, Glauser MP, Tomasz A. Gram-positive cell walls stimulate synthesis of tumor necrosis factor α and interleukin-6 by human monocytes. Infect Immun. 1994;62:2715-2721. [PubMed] [Cited in This Article: ] |
| 20. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1361] [Cited by in F6Publishing: 1440] [Article Influence: 84.7] [Reference Citation Analysis (1)] |
| 21. | Isayama F, Hines IN, Kremer M, Milton RJ, Byrd CL, Perry AW, McKim SE, Parsons C, Rippe RA, Wheeler MD. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1318-G1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 378] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 23. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 24. | Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 260] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 25. | Aoyama T, Paik YH, Seki E. Toll-like receptor signaling and liver fibrosis. Gastroenterol Res Pract. 2010;2010. [PubMed] [Cited in This Article: ] |
| 26. | Gao B, Seki E, Brenner DA, Friedman S, Cohen JI, Nagy L, Szabo G, Zakhari S. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516-G525. [PubMed] [Cited in This Article: ] |
| 27. | Tsukamoto H, Takei Y, McClain CJ, Joshi-Barve S, Hill D, Schmidt J, Deaciuc I, Barve S, Colell A, Garcia-Ruiz C. How is the liver primed or sensitized for alcoholic liver disease? Alcohol Clin Exp Res. 2001;25:171S-181S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Nagata K, Suzuki H, Sakaguchi S. Common pathogenic mechanism in development progression of liver injury caused by non-alcoholic or alcoholic steatohepatitis. J Toxicol Sci. 2007;32:453-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 29. | Csak T, Dolganiuc A, Kodys K, Nath B, Petrasek J, Bala S, Lippai D, Szabo G. Mitochondrial antiviral signaling protein defect links impaired antiviral response and liver injury in steatohepatitis in mice. Hepatology. 2011;53:1917-1931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Petrasek J, Dolganiuc A, Csak T, Kurt-Jones EA, Szabo G. Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology. 2011;140:697-708.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Petrasek J, Dolganiuc A, Csak T, Nath B, Hritz I, Kodys K, Catalano D, Kurt-Jones E, Mandrekar P, Szabo G. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology. 2011;53:649-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5683] [Cited by in F6Publishing: 5906] [Article Influence: 347.4] [Reference Citation Analysis (1)] |
| 33. | Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, Catalano D, Mandrekar P, Szabo G. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Bergheim I, Weber S, Vos M, Krämer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol. 2008;48:983-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 382] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 35. | Majdalawieh A, Ro HS. LPS-induced suppression of macrophage cholesterol efflux is mediated by adipocyte enhancer-binding protein 1. Int J Biochem Cell Biol. 2009;41:1518-1525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 472] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 37. | Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 510] [Cited by in F6Publishing: 522] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 38. | Sato N. Central role of mitochondria in metabolic regulation of liver pathophysiology. J Gastroenterol Hepatol. 2007;22 Suppl 1:S1-S6. [PubMed] [Cited in This Article: ] |
| 39. | Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511-12516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 770] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 40. | Solga SF, Diehl AM. Non-alcoholic fatty liver disease: lumen-liver interactions and possible role for probiotics. J Hepatol. 2003;38:681-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, Keshavarzian A. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026-1033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 42. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 938] [Cited by in F6Publishing: 982] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 43. | Shanab AA, Scully P, Crosbie O, Buckley M, O'Mahony L, Shanahan F, Gazareen S, Murphy E, Quigley EM. Small intestinal bacterial overgrowth in nonalcoholic steatohepatitis: association with toll-like receptor 4 expression and plasma levels of interleukin 8. Dig Dis Sci. 2011;56:1524-1534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 44. | Sabaté JM, Jouët P, Harnois F, Mechler C, Msika S, Grossin M, Coffin B. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg. 2008;18:371-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 45. | Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20-26. [PubMed] [Cited in This Article: ] |
| 46. | Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1860] [Cited by in F6Publishing: 2136] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 47. | Pålsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 847] [Cited by in F6Publishing: 889] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 48. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4095] [Cited by in F6Publishing: 4129] [Article Influence: 242.9] [Reference Citation Analysis (0)] |
| 49. | Csak T, Velayudham A, Hritz I, Petrasek J, Levin I, Lippai D, Catalano D, Mandrekar P, Dolganiuc A, Kurt-Jones E. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433-G441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 50. | Singh R, Bullard J, Kalra M, Assefa S, Kaul AK, Vonfeldt K, Strom SC, Conrad RS, Sharp HL, Kaul R. Status of bacterial colonization, Toll-like receptor expression and nuclear factor-kappa B activation in normal and diseased human livers. Clin Immunol. 2011;138:41-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Nazim M, Stamp G, Hodgson HJ. Non-alcoholic steatohepatitis associated with small intestinal diverticulosis and bacterial overgrowth. Hepatogastroenterology. 1989;36:349-351. [PubMed] [Cited in This Article: ] |
| 52. | Riordan SM, McIver CJ, Williams R. Liver damage in human small intestinal bacterial overgrowth. Am J Gastroenterol. 1998;93:234-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Zhao LF, Jia JM, Han DW. [The role of enterogenous endotoxemia in the pathogenesis of non-alcoholic steatohepatitis]. Zhonghua Ganzangbing Zazhi. 2004;12:632. [PubMed] [Cited in This Article: ] |
| 54. | Fu JF, Fang YL, Liang L, Wang CL, Hong F, Dong GP. A rabbit model of pediatric nonalcoholic steatohepatitis: the role of adiponectin. World J Gastroenterol. 2009;15:912-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Soza A, Riquelme A, González R, Alvarez M, Pérez-Ayuso RM, Glasinovic JC, Arrese M. Increased orocecal transit time in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2005;50:1136-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor α in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 598] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 57. | Shen J, Sakaida I, Uchida K, Terai S, Okita K. Leptin enhances TNF-α production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005;77:1502-1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3880] [Cited by in F6Publishing: 4230] [Article Influence: 302.1] [Reference Citation Analysis (0)] |
| 59. | Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1162] [Cited by in F6Publishing: 1302] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 60. | Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 778] [Cited by in F6Publishing: 818] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 61. | Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-α-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009;51:168-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 62. | Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Königsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138:1452-1455. [PubMed] [Cited in This Article: ] |
| 63. | Ruiz AG, Casafont F, Crespo J, Cayón A, Mayorga M, Estebanez A, Fernadez-Escalante JC, Pons-Romero F. Lipopolysaccharide-binding protein plasma levels and liver TNF-α gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg. 2007;17:1374-1380. [PubMed] [Cited in This Article: ] |
| 64. | Li H, Förstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 65. | Pateron D, Tazi KA, Sogni P, Heller J, Chagneau C, Poirel O, Philippe M, Moreau R, Lebrec D. Role of aortic nitric oxide synthase 3 (eNOS) in the systemic vasodilation of portal hypertension. Gastroenterology. 2000;119:196-200. [PubMed] [Cited in This Article: ] |
| 66. | Tazi KA, Moreau R, Hervé P, Dauvergne A, Cazals-Hatem D, Bert F, Poirel O, Rabiller A, Lebrec D. Norfloxacin reduces aortic NO synthases and proinflammatory cytokine up-regulation in cirrhotic rats: role of Akt signaling. Gastroenterology. 2005;129:303-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Heller J, Sogni P, Barrière E, Tazi KA, Chauvelot-Moachon L, Guimont MC, Bories PN, Poirel O, Moreau R, Lebrec D. Effects of lipopolysaccharide on TNF-α production, hepatic NOS2 activity, and hepatic toxicity in rats with cirrhosis. J Hepatol. 2000;33:376-381. [PubMed] [Cited in This Article: ] |
| 68. | Bellot P, García-Pagán JC, Francés R, Abraldes JG, Navasa M, Pérez-Mateo M, Such J, Bosch J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology. 2010;52:2044-2052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 69. | Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 211] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 70. | Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 302] [Cited by in F6Publishing: 294] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 71. | Natarajan SK, Ramamoorthy P, Thomas S, Basivireddy J, Kang G, Ramachandran A, Pulimood AB, Balasubramanian KA. Intestinal mucosal alterations in rats with carbon tetrachloride-induced cirrhosis: changes in glycosylation and luminal bacteria. Hepatology. 2006;43:837-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Appenrodt B, Lehmann LE, Thyssen L, Gentemann M, Rabe C, Molitor E, Trebicka J, Stüber F, Sauerbruch T. Is detection of bacterial DNA in ascitic fluid of clinical relevance? Eur J Gastroenterol Hepatol. 2010;22:1487-1494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Thalheimer U, De Iorio F, Capra F, del Mar Lleo M, Zuliani V, Ghidini V, Tafi MC, Caburlotto G, Gennari M, Burroughs AK. Altered intestinal function precedes the appearance of bacterial DNA in serum and ascites in patients with cirrhosis: a pilot study. Eur J Gastroenterol Hepatol. 2010;22:1228-1234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Papp M, Norman GL, Vitalis Z, Tornai I, Altorjay I, Foldi I, Udvardy M, Shums Z, Dinya T, Orosz P. Presence of anti-microbial antibodies in liver cirrhosis--a tell-tale sign of compromised immunity? PLoS One. 2010;5:e12957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Lauritano EC, Valenza V, Sparano L, Scarpellini E, Gabrielli M, Cazzato A, Ferraro PM, Gasbarrini A. Small intestinal bacterial overgrowth and intestinal permeability. Scand J Gastroenterol. 2010;45:1131-1132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, Dal Lago A, Ojetti V, Ainora ME, Santoro M. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 77. | Jun DW, Kim KT, Lee OY, Chae JD, Son BK, Kim SH, Jo YJ, Park YS. Association between small intestinal bacterial overgrowth and peripheral bacterial DNA in cirrhotic patients. Dig Dis Sci. 2010;55:1465-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 78. | Neugebauer H, Hartmann P, Krenn S, Glück T, Schölmerich J, Straub R, Wiest R. Bacterial translocation increases phagocytic activity of polymorphonuclear leucocytes in portal hypertension: priming independent of liver cirrhosis. Liver Int. 2008;28:1149-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 79. | Márquez M, Fernández-Gutiérrez C, Montes-de-Oca M, Blanco MJ, Brun F, Rodríguez-Ramos C, Girón-González JA. Chronic antigenic stimuli as a possible explanation for the immunodepression caused by liver cirrhosis. Clin Exp Immunol. 2009;158:219-229. [PubMed] [Cited in This Article: ] |
| 80. | Calvano SE, Thompson WA, Marra MN, Coyle SM, de Riesthal HF, Trousdale RK, Barie PS, Scott RW, Moldawer LL, Lowry SF. Changes in polymorphonuclear leukocyte surface and plasma bactericidal/permeability-increasing protein and plasma lipopolysaccharide binding protein during endotoxemia or sepsis. Arch Surg. 1994;129:220-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1164] [Cited by in F6Publishing: 1155] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 82. | Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006;8:946-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 83. | González-Navajas JM, Bellot P, Francés R, Zapater P, Muñoz C, García-Pagán JC, Pascual S, Pérez-Mateo M, Bosch J, Such J. Presence of bacterial-DNA in cirrhosis identifies a subgroup of patients with marked inflammatory response not related to endotoxin. J Hepatol. 2008;48:61-67. [PubMed] [Cited in This Article: ] |
| 84. | Cesaro C, Tiso A, Del Prete A, Cariello R, Tuccillo C, Cotticelli G, Del Vecchio Blanco C, Loguercio C. Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis. 2011;43:431-438. [PubMed] [DOI] [Cited in This Article: ] |
| 85. | Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011;35:14S-20S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 86. | Zhou HJ, Yin L, Chen CQ, Shi MM, Zhang MJ. Administration of probiotics reduces bacterial translocation after intestinal transplantation in rats. Transplant Proc. 2010;42:4643-4647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 87. | Generoso SV, Viana M, Santos R, Martins FS, Machado JA, Arantes RM, Nicoli JR, Correia MI, Cardoso VN. Saccharomyces cerevisiae strain UFMG 905 protects against bacterial translocation, preserves gut barrier integrity and stimulates the immune system in a murine intestinal obstruction model. Arch Microbiol. 2010;192:477-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 88. | Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, Desimone C, Song XY, Diehl AM. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 658] [Cited by in F6Publishing: 662] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 89. | Li YT, Wang L, Chen Y, Chen YB, Wang HY, Wu ZW, Li LJ. Effects of gut microflora on hepatic damage after acute liver injury in rats. J Trauma. 2010;68:76-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Bedirli A, Kerem M, Ofluoglu E, Salman B, Katircioglu H, Bedirli N, Yilmazer D, Alper M, Pasaoglu H. Administration of Chlorella sp. microalgae reduces endotoxemia, intestinal oxidative stress and bacterial translocation in experimental biliary obstruction. Clin Nutr. 2009;28:674-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 91. | Bereswill S, Muñoz M, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Loddenkemper C, Göbel UB, Heimesaat MM. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One. 2010;5:e15099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 92. | dos Santos RG, Viana ML, Generoso SV, Arantes RE, Davisson Correia MI, Cardoso VN. Glutamine supplementation decreases intestinal permeability and preserves gut mucosa integrity in an experimental mouse model. JPEN J Parenter Enteral Nutr. 2010;34:408-413. [PubMed] [Cited in This Article: ] |
| 93. | Viana ML, Santos RG, Generoso SV, Arantes RM, Correia MI, Cardoso VN. Pretreatment with arginine preserves intestinal barrier integrity and reduces bacterial translocation in mice. Nutrition. 2010;26:218-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 94. | Faber J, van Limpt K, Kegler D, Luiking Y, Garssen J, van Helvoort A, Vos AP, Knol J. Bacterial translocation is reduced by a specific nutritional combination in mice with chemotherapy-induced neutropenia. J Nutr. 2011;141:1292-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Gencay C, Kilicoglu SS, Kismet K, Kilicoglu B, Erel S, Muratoglu S, Sunay AE, Erdemli E, Akkus MA. Effect of honey on bacterial translocation and intestinal morphology in obstructive jaundice. World J Gastroenterol. 2008;14:3410-3415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 96. | Francés R, Chiva M, Sánchez E, González-Navajas JM, Llovet T, Zapater P, Soriano G, Muñoz C, Balanzó J, Pérez-Mateo M. Bacterial translocation is downregulated by anti-TNF-α monoclonal antibody administration in rats with cirrhosis and ascites. J Hepatol. 2007;46:797-803. [PubMed] [Cited in This Article: ] |
| 97. | Nastos C, Kalimeris K, Papoutsidakis N, Defterevos G, Pafiti A, Kalogeropoulou H, Zerva L, Nomikos T, Kostopanagiotou G, Smyrniotis V. Antioxidant treatment attenuates intestinal mucosal damage and gut barrier dysfunction after major hepatectomy. Study in a porcine model. J Gastrointest Surg. 2011;15:809-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Duan L, Chen X, Alexander JW. Regulatory effect of histamine on the barrier function of intestinal mucosal. J Gastrointest Surg. 2010;14:1180-1185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Worlicek M, Knebel K, Linde HJ, Moleda L, Schölmerich J, Straub RH, Wiest R. Splanchnic sympathectomy prevents translocation and spreading of E coli but not S aureus in liver cirrhosis. Gut. 2010;59:1127-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, Szabo G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 101. | Chung MY, Yeung SF, Park HJ, Volek JS, Bruno RS. Dietary α- and γ-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis. J Nutr Biochem. 2010;21:1200-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 102. | Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N, Sarasqueta C, Cosme A, Irastorza B, González A. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008;8:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 103. | Piao N, Ikejima K, Kon K, Aoyama T, Osada T, Takei Y, Sato N, Watanabe S. Synthetic triglyceride containing an arachidonic acid branch (8A8) prevents lipopolysaccharide-induced liver injury. Life Sci. 2009;85:617-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 104. | Duman DG, Ozdemir F, Birben E, Keskin O, Ekşioğlu-Demiralp E, Celikel C, Kalayci O, Kalayci C. Effects of pentoxifylline on TNF-α production by peripheral blood mononuclear cells in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2007;52:2520-2524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Mizrahi M, Shabat Y, Adar T, Ben Ya'acov A, Ilan Y. Alleviation of insulin resistance and liver damage by oral administration of etec colostrums is mediated by increased GLP-1, adiponectin serum levels and tregs: Results of a phase I/II clinical trial in NASH. Hepatology. 2010;52:163A. [Cited in This Article: ] |