Published online Mar 21, 2012. doi: 10.3748/wjg.v18.i11.1273
Revised: September 20, 2011
Accepted: January 18, 2012
Published online: March 21, 2012
Non-hepatocellular carcinoma (non-HCC) with macroscopic bile duct tumor thrombus (BDTT) formation is rare, few radiological studies have been reported. In this case report, we retrospectively analyzed the imaging findings of three cases of non-HCC with macroscopic BDTT on dynamic enhanced multislice computed tomography (MSCT) scan. One case of primary hepatic carcinosarcoma was presented as a solitary, large well-defined tumor with significant necrotic changes. One case of liver metastasis from colon cancer was presented as a lobulated, large ill-defined tumor. One case of intraductal oncocytic papillary neoplasm involved the entire pancreas, presented as a cystic and solid mass with multilocular changes (the individual loculi were less than 5.0 mm in diameter). The bile duct was dilated due to expansible growth of the BDTT in all three patients. The BDTT was contiguous with hepatic or pancreatic tumor, and both of them showed the same enhancement patterns on dynamic contrast-enhanced computed tomography scan: early enhancement in the hepatic arterial phase and a quick wash-out of contrast agent in the portal and equilibrium phases. Macroscopic BDTT in non-HCC patient is rare, dynamic enhanced MSCT scan may be valuable in the diagnosis of non-HCC with BDTT.
- Citation: Liu QY, Lin XF, Li HG, Gao M, Zhang WD. Tumors with macroscopic bile duct thrombi in non-HCC patients: Dynamic multi-phase MSCT findings. World J Gastroenterol 2012; 18(11): 1273-1278
- URL: https://www.wjgnet.com/1007-9327/full/v18/i11/1273.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i11.1273
Hepatocellular carcinoma (HCC) associated with bile duct invasion and subsequent bile duct tumor thrombus (BDTT) formation occurs in only 0.79%-4% of patients with primary HCC[1,2]. Because HCC is one of the most prevalent malignant tumors, HCC with BDTT is not uncommonly encountered in clinical practice and its clinical characteristics have been well described[3]. Non-HCC liver tumor can also invade the biliary tree, such as combined hepatocellular and cholangiocellular carcinoma and liver metastasis[4-10]. However, only few cases of non-HCC with BDTT have been reported in the radiological literatures[5-7]. In this study, we report three cases of macroscopic BDTT: one case of primary hepatic carcinosarcoma, one case of liver metastasis from colon cancer, and one case of intraductal oncocytic papillary neoplasm (IOPN) of the pancreas. The cases of primary hepatic carcinosarcoma or IOPN of the pancreas associated with macroscopic BDTT have not been previously reported.
All three non-HCC patients with macroscopic BDTT were treated in our hospital. They had jaundice on admission. Liver function tests showed that aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase and alkaline phosphatase were elevated. Total serum bilirubin (TBil) was significantly increased mainly due to direct bilirubin (DBil). Serum markers of hepatitis B and hepatitis C and tumor markers alpha-fetoprotein (AFP), carcino-embryonic antigen (CEA) and CA125 were all normal. However, CA19-9 was elevated in a patient with primary hepatic carcinosarcoma and a patient with IOPN of the pancreas. All three cases of non-HCC with macroscopic BDTT were pathologically confirmed. We retrospectively analyzed the dynamic enhanced multislice computed tomography (MSCT) findings in these three cases.
The computed tomography (CT) examination was performed using a 64-slice spiral CT scanner (Sensation 64, Siemens Medical Solutions), the technical parameters were as follows: 120 kVp tube voltage, 200 effective mAs, 64 mm × 0.6 mm beam collimation, pitch of 0.9, rotation time of 0.5 s, 5 mm reconstruction slice thickness. After acquisition of unenhanced images, nonionic iodinated contrast agent (iodipamide, 370 mg I/mL, Bracco) was injected through a dual-head injector at a rate of 3.5 mL/s and followed by a 20-mL saline flush, with a dose of 2.0 mL/kg body weight. To determine the timing for the hepatic arterial phase (HAP) scanning, a bolus-tracking technique was used with a region of interest in the descending aorta. After achieving enhancement of the descending aorta up to 100 HU, the HAP images were acquired with the scanning delay of 5 s. Portal venous phase (PVP) and equilibrium phase (EP) images were obtained with a delayed time of 30 s and 120 s, respectively.
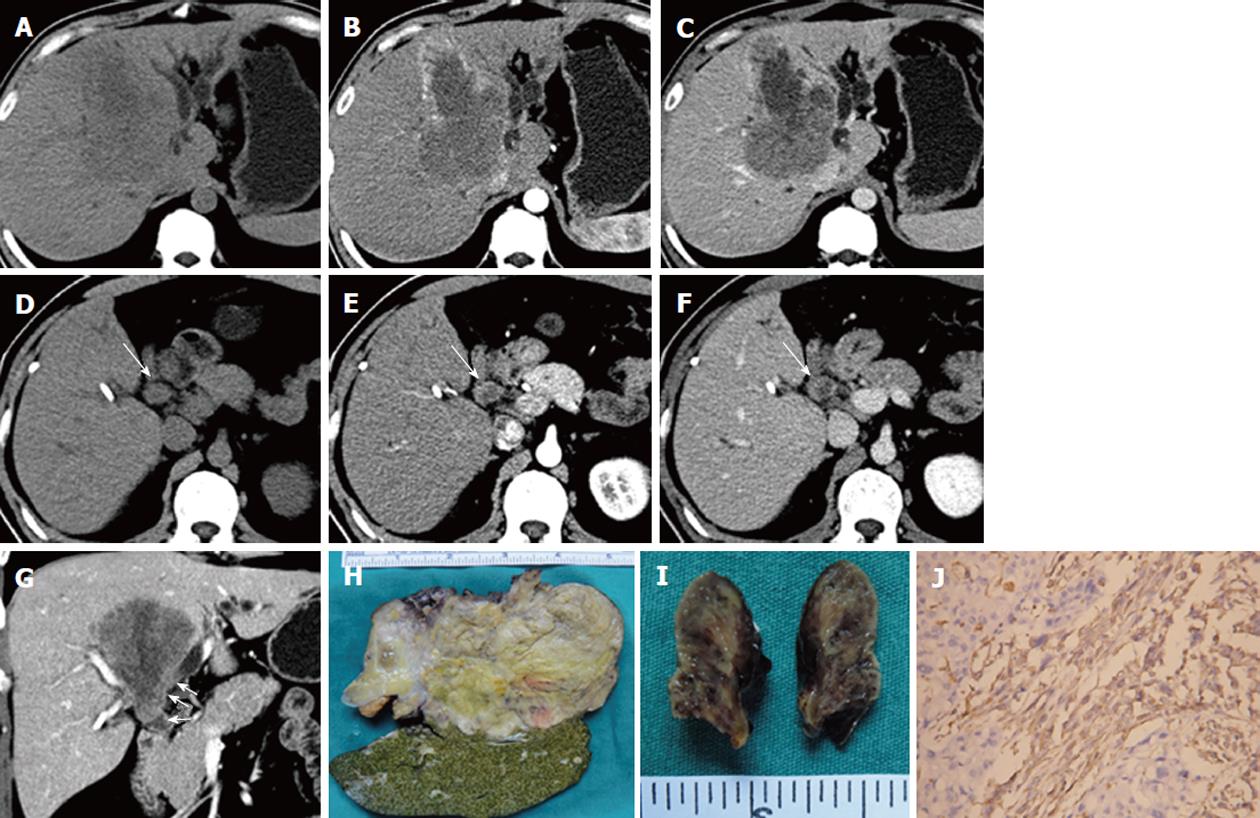
A 41-year-old man was admitted to our hospital on November 18, 2010 with a history of jaundice and right upper abdominal discomfort for one month. Laboratory examination showed that serum TBil was 282.1 μmol/L (normal range: 5-24 μmol/L), DBil was 221.4 μmol/L (normal range: ≤ 11 μmol/L), and tumor marker CA19-9 was 953.4 U/mL (normal range: ≤ 35 U/mL). A well-defined tumor with a size of 10.8 cm × 6.9 cm in the middle hepatic lobe (Segments IV, V and VIII) was revealed on CT scan. The tumor showed hypo-attenuation (relative to normal liver parenchyma) on pre-contrast CT scan, early rim enhancement in the HAP images and hypo-attenuation in the PVP and EP images. A large necrotic area was noted in the tumor, and showed no enhancement after contrast administration. A tumor thrombus was revealed in the dilated left and right hepatic duct and the common hepatic duct, which was contiguous with the hepatic tumor. No bile duct wall invasion was noted. The enhancement pattern of the intraductal mass was consistent with intrahepatic tumor on dynamic enhanced CT scan. Biliary tree dilation was seen distal to the intraductal mass. The patient underwent left trisectionectomy and right posterior hepatic bile duct-jejunum anastomosis, and intraductal mass was removed through resection of the extrahepatic bile duct. The intrahepatic tumor was not encapsulated. The intraductal mass was not adhered to the bile duct wall, and was easily removed. Primary hepatic carcinosarcoma with BDTT was pathologically confirmed. The carcinomatous and sarcomatous elements were HCC and fibrosarcoma, respectively. The immunohistochemical results revealed that the sarcomatous elements were Vimentin positive, and negative results for cytokeratin, epithelial membrane antigen, hepatocyte, CD34, AFP, AAT, S-100, actin and CD117 (Figure 1). The patient died two months after surgery due to hepatorenal syndrome.
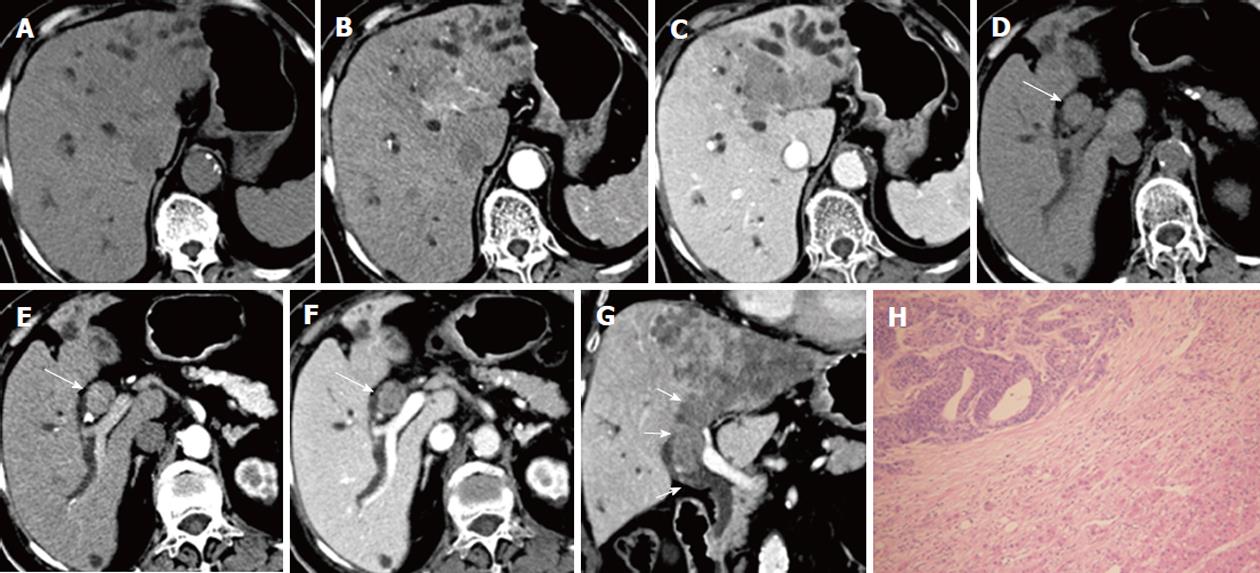
A 75-year-old woman was admitted on November 19, 2010 with epigastric pain and progressive jaundice for seven days. The patient underwent radical resection of colon cancer six years ago. Laboratory results showed significantly elevated serum TBil (196.5 μmol/L) and DBil (131.3 μmol/L). The tumor markers AFP, CEA, CA125 and CA19-9 were all in the normal range. A large lobulated tumor of 8.7 cm × 9.5 cm was displayed on CT scan in the left hepatic lobe with an ill-defined margin. The tumor demonstrated hypo-attenuation on precontrast CT scan, early mild enhancement in the HAP images and inhomogeneous hypo-attenuation in the PVP and EP images. An intraductal tumor thrombus was seen extending from the dilated left intrahepatic bile duct to the upper common bile duct, and was contiguous with intrahepatic tumor. The intraductal thrombus showed similar enhancement patterns with intrahepatic tumor on dynamic enhanced CT scans. Intrahepatic ductal dilation was found distal to the intraductal mass. Tumor thrombi were also seen in the inferior vena cava, the middle hepatic vein and the left hepatic vein. No enlarged lymph node was observed. The patient received percutanoeus transhepatic biliary drainage instead of surgery due to poor liver reserve. However, jaundice was not effectively relieved, thus palliative thrombectomy through choledochotomy was performed. The intraductal tumor thrombus was easily removed as it was not adhered to the bile duct wall. Resection specimen revealed to be metastatic adenocarcinoma pathologically, and was positive for CDX2 and CEA, and negative for hepatocyte, AFP, HBsAg, and CD34 on immunohistochemical staining (Figure 2). The patient was still alive 10 months after the operation.
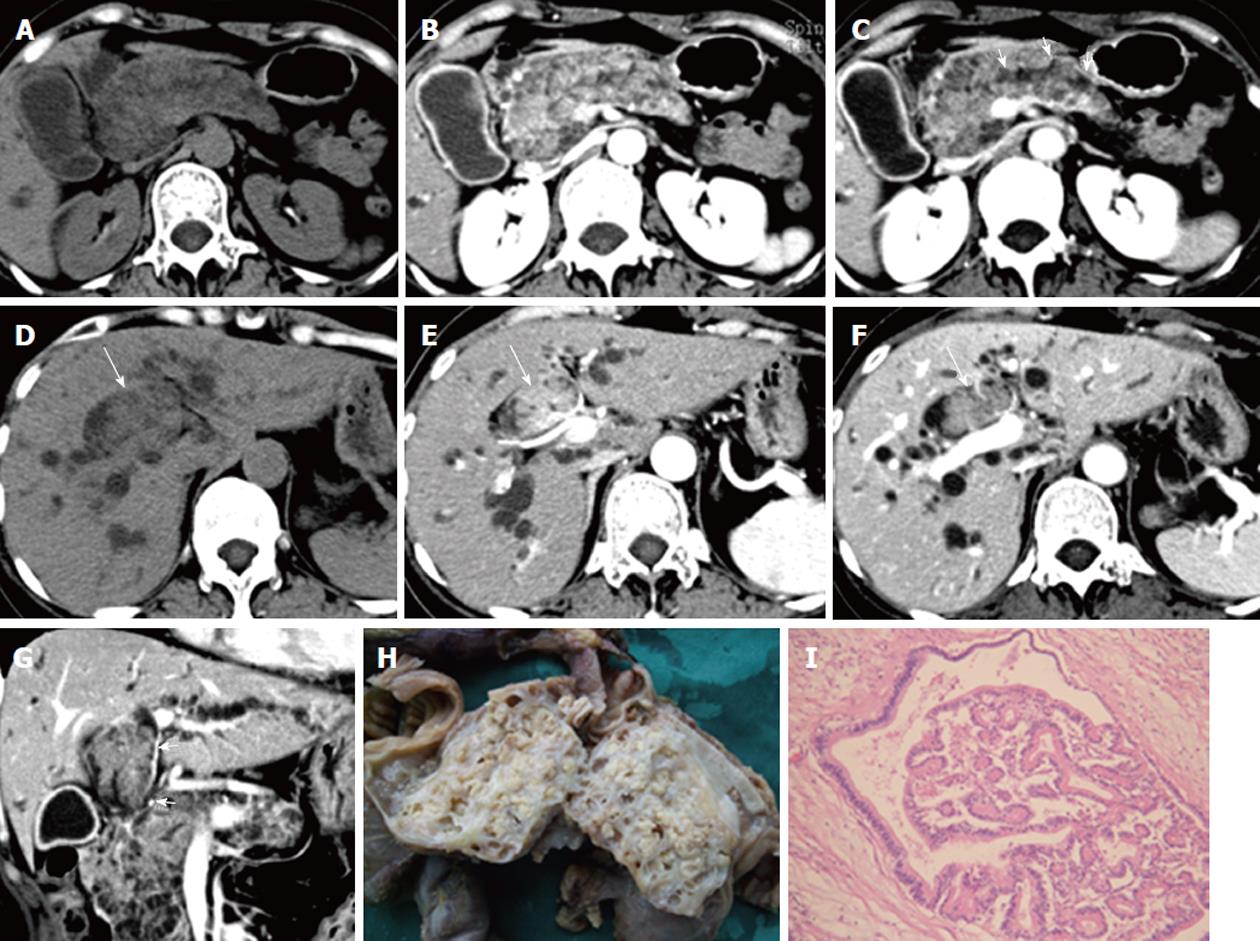
A 43-year-old woman was admitted on December 29, 2009 with a history of jaundice for 10 d. The laboratory results showed a high level of serum TBil (174.6 μmol/L) and DBil (141.3 μmol/L). The tumor marker CA19-9 was slightly elevated, with a level of 36.5 U/mL. CT scan showed enlargement of the pancreas which consisted of a mixed solid and cystic tumor. The solid components showed hypo-attenuation on precontrast CT scan, early enhancement on the HAP images and hypo-attenuation on the PVP and EP images. The cystic components were multilocular, with the loculi less than 5.0 mm in diameter, and showed no enhancement after contrast administration. The main pancreatic duct was mildly dilated and communicated with pancreatic tumor. An intraductal tumor thrombus appeared in the dilated right hepatic duct and the common bile duct on CT scan, and it was contiguous with the pancreatic tumor. The enhancement patterns of the intraductal thrombus were consistent with pancreatic tumor on dynamic enhanced CT scans. No enlarged lymph node was detected. Thrombectomy through choledochotomy and pancreatic-duodenal resection was performed in this patient. IOPN of the pancreas with BDTT formation was confirmed pathologically. Tumor invasion was only observed in the lower segment of the common bile duct wall (Figure 3). The patient was still alive 21 mo after the operation.
Macroscopic BDTT secondary to liver tumors is not commonly seen clinically and may occur in HCC, combined hepatocellular and cholangiocellular carcinoma and hepatic metastasis[4-10]. The mechanism of tumor thrombus formation in the bile duct is unclear. It is widely accepted that hepatic tumors (primary or secondary) can directly invade the bile duct, even extrabiliary malignant tumors can directly metastasize to the bile duct[10]. Consequently, tumors can develop along the bile duct and form intraductal thrombus. However, Peng et al[11] indicated that the tumor cells in HCC with BDTT might be originally derived from the canals of Hering and hepatic stem cells or primitive progenitor cells. We retrospectively analyzed three cases of BDTT secondary to primary hepatic carcinosarcoma, hepatic metastasis from colon cancer and IOPN of the pancreas and reviewed their clinical and imaging findings.
World Health Organization defined primary hepatic carcinosarcomas as tumors containing both carcinomatous (either hepatocellular or cholangiocellular) and sarcomatous components and without obvious transition zone between them[12]. Primary hepatic carcinosarcoma is extremely rare and aggressive. To our knowledge, only about 20 cases have been reported in the English literature, and the pathogenesis is still controversial[12,13]. This tumor occurs between 34 and 84 years of age and presented no specific clinical manifestations[12,13]. More than half of the patients had a background of hepatic cirrhosis or fibrosis[12-14]. Elevated serum AFP and CA19-9 could be observed in some cases[14]. Patients usually have poor prognosis due to the highly invasive and metastatic features of the tumors[14].
The CT characteristics of primary hepatic carcinosarcomas reported in the literature include a solitary mass with central necrosis and myxoid changes, round, oval or lobulated in shape, and without capsule formation. They show iso-attenuation or hypo-attenuation on precontrast CT scan. Dynamic enhanced CT scan revealed early rim or peripheral ring enhancement in the HAP images, hypo-attenuation in the PVP images and iso-attenuation in the EP images[12,13,15]. If the sarcomatous components were osteosarcoma or chondrosarcoma, dense rocky calcifications or bone formations may be presented within the mass radiologically[15]. The case of primary hepatic carcinosarcoma described here has similar CT findings as reported in the literature. Interestingly, this case was also associated with BDTT, which has not been previously reported. The BDTT grew expansively without invasion of the bile duct wall, and was contiguous with the hepatic carcinosarcoma. Both of them showed the same enhancement pattern on dynamic enhanced CT scan as they shared the same blood supply. We found similar phenomena in HCC with BDTT, i.e., the enhancement pattern of BDTT was the same as HCC on dynamic enhanced CT scan, intraductal tumor thrombus was continuous with the main intrahepatic HCC mass, no thickened bile duct wall adjacent to the thrombus was observed[16]. The differential diagnosis of primary hepatic carcinosarcoma with BDTT from HCC with BDTT is difficult. In the presence of large amounts of tumor necrosis, tumor calcification or ossification and negative serum AFP, hepatic carcinosarcoma should be considered in the differential diagnosis.
Macroscopic intrabiliary tumor growth is a peculiar mode of intrahepatic spread in patients with colorectal liver metastasis. Metastatic hepatic tumors from colorectal cancer demonstrated macroscopic BDTT in approximately 5.8%-12.1% of resected tumors[6,8,9]. The growth of BDTT included two components: intraluminal and intraepithelial extension[8]. The diagnosis of metastatic hepatic tumors from colorectal cancer associated with macroscopic BDTT is important because macroscopic BDTT is an independent indicator of favorable prognosis in such patients. Aggressive surgical treatment can improve the chances of long-term survival[8,9]. Currently, there are few radiological studies about hepatic metastasis from colorectal cancer with bile duct invasion[5-7]. Okano et al[6] reported the following radiological findings on CT scan in detecting the presence of intrabiliary tumor growth in patients with liver metastases from colorectal cancer: intrahepatic bile duct dilatation, thickened portal tract and wedge-shaped area with enhancement. A thickened portal tract around the tumor corresponded to intrabiliary thrombus itself, and the presence of this sign depended on the length of the intraductal thrombus. If the length of the thrombus is larger than 30 mm, thickened portal tract will likely be observed[6]. However, in our cases and in 8 cases of hepatic metastasis with BDTT from colon cancer reported by Lee et al[5], no such sign was observed. This discrepancy was likely due to the use of an obsolete CT scanner as reported by Okano et al[6], which was limited by low spatial resolution and thicker slice scanning (7-10 mm), thus the intraductal thrombus itself could not be clearly detected. The wedge-shaped area with enhancement is assumed to be caused by reduced portal flow and a compensatory increase in arterial blood flow caused by tumor compression or arterioportal shunt in the involved portal venous branch[6]. Jinzaki et al[7] reported four cases of liver metastasis from colorectal cancer with intraductal invasion. Two of the cases revealed hypo-dense hepatic tumors with bile duct dilation on an enhanced CT scan without presence of intraductal thrombus, while in the other two cases, intraductal thrombus itself appeared on CT scans, which were contiguous with the main intrahepatic tumors. The intraductal thrombus was isodense on unenhanced and enhanced CT compared with the main intrahepatic tumor. In our case of liver metastasis from colon cancer with macroscopic BDTT, the BDTT showed similar expansible growth pattern, and was contiguous with the hepatic metastatic tumor. Both of them showed the same enhancement pattern on CT scan as they shared a common blood supply.
In clinical practice, it is important to differentiate metastatic tumor from primary intraductal cholangiocarcinoma in patients with a history of colorectal cancer when an intraductal mass is presented in the bile duct. The expansible growth of intraductal mass and its direct connection with hepatic tumors are very valuable features in the differential diagnosis[5]. The diagnosis of liver metastasis with BDTT should also be differentiated from HCC with BDTT. Hepatic cirrhosis background and a rise in AFP level may aid in the diagnosis of HCC[16].
The term “intraductal oncocytic papillary neoplasm”was proposed by Adsay et al[17] in 1996 to describe a rare type of pancreatic tumor. Currently, only about 20 cases of IOPN of the pancreas have been reported, but the accurate incidence and etiology of these cases are still unknown[18,19]. One of the distinguishing features of IOPN of the pancreas is the abundant, finely granular, eosinophilic cytoplasm of the cells. The tumors usually form complex, branching, and arborizing papillaries[17,18]. IOPN of the pancreas is often found in the elderly (mean age, 63.9 years), with no gender predilection and specific clinical features[18]. Tumors mainly involve the main pancreatic duct. They are more commonly observed in the head of the pancreas although they can occur in any part of the pancreas. Multiple sites involved have also been reported in the literature[17-20]. In general, IOPN of the pancreas usually presents as either unilocular or multilocular cystic tumor with individual loculi ranging from 0.2 to 4.0 cm. The cystic loculi are filled with mucin and have prominent papillary growth. Cystic tumors usually communicate with the pancreatic duct system[17-20]. Most IOPNs of the pancreas exhibit severe dysplasia histopathologically to warrant the term intraductal oncocytic papillary carcinoma, and therefore they should be surgically resected completely[17].
Radiologically IOPN of the pancreas can be presented as a well-defined mass with unilocular or multilocular cystic changes and with mural or solid nodules. The tumors always communicated with the dilated pancreatic duct[18-20]. Mural nodules or solid tumor components may show enhancement on CT or magnetic resonance imaging imaging[19,20]. Adsay et al[17] reported one case of IOPN of the pancreas with common bile duct involvement, and Fischer et al[19] reported one case of IOPN of the pancreas with dilation of the common bile duct and intrahepatic ducts. However, no further description has been mentioned in both cases. Currently, no case of IOPN of the pancreas with macroscopic BDTT has been reported. The case we reported here has the following features: tumor involved the entire pancreas with multilocular, mixed cystic and solid changes, and the individual loculi were less than 5.0 mm in diameter. The pancreatic tumor invaded the lower segment of the common bile duct, and hence intraductal thrombus was formed, extending upward along the bile duct with expansible growth pattern. On dynamic enhanced CT scans, pancreatic tumor and BDTT have the same enhancement patterns, which might be due to direct connection of BDTT with main pancreatic tumor, and both sharing the same blood supply. Intraductal papillary mucinous neoplasm (IPMN) of pancreas has similar radiological features as IOPN. A fluorodeoxyglucose (FDG) positron emission tomography scan can help differentiate between IOPN and IPMN of the pancreas. A strong uptake of FDG was reported in IOPN of the pancreas, which suggested high glucose metabolic activity of tumor cells[19,20].
In conclusion, non-HCC with macroscopic BDTT is a rare clinical entity. All three cases in this study shared the following imaging features: expansible growth of the BDTT; the intrahepatic tumor or pancreatic tumor contiguous with the BDTT, and both showing the same enhancement patterns on CT scans; and intrahepatic bile duct dilation distal to the BDTT. MSCT may be a valuable modality in the diagnosis of non-HCC with BDTT.
Peer reviewers: Sundeep Singh Saluja, MS, MCh, Assistant Professor, Department of Gastrointestinal Surgery, GB Pant Hospital and Maulana azad Medical College, Bahadur Shah Zafar Marg, New Delhi 110002, India; Sophoclis Alexopoulos, MD, Assistant Professor of Surgery, Department of Surgery, Division of Hepatobiliary/Pancreatic and Abdominal Organ Transplant Surgery, University of Southern California, 1510 San Pablo Street, Suite 200, Los Angeles, CA 90033-4612, United States
S- Editor Shi ZF L- Editor Ma JY E- Editor Li JY
| 1. | Qin LX, Ma ZC, Wu ZQ, Fan J, Zhou XD, Sun HC, Ye QH, Wang L, Tang ZY. Diagnosis and surgical treatments of hepatocellular carcinoma with tumor thrombosis in bile duct: experience of 34 patients. World J Gastroenterol. 2004;10:1397-1401. [PubMed] [Cited in This Article: ] |
| 2. | Noda T, Nagano H, Tomimaru Y, Murakami M, Wada H, Kobayashi S, Marubashi S, Eguchi H, Takeda Y, Tanemura M. Prognosis of hepatocellular carcinoma with biliary tumor thrombi after liver surgery. Surgery. 2011;149:371-377. [PubMed] [Cited in This Article: ] |
| 3. | Ikenaga N, Chijiiwa K, Otani K, Ohuchida J, Uchiyama S, Kondo K. Clinicopathologic characteristics of hepatocellular carcinoma with bile duct invasion. J Gastrointest Surg. 2009;13:492-497. [PubMed] [Cited in This Article: ] |
| 4. | Saito M, Hige S, Takeda H, Tomaru U, Shibata M, Asaka M. Combined hepatocellular carcinoma and cholangiocarcinoma growing into the common bile duct. J Gastroenterol. 2001;36:842-847. [PubMed] [Cited in This Article: ] |
| 5. | Lee YJ, Kim SH, Lee JY, Kim MA, Lee JM, Han JK, Choi BI. Differential CT features of intraductal biliary metastasis and double primary intraductal polypoid cholangiocarcinoma in patients with a history of extrabiliary malignancy. AJR Am J Roentgenol. 2009;193:1061-1069. [PubMed] [Cited in This Article: ] |
| 6. | Okano K, Yamamoto J, Okabayashi T, Sugawara Y, Shimada K, Kosuge T, Yamasaki S, Furukawa H, Muramatsu Y. CT imaging of intrabiliary growth of colorectal liver metastases: a comparison of pathological findings of resected specimens. Br J Radiol. 2002;75:497-501. [PubMed] [Cited in This Article: ] |
| 7. | Jinzaki M, Tanimoto A, Suzuki K, Seki T, Satoh Y, Hiramatsu K, Mukai M, Nakanishi I. Liver metastases from colon cancer with intra-bile duct tumor growth: radiologic features. J Comput Assist Tomogr. 1997;21:656-660. [PubMed] [Cited in This Article: ] |
| 8. | Sugiura T, Nagino M, Oda K, Ebata T, Nishio H, Arai T, Nimura Y. Hepatectomy for colorectal liver metastases with macroscopic intrabiliary tumor growth. World J Surg. 2006;30:1902-1908. [PubMed] [Cited in This Article: ] |
| 9. | Kubo M, Sakamoto M, Fukushima N, Yachida S, Nakanishi Y, Shimoda T, Yamamoto J, Moriya Y, Hirohashi S. Less aggressive features of colorectal cancer with liver metastases showing macroscopic intrabiliary extension. Pathol Int. 2002;52:514-518. [PubMed] [Cited in This Article: ] |
| 10. | Colovic RB, Grubor NM, Jovanovic MD, Micev MT, Colovic NR. Metastatic melanoma to the common bile duct causing obstructive jaundice: a case report. World J Gastroenterol. 2007;13:813-815. [PubMed] [Cited in This Article: ] |
| 11. | Peng N, Li L, Cai X, Tan S, Wu T. Liver stem/progenitor cells in the canals of Hering: cellular origin of hepatocellular carcinoma with bile duct tumor thrombi? Stem Cell Rev. 2010;6:579-584. [PubMed] [Cited in This Article: ] |
| 12. | Shu RY, Ye M, Yu WY. A case of primary liver carcinosarcoma: CT findings. Chin J Cancer. 2010;29:346-348. [PubMed] [Cited in This Article: ] |
| 13. | Goto H, Tanaka A, Kondo F, Takeshita K, Nagashima I, Hanawa N, Aiso M, Takamori Y, Kato K, Takahashi Y. Carcinosarcoma of the liver. Intern Med. 2010;49:2577-2582. [PubMed] [Cited in This Article: ] |
| 14. | Lao XM, Chen DY, Zhang YQ, Xiang J, Guo RP, Lin XJ, Li JQ. Primary carcinosarcoma of the liver: clinicopathologic features of 5 cases and a review of the literature. Am J Surg Pathol. 2007;31:817-826. [PubMed] [Cited in This Article: ] |
| 15. | Kwon JH, Kang YN, Kang KJ. Carcinosarcoma of the liver: a case report. Korean J Radiol. 2007;8:343-347. [PubMed] [Cited in This Article: ] |
| 16. | Liu QY, Zhang WD, Chen JY, Li HG, Liu C, Liang BL. Hepatocellular carcinoma with bile duct tumor thrombus: dynamic computed tomography findings and histopathologic correlation. J Comput Assist Tomogr. 2010;35:187-194. [PubMed] [Cited in This Article: ] |
| 17. | Adsay NV, Adair CF, Heffess CS, Klimstra DS. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980-994. [PubMed] [Cited in This Article: ] |
| 18. | Liszka L, Pajak J, Zielińska-Pajak E, Krzych L, Gołka D, Mrowiec S, Lampe P. Intraductal oncocytic papillary neoplasms of the pancreas and bile ducts: a description of five new cases and review based on a systematic survey of the literature. J Hepatobiliary Pancreat Sci. 2010;17:246-261. [PubMed] [Cited in This Article: ] |
| 19. | Fischer MA, Donati O, Heinrich S, Weber A, Hany TF, Soldini D, Alkadhi H, Marincek B, Scheffel H. Intraductal oncocytic papillary neoplasm of the pancreas: a radio-pathological case study. JOP. 2010;11:49-54. [PubMed] [Cited in This Article: ] |
| 20. | Kato Y, Nakagouri T, Konishi M, Takahashi S, Gotoda N, Hasebe T, Kinosita T. Intraductal oncocytic papillary neoplasm of the pancreas with strong accumulation on FDG-PET. Hepatogastroenterology. 2008;55:900-902. [PubMed] [Cited in This Article: ] |