Published online May 28, 2012. doi: 10.3748/wjg.v18.i20.2576
Revised: February 10, 2012
Accepted: March 10, 2012
Published online: May 28, 2012
AIM: To investigate the effects and mechanisms of action of glycine on phagocytosis and tumor necrosis factor (TNF)-α secretion by Kupffer cells in vitro.
METHODS: Kupffer cells were isolated from normal rats by collagenase digestion and Percoll density gradient differential centrifugation. After culture for 24 h, Kupffer cells were incubated in fresh Dulbecco's Modification of Eagle’s Medium containing glycine (G1: 1 mmol/L, G2: 10 mmol/L, G3: 100 mmol/L and G4: 300 mmol/L) for 3 h, then used to measure phagocytosis by a bead test, TNF-α secretion after lipopolysaccharide stimulation by radioactive immunoassay, and microfilament and microtubule expression by staining with phalloidin-fluorescein isothiocyanate (FITC) or a monoclonal anti-α tubulin-FITC antibody, respectively, and evaluated under a ultraviolet fluorescence microscope.
RESULTS: Glycine decreased the phagocytosis of Kupffer cells at both 30 min and 60 min (P < 0.01, P < 0.05). The numbers of beads phagocytosed by Kupffer cells in 30 min were 16.9 ± 4.0 (control), 9.6 ± 4.1 (G1), 12.1 ± 5.7 (G2), 8.1 ± 3.2 (G3) and 7.5 ± 2.0 (G4), and were 22.5 ± 7.9 (control), 20.1 ± 5.8 (G1), 19.3 ± 4.8 (G2), 13.5 ± 4.7 (G3) and 9.2 ± 3.1 (G4) after 60 min. TNF-α secretion by Kupffer cells in G1 (0.19 ± 0.03), G2 (0.16 ± 0.04), G3 (0.14 ± 0.03) and G4 (0.13 ± 0.05) was significantly less than that in controls (0.26 ± 0.03, P < 0.01), and the decrease in secretion was dose-dependent (P < 0.05). Microfilaments of Kupffer cells in G2, G3 and G4 groups were arranged in a disorderly manner. The fluorescence densities of microtubules in G1 (53.4 ± 10.5), G2 (54.1 ± 14.6), G3 (64.9 ± 12.1) and G4 (52.1 ± 14.2) were all lower than those in the controls (102.2 ± 23.7, P < 0.01), but the decrease in microtubule fluorescence density was not dose-dependant.
CONCLUSION: Glycine can decrease the phagocytosis and secretion by Kupffer cells in vitro, which may be related to the changes in the expression of microfilaments and microtubules induced by Kupffer cells.
-
Citation: Wu HW, Yun KM, Han DW, Xu RL, Zhao YC. Effects of glycine on phagocytosis and secretion by Kupffer cells
in vitro . World J Gastroenterol 2012; 18(20): 2576-2581 - URL: https://www.wjgnet.com/1007-9327/full/v18/i20/2576.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i20.2576
Glycine has been well characterized in the spinal cord as an inhibitory neurotransmitter which activates expression of the glycine-gated chloride channel (GlyR) in postsynaptic membranes. Kupffer cells contain a GlyR similar to that described previously in the central nervous system[1,2]. Many studies have shown that dietary or intravenous glycine has a protective effect in rat models against endotoxic shock, hemorrhagic shock, liver ischemia-reperfusion, liver transplantation, and alcohol-induced liver injury and is most likely to exert this effect by inactivating the Kupffer cells via this newly identified GlyR[3-10]. Our previous studies also indicated that glycine protected rats from thioacetamide-induced liver injury and intestinal endotoxemia[11,12]. The mechanism involved may be related to inhibition of the release of pro-inflammatory cytokines by Kupffer cells induced by glycine. In vivo and in vitro experiments have found that glycine inhibits the secretion of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in Kupffer cells[13-15]. However, the impact of glycine on phagocytosis by Kupffer cells has not been reported, and the mechanisms underlying the effect of glycine on TNF-α secretion by Kupffer cells have not been fully understood. Our in vitro study showed that lipopolysaccharide (LPS) probably enhanced or inhibited the phagocytosis of Kupffer cells by acting through mechanisms involving microfilaments or microtubules[16]. This study aimed to investigate the effects of glycine on phagocytosis and the mechanisms underlying TNF-α secretion by Kupffer cells in vitro.
Adult male Wistar rats weighing 300-330 g were obtained from the Experimental Animal Center of Shanxi Medical University (China). All animals were fed with standard laboratory chow and water was available ad libitum. The experimental protocols were approved by the Shanxi Animal Research Ethics Committee.
Polystyrene beads (1.1 μm), monoclonal anti-α tubulin-fluorescein isothiocyanate (FITC) conjugate, LPS (Escherichia coli Serotype 0128:B12), collagenase IV, phalloidin-FITC, hydroxyethyl piperazine ethanesulfonic acid (HEPES) Percoll, and Dulbecco’s Modification of Eagle’s Medium (DMEM) were purchased from Sigma (St. Louis, United States); a radioimmunoassay kit for TNF-α measurement was purchased from the Radio-Immunity Institute of the Chinese Liberation Army Omni-hospital (Beijing, China); glycine, sodium pentobarbital, fetal bovine serum (FBS), penicillin G, streptaquaine, insulin, glutamine, trypan blue, and all other reagents not specifically mentioned elsewhere were prepared by Beijing Chemical Inc. (Beijing, China).
Kupffer cells from Wistar rats were isolated by collagenase digestion and differential centrifugation, using Percoll density gradients as described previously with slight modifications[17]. Briefly, the liver was perfused in situ through the portal vein with Ca2+ and Mg2+ free Hanks’ balanced salt solution (HBSS) containing 0.5 mmol/L ethylene glycol-bis (β-aminoethyl ether)-N,N,N,N- tetraacetic acid (EGTA) at 37 °C for 5 min at a flow rate of 26 mL/min. Subsequently, perfusion was performed with HBSS containing 0.05% collagenase IV at 37 °C for 5 min. After the liver was digested, it was excised and cut into small pieces in collagenase buffer. The suspension was filtered through nylon gauze, and the filtrate was centrifuged twice at 50 ×g at 4 °C for 3 min to remove parenchymal cells. The nonparenchymal cell fraction was washed with buffer and centrifuged on a density cushion of Percoll at 1000 ×g at 4 °C for 20 min to obtain the Kupffer cell fraction, and the cells obtained were washed with buffer again. The viability of isolated Kupffer cells was determined by trypan blue exclusion and routinely exceeded 90%. Cells were seeded onto 24-well culture plates (Corning, NY) or 25 mm × 25 mm glass coverslips at a density of 1 × 106 or 5 × 105 and cultured in DMEM supplemented with 10% FBS, antibiotics (100 U/mL penicillin G and 100 μg/mL streptomycin sulfate), 0.1 U/100 mL insulin and 15 mmol/L glutamine at 37 °C with 5% CO2. Non-adherent cells were removed after 1 h by replacing the culture medium. All adherent cells phagocytosed latex beads and stained positive for catalase, confirming that they were Kupffer cells, and cells were cultured for 24 h before experiment.
Cells were seeded onto 24-well plates and 12 mm × 12 mm glass coverslips, and incubated with fresh medium containing glycine (G1: 1 mmol/L, G2: 10 mmol/L, G3: 100 mmol/L and G4: 300 mmol/L) at 37 °C with 5% CO2 for 3 h. Phagocytosis and expression of microfilaments and microtubules by Kupffer cells were measured by the bead phagocytosis test, fluorescence staining and immunofluorescence staining, as described below.
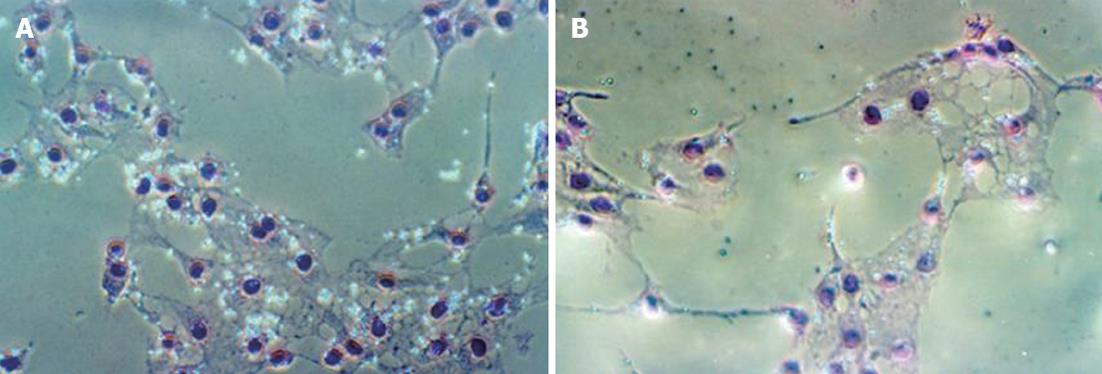
Phagocytosis by Kupffer cells was evaluated by the Kupffer cell’s ability to ingest polystyrene beads according to the modified method of Hirose et al[18]. Briefly, cells were seeded onto 12 mm × 12 mm glass coverslips or glass plates and incubated with fresh medium containing 0.05% polystyrene beads for 30 min or 60 min at 37 °C with 5% CO2. Following vigorous pipetting to remove non-phagocytosed latex beads, the coverslips or glass plates were washed 3 times with PBS and fixed with 2% formaldehyde or methanol for 5 min. After staining by Giemsa’s method for 15 min at room temperature and washing 3 times with PBS, the coverslips were inverted onto glass slides and observed under phase contrast microscope. The mean number of latex beads phagocytosed by each Kupffer cell was counted in at least 20 Kupffer cells per field at magnification of 200 times, 5 fields per coverslip in 6 coverslips.
Kupffer cells were seeded into 24-well plates at a density of 1 × 106/well and incubated with fresh DMEM containing 100 ng/mL LPS for 60 min at 37 °C with 5% CO2. At the end of this period, the medium was collected, centrifuged at 1000 ×g at 4 °C for 10 min, and the supernatant was stored at -80 °C until used for TNF-α assay. TNF-α in medium was measured using the radioimmunoassay kit. The levels of TNF-α in the wells represented the secretion of Kupffer cells.
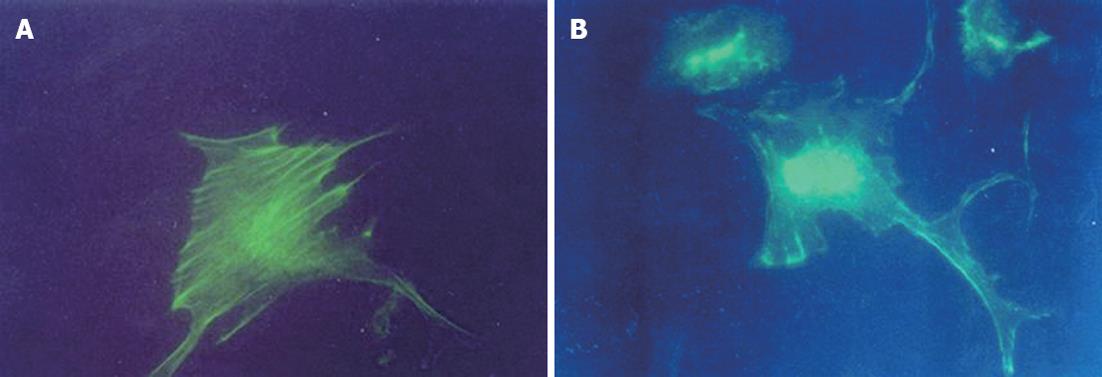
Kupffer cells were stained with phalloidin-FITC according to the modified method of Wulf et al[19]. Briefly, Kupffer cells were seeded onto 12 mm × 12 mm glass coverslips at a density of 5 × 105 (1 × 104 to 2 × 104 cells/coverslip), fixed with 2% formaldehyde for 20 min and extracted with 0.5% Triton X-100 for 15 min. The fixed cells were then washed 3 times with PBS (10 mmol/L, pH 7.4) and stained with phalloidin-FITC for 45 min at room temperature in the dark. They were then washed for a further 3 times with PBS, the coverslips were inverted onto mounting medium applied to glass slides, and they were observed and photographed under a ultraviolet (UV) fluorescence microscope with a high magnification of 400 times. Mounted preparations could be stored in the dark at 2 °C-8 °C.
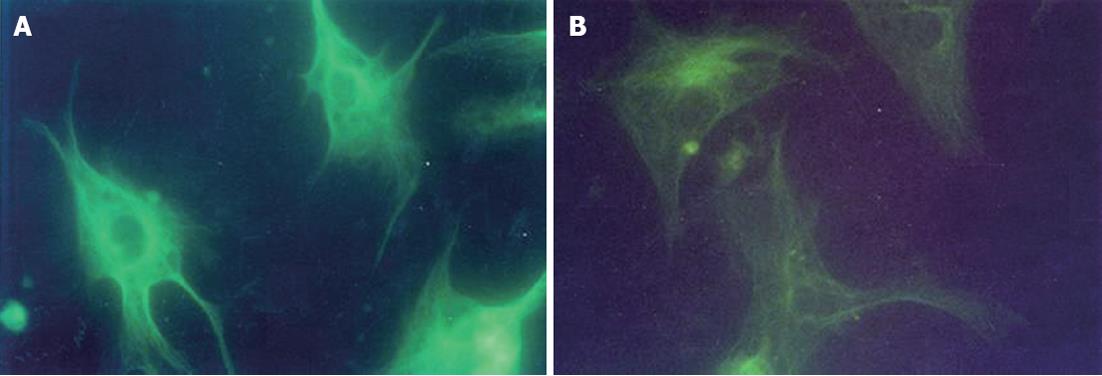
Microtubules in Kupffer cells were stained with a monoclonal anti-α tubulin-FITC antibody according to the method recommended by the producer. Briefly, Kupffer cells were seeded onto 12 mm × 12 mm glass coverslips at a density of 5 × 105 (1 × 104 to 2 × 104 cells/coverslip). They were then fixed with cold methanol for 10 min at -20 °C and rinsed twice with cold acetone (-20 °C) for 10 s, then the cell layer was rehydrated in PBS (10 mmol/L, pH 7.4) for at least 30 min and stained with monoclonal anti-α tubulin-FITC (1:25 diluted with PBS containing 1% bovine serum albumin) in a dark-room for 60 min at room temperature. The stained cells were washed 3 times with PBS, the coverslips were inverted onto mounting medium applied to glass slides and observed and photographed under a UV fluorescence microscope. Mounted preparations could be stored in the dark at 2-8 °C. The fluorescence density was measured in 10 cells using the MIAS-300 picture analysis system from at least 5 fields in each picture at a high magnification of 400 times.
All results were expressed as mean ± SD. Statistical differences between means were analyzed by one-way analysis of variance or t test using the SPSS 12.0 statistical package. Statistical significance level was set at P < 0.05.
When incubated in 5% CO2 with fresh medium containing glycine at 37 °C for 30 min or 60 min, phagocytosis by Kupffer cells decreased significantly. The number of beads phagocytosed by Kupffer cells in groups G3 and G4 was less than that of group G1 in 60 min. There were no significant differences in the amount of beads phagocytosed by Kupffer cells among the G2, G3 and G4 groups (Table 1 and Figure 1).
When incubated in 5% CO2 with fresh medium containing glycine at 37 °C for 3 h, TNF-α secretion by Kupffer cells decreased significantly, and the decrease in secretion was dose dependent. TNF-α concentrations detected in the medium of groups G3 and G4 were significantly lower than in the medium of group G1 (Table 2).
After 3 h incubation in 5% CO2 with fresh medium containing glycine at 37 °C, Kupffer cells stained with FITC-Phalloidin did not demonstrate organized microfilaments in groups G2, G3 or G4. There were no significant differences in the microfilament fluorescence densities among Kupffer cells in control, G1, G2, G3 and G4 groups (Figure 2).
Following 3 h incubation in fresh medium containing glycine at 37 °C with 5% CO2, Kupffer cells were stained with monoclonal anti-α tubulin-FITC. A significant decrease in the fluorescence density of microtubules was observed in Kupffer cells incubated with glycine as compared with the controls. However, the fluorescence density of the microtubules did not show a dose-dependent decrease among G1, G2, G3 and G4 groups (Table 2 and Figure 3).
Kupffer cells are the main component of the host monocyte-macrophage system, and their two main functions are phagocytosis and secretion. There is much evidence indicating that activation of Kupffer cells and their production of pro-inflammatory cytokines contribute to the pathogenesis of different liver injuries, including alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD) and liver failure among others[20-22]. Tsujimoto et al[23] showed that phagocytic activity of Kupffer cells was decreased in a rat model of nonalcoholic steatohepatitis. Glycine is a nonessential amino acid and an inhibitory neurotransmitter in the central nervous system. Many studies have shown that dietary or intravenous glycine can protect against a variety of liver injuries[3-10]. In this study, we found that glycine decreases the phagocytosis and secretion of Kupffer cells in vitro.
The mechanisms of Kupffer cell phagocytosis are still not completely understood. The ruffling of the cell membrane and formation of pseudopodia may play an important role and these effects are believed to be accomplished by the cytoskeleton. In the cytoskeleton, actin-myosin interaction through the calcium-calmodulin system plays a major role in this activity[24]. In this system, intracellular Ca2+ combines with calmodulin to form the active calcium-calmodulin complex, which activates an enzyme, myosin light chain kinase, to phosphorylate the light chain of myosin. Phosphorylated myosin, but not unphosphorylated myosin, can interact with actin to induce activity of the cell membrane and pseudopodia, leading to phagocytosis. This process is reversible, in that a phosphatase can catalyze dephosphorylation of myosin, restoring it to a form that can not be activated by actin.
Previous studies have shown that integrity of the cytoskeletal system is important for phagocytosis of Kupffer cells. Depolymerization of the cytoskeleton decreased phagocytosis by Kupffer cells[25-27]. However, the effects of glycine on phagocytosis by Kupffer cells have not been reported.
The present experiments show that glycine decreases phagocytosis by Kupffer cells in vitro, causes disordering of the microfilaments in Kupffer cells, and reduces their expression of microtubules. All these results show that glycine can decrease the phagocytosis of Kupffer cells by acting on the microfilaments and microtubules.
Some studies have shown that both CD14 and non-CD14 mechanisms are involved in the TNF-α secretion of monocytes and Kupffer cells, and that both endocytosis and Ca2+ are required for endotoxin-stimulated TNF-α release by Kupffer cells in rats[28-30]. Previous studies have shown that glycine can protect against many injuries and illnesses in rat models, most likely by inactivating Kupffer cells and decreasing TNF-α secretion[3-15]. An in vitro study has shown that glycine prevents the increases in [Ca2+]i caused to LPS by activating chloride influx-reduced synthesis and release of toxic mediators by Kupffer cells[2]. Thus, glycine can activate the chloride influx, prevent the increases in [Ca2+]i and reduce the TNF-α secretion of Kupffer cells.
Other studies have demonstrated the involvement of a microtubule-dependent mechanism in TNF-α secretion by monocytes. Taxol, a microtubule-stabilizing antineoplastic agent, induced expression of tumor TNF-α in macrophages[31]. Microtubule-disrupting agents such as colchicine had opposite effects on TNF-α production[32-34]. The present experiments showed that glycine significantly decreased TNF-α secretion and microtubule expression. Some of our results are consistent with previous reports[13-15], leading us to believe that glycine can prevent TNF-α secretion by Kupffer cells through disruption of microtubules.
In summary, glycine decreases both phagocytosis and secretion by Kupffer cells in vitro, which is probably related to glycine-induced changes in expression of microfilaments and microtubules in Kupffer cells.
The authors thank Chun-Ni Zhao for performing the statistical analysis.
Activated Kupffer cells are most likely involved in the pathogenesis of different liver injuries. Glycine generally is considered as a protective agent for liver injuries. The mechanism may be related to the fact that glycine inhibits the release of pro-inflammatory cytokines by Kupffer cells. So, it is very important to clarify the impact of glycine on the phagocytosis and secretion by Kupffer cells.
It is believed that cytoskeleton plays a vital physiological role in phagocytosis by Kupffer cells, and depolymerization of cytoskeleton decreases the phagocytosis by Kupffer cells. Glycine protects against liver injuries by preventing the elevation of intracellular Ca2+ and reducing pro-inflammatory cytokines production by Kupffer cells. But the impact of glycine on phagocytosis by Kupffer cells is still unclear, and the mechanisms of glycine on tumor necrosis factor-α secretion by Kupffer cells have not been completely understood.
This is the first study to report that glycine decreases the phagocytosis of Kupffer cells by acting on the microfilaments and microtubules in vitro.
This study suggests that glycine may be an effective agent which could provide a future strategy for therapeutic intervention in the treatment of liver injuries induced by activated Kupffer cells.
It is an interesting study with appropriate methodology and the results are clear and of great importance.
Peer reviewer: Yujin Hoshida, MD, PhD, Cancer Program, Broad Institute, 7 Cambridge Center, Cambridge, MA 02142, United States
S- Editor Lv S L- Editor Ma JY E- Editor Zheng XM
| 1. | Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G856-G863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Ikejima K, Qu W, Stachlewitz RF, Thurman RG. Kupffer cells contain a glycine-gated chloride channel. Am J Physiol. 1997;272:G1581-G1586. [PubMed] [Cited in This Article: ] |
| 3. | Matilla B, Mauriz JL, Culebras JM, González-Gallego J, González P. [Glycine: a cell-protecting anti-oxidant nutrient]. Nutr Hosp. 2002;17:2-9. [PubMed] [Cited in This Article: ] |
| 4. | Ikejima K, Iimuro Y, Forman DT, Thurman RG. A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol. 1996;271:G97-103. [PubMed] [Cited in This Article: ] |
| 5. | Zhong Z, Enomoto N, Connor HD, Moss N, Mason RP, Thurman RG. Glycine improves survival after hemorrhagic shock in the rat. Shock. 1999;12:54-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Currin RT, Caldwell-Kenkel JC, Lichtman SN, Bachmann S, Takei Y, Kawano S, Thurman RG, Lemasters JJ. Protection by Carolina rinse solution, acidotic pH, and glycine against lethal reperfusion injury to sinusoidal endothelial cells of rat livers stored for transplantation. Transplantation. 1996;62:1549-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Thurman RG, Schemmer P, Zhong Z, Bunzendahl H, von Frankenberg M, Lemasters JJ. Kupffer cell-dependent reperfusion injury in liver transplantation: new clinically relevant use of glycine. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:185-190. [PubMed] [Cited in This Article: ] |
| 8. | Schemmer P, Bradford BU, Rose ML, Bunzendahl H, Raleigh JA, Lemasters JJ, Thurman RG. Intravenous glycine improves survival in rat liver transplantation. Am J Physiol. 1999;276:G924-G932. [PubMed] [Cited in This Article: ] |
| 9. | Iimuro Y, Bradford BU, Forman DT, Thurman RG. Glycine prevents alcohol-induced liver injury by decreasing alcohol in the rat stomach. Gastroenterology. 1996;110:1536-1542. [PubMed] [Cited in This Article: ] |
| 10. | Yin M, Ikejima K, Arteel GE, Seabra V, Bradford BU, Kono H, Rusyn I, Thurman RG. Glycine accelerates recovery from alcohol-induced liver injury. J Pharmacol Exp Ther. 1998;286:1014-1019. [PubMed] [Cited in This Article: ] |
| 11. | Zhang W, Yun KM, Han DW. [Study on the protective mechanism of glycine on rat intestinal endotoxemia]. Shanxi Yike Daxue Xuebao. 2003;34:97-98. [Cited in This Article: ] |
| 12. | Zhang W, Yun KM, Han DW. [Study on protection of glycine from thioacetamide induced acute liver injury]. Shanxi Yike Daxue Xuebao. 2003;34:210-211. [Cited in This Article: ] |
| 13. | Alarcon-Aguilar FJ, Almanza-Perez J, Blancas G, Angeles S, Garcia-Macedo R, Roman R, Cruz M. Glycine regulates the production of pro-inflammatory cytokines in lean and monosodium glutamate-obese mice. Eur J Pharmacol. 2008;599:152-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Wang G, Wang Y, Guan FL, Ren GC, Wang YZ. [The effect of combination of glycine and methylprednisolone on Kupffer cells of liver after hemorrhagic shock in rats]. Zhonghua Waike Zazhi. 2006;44:349-352. [PubMed] [Cited in This Article: ] |
| 15. | Garcia-Macedo R, Sanchez-Muñoz F, Almanza-Perez JC, Duran-Reyes G, Alarcon-Aguilar F, Cruz M. Glycine increases mRNA adiponectin and diminishes pro-inflammatory adipokines expression in 3T3-L1 cells. Eur J Pharmacol. 2008;587:317-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Yuan KM, Han DW, Xu RL, Zhao YC. Effect of LPS on phagocytosis of rat Kupffer cells in vitro. Chin J Pathophysiol. 2003;19:795-798. [Cited in This Article: ] |
| 17. | Smedsrød B, Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol. 1985;38:213-230. [PubMed] [Cited in This Article: ] |
| 18. | Hirose M, Watanabe S, Ueno T, Kitami N, Sato N. Pertussis toxin-induced redistribution of cortical actomyosin and inhibition of phagocytosis in rat Kupffer cells. J Gastroenterol Hepatol. 1993;8:348-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Wulf E, Deboben A, Bautz FA, Faulstich H, Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci USA. 1979;76:4498-4502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 493] [Cited by in F6Publishing: 542] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335-1349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 289] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Solga SF, Diehl AM. Non-alcoholic fatty liver disease: lumen-liver interactions and possible role for probiotics. J Hepatol. 2003;38:681-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166-176. [PubMed] [Cited in This Article: ] |
| 23. | Tsujimoto T, Kawaratani H, Kitazawa T, Hirai T, Ohishi H, Kitade M, Yoshiji H, Uemura M, Fukui H. Decreased phagocytic activity of Kupffer cells in a rat nonalcoholic steatohepatitis model. World J Gastroenterol. 2008;14:6036-6043. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Bretscher A. Microfilament structure and function in the cortical cytoskeleton. Annu Rev Cell Biol. 1991;7:337-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 228] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Sun WB, Han BL, Peng ZM, Li K, Ji Q, Chen J, Wang HZ, Ma RL. Effect of aging on cytoskeleton system of Kupffer cell and its phagocytic capacity. World J Gastroenterol. 1998;4:77-79. [PubMed] [Cited in This Article: ] |
| 26. | Watanabe S, Hirose M, Ueno T, Kominami E, Namihisa T. Integrity of the cytoskeletal system is important for phagocytosis by Kupffer cells. Liver. 1990;10:249-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Dijkstra J, van Galen M, Scherphof G. Effects of (dihydro)cytochalasin B, colchicine, monensin and trifluoperazine on uptake and processing of liposomes by Kupffer cells in culture. Biochim Biophys Acta. 1985;845:34-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256-G265. [PubMed] [Cited in This Article: ] |
| 29. | Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448-2457. [PubMed] [Cited in This Article: ] |
| 30. | Lichtman SN, Wang J, Zhang C, Lemasters JJ. Endocytosis and Ca2+ are required for endotoxin-stimulated TNF-alpha release by rat Kupffer cells. Am J Physiol. 1996;271:G920-G928. [PubMed] [Cited in This Article: ] |
| 31. | Wang J, Kobayashi M, Han M, Choi S, Takano M, Hashino S, Tanaka J, Kondoh T, Kawamura K, Hosokawa M. MyD88 is involved in the signalling pathway for Taxol-induced apoptosis and TNF-alpha expression in human myelomonocytic cells. Br J Haematol. 2002;118:638-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Li Z, Davis GS, Mohr C, Nain M, Gemsa D. Suppression of LPS-induced tumor necrosis factor-alpha gene expression by microtubule disrupting agents. Immunobiology. 1996;195:640-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Li Z, Davis GS, Mohr C, Nain M, Gemsa D. Inhibition of LPS-induced tumor necrosis factor-alpha production by colchicine and other microtubule disrupting drugs. Immunobiology. 1996;195:624-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Allen JN, Herzyk DJ, Wewers MD. Colchicine has opposite effects on interleukin-1 beta and tumor necrosis factor-alpha production. Am J Physiol. 1991;261:L315-L321. [PubMed] [Cited in This Article: ] |