Published online Mar 14, 2012. doi: 10.3748/wjg.v18.i10.1130
Revised: July 27, 2011
Accepted: October 14, 2011
Published online: March 14, 2012
AIM: To evaluate the feasibility of quantifying liver choline concentrations in both normal and apoptotic rabbit livers in vivo, using 1H magnetic resonance spectroscopy (1H-MRS).
METHODS: 1H-MRS was performed in 18 rabbits using a 1.5T GE MR system with an eight-channel head/neck receiving coil. Fifteen rabbits were injected with sodium selenite at a dose of 10 μmol/kg to induce the liver cell apoptosis. Point-resolved spectroscopy sequence-localized spectra were obtained from 10 livers once before and once 24 h after sodium selenite injection in vivo. T1 and T2 relaxation time of water and choline was measured separately in the livers of three healthy rabbits and three selenite-treated rabbits. Hematoxylin and eosin and dUTP-biotin nick end labeling (TUNEL) staining was used to detect and confirm apoptosis. Choline peak areas were measured relative to unsuppressed water using LCModel. Relaxation attenuation was corrected using the average of T1 and T2 relaxation time. The choline concentration was quantified using a formula, which was tested by a phantom with a known concentration.
RESULTS: Apoptosis of hepatic cells was confirmed by TUNEL assay. In phantom experiment, the choline concentration (3.01 mmol/L), measured by 1H-MRS, was in good agreement with the actual concentration (3 mmol/L). The average T1 and T2 relaxation time of choline was 612 ± 15 ms and 74 ± 4 ms in the control group and 670 ± 27 ms and 78 ± 5 ms in apoptotic livers in vivo, respectively. Choline was quantified in 10 rabbits, once before and once after the injection with sodium selenite. The choline concentration decreased from 14.5 ± 7.57 mmol/L before sodium selenite injection to 10.8 ± 6.58 mmol/L (mean ± SD, n = 10) after treatment (Z = -2.395, P < 0.05, two-sample paired Wilcoxon test).
CONCLUSION: 1H-MRS can be used to quantify liver choline in vivo using unsuppressed water as an internal reference. Decreased liver choline concentrations are found in sodium selenite-treated rabbits undergoing liver cell apoptosis.
- Citation: Shen ZW, Cao Z, You KZ, Yang ZX, Xiao YY, Cheng XF, Chen YW, Wu RH. Quantification of choline concentration following liver cell apoptosis using 1H magnetic resonance spectroscopy. World J Gastroenterol 2012; 18(10): 1130-1136
- URL: https://www.wjgnet.com/1007-9327/full/v18/i10/1130.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i10.1130
Cell apoptosis is a form of programmed cell death. Compared with necrosis, cell apoptosis does not damage the neighboring cells. Primary tumors are thought to be a result of inadequate apoptosis[1]. Through detecting apoptosis, the progression of a tumor can be evaluated and drug effects can be determined so as to adopt appropriate therapies[2]. It is, therefore, essential to develop a non-invasive method that can be used to assess apoptosis of tissues in vivo.
Proton magnetic resonance spectroscopy (1H-MRS) is a powerful tool which can be used for non-invasive measurement of compounds in vivo. Several previous studies[3-6], evaluated the role of 1H-MRS in characterization of apoptosis with lipid signals. The most important non-lipid signals are from the choline-containing compounds at 3.2 ppm. However, changes in these signals were not universal, at least in the early stages of apoptosis[7]. One of our previous studies, using in vitro 9.4T high resolution magnetic resonance spectroscopy, has shown decreased total choline compounds and free choline in the rat apoptotic liver tissues as compared with the healthy rats[8].
The liver is a particularly suitable and interesting organ for metabolic studies using in vivo magnetic resonance spectroscopy due to its rich metabolic activities and location at the body surface[9]. However, difficulties in measurement may mostly come from respiratory movement, which leads to limited spectral resolution, especially in the clinical environment. Several previous in vivo studies[10,11] focused on choline-to-lipid ratios in liver tumors. These studies have shown increased choline-to-lipid ratios with the progression of hepatocarcinogenesis[10], and decreased choline-to-lipid ratios after treatment[11]. However, quantitative results are more suitable for horizontal and vertical comparison. Two preliminary reports[12,13] have quantified the choline concentrations in human hepatic tumors using separate reference standards of an external phantom and internal water signal. Tissue water reference method has been used for quantification of the choline level in 1H-MR spectra of brain[14], breast, muscle[15], and ovary[16], and is considered as a simple and practical approach in the clinical environment. Fischbach et al[17] applied LCModel to quantify choline relative to water, although the relaxation attenuation was not corrected. Accurate quantification requires correction for the relaxation attenuation, especially for point-resolved spectroscopy sequence (PRESS) sequence[18,19].
The aim of this study was to use an in vivo rabbit model (1) to evaluate the feasibility of quantifying choline concentrations of healthy and apoptotic livers with in vivo1H-MRS using the unsuppressed water signal as the internal reference, and to correct the relaxation attenuation, and (2) to compare the observed changes of choline with our previous in vitro results.
This study was approved ethically by the Animal Ethics Committee of Shantou University Medical College.
A standard spectroscopy phantom provided by GE Medical Systems (25-cm-diameter MRS HD sphere; General Electric Company) was used. The metabolites in the phantom are 3.0 mmol/L choline chloride, 10.0 mmol/L creatine hydrate, 12.5 mmol/L N-acetylaspartic acid, 7.5 mmol/L myo-inositol, 12.5 mmol/L L-Glutamic acid and 5 mmol/L lactate, containing 0.1% sodium azide, 0.1% Magnavis, 50 mmol/L potassium dihydrogen phosphate and 56 mmol/L sodium hydroxide. This phantom was used in previous studies in the brain[20] and liver[13] as the quantification standard.
New Zealand white rabbits (n = 18) weighing 1.9 ± 0.3 kg were used in this study. In the control group, rabbits (n = 3) were injected with saline and were sacrificed for the liver histological analysis after the 1H-MRS measurement. In the experimental group, the rabbits (n = 15) were injected with sodium selenite at a dose of 10 μmol/kg. Spectra were obtained once before and once 24 h after sodium selenite injection, and the rabbits were sacrificed after MRS. Prior to the MRS, rabbits were fasted for six hours and anesthetized with 1 mL/kg sodium pentobarbital through ear vein injection. Shen et al[21] observed that Se was able to induce apoptosis in in vitro HepG2 cells and found that selenite-induced apoptosis was both time- and dose-dependent[22]. The duration and dose of sodium selenite were determined according to their studies. All the rabbits were supplied from the Laboratory Animal Center of Shantou University Medical College.
All studies were performed on a 1.5-T HDxt MR scanner (Signa Systems, GE Healthcare) using an eight-channel head/neck receiving coil. At first, the phantom was used for the regular system stability check and served as a test to confirm the quantification strategy in vivo. For the phantom measurement, magnetic resonance imaging (MRI) consisted of axial and coronal localizer sequences for positioning the single voxel (20 mm × 20 mm × 20 mm) within the phantom solution. For in vivo measurement, rabbits were placed in the prone position and the liver region was situated in the center of the coil. The abdomen was immobilized with cushions to reduce respiratory movement. MRI consisted of images followed by the Propeller fast spin echo T2-weight sequence (TE/TR: 110 ms/6000 ms; field of view: 20 cm × 20 cm; slice thickness: 4 mm; slice space: 0.5 mm) to define the position of volume of interest (VOI).
Proton MRS was performed using a PRESS (TE/TR: 35 ms/1500 ms; total scan number: 128; VOI: 15 mm × 15 mm × 15 mm). Water suppression was obtained with chemical shift selective saturation. The volume saturation suppression (VSS) pulse was oblique and placed on the edge of the voxel for shimming and reducing motion artifact. T1 and T2 relaxation time of choline and water was separately measured in the phantom, three rabbits in the control group, and three selenite-injected rabbits. For T1 measurement, the TE was kept constant at 35 ms and the TR varied from 1130 ms to 3000 ms for five measurements. For T2 measurement, the TR was kept constant at 1500 ms and the TE varied from 30 ms to 135 ms for five measurements.
The raw spectral data were input into a SAGE software package (GE Healthcare). The peak amplitudes of the unsuppressed water signal at 4.7 ppm and choline compound signals at 3.2 ppm were measured with SAGE. The T2 relaxation time of MR-visible water and choline was measured in SAGE using the macro T2 fit. T1 time was obtained by fitting the data to a mono-exponential model as a function of TR, and TE to a mono-exponential model with an in-house program. The average T1 and T2 values for Cho and water were used for correcting the relaxation attenuation with the following equation.
Choline was quantified using commercially available LCModel (version 6.2-2B) software, according to Dr. Provencher[17], which is suitable for fitting the spectra collected by PRESS sequence (TE = 35 ms) in a GE 1.5T scanner. The mode of only-choline-2 was selected for phantom analysis. Using the prior knowledge incorporated into the spectral fitting, phase and baseline correction were automatically processed with LCModel. The control parameters were adjusted according to the LCModel manual.
Spectra were discarded if the reported signal-to-noise ratio (SNR) from LCModel was less than 15, or if the SD of choline was more than 20%. The ratio of the choline resonance area to the unsuppressed water resonance area was acquired. These ratios were converted to approximate mmol/L units by setting the concentration of water in the phantom as 55 556 mmol/L according to the manual. This ratio was measured in vivo using the analysis mode of liver-6. The water concentration was assumed to be 47 778 mmol/L for an 86% water content of the liver. The absolute concentration of choline was measured by the following formula modified from the reference[15].
Where ACho/H2O = the area ratio of choline to unsuppressed water, nH2O = 2, nCho = 9 (from three CH3 groups), fT1 = 1 - exp(-TR/T1), and fT2 = exp(-TE/T2). CFLipid represents a correction factor that is equivalent to AH2O/(AH2O + ALipid) measured from the non-suppressed water.
All animals were sacrificed under anesthesia after the MRS. Livers were removed and washed with physiological saline. Macroscopic examination followed by visual inspection was performed, and representative sections were taken. Formalin fixed samples were embedded in paraffin and 2-mm thin sections were cut. Samples were stained with hematoxylin and eosin and examined under light microscopy to identify apoptotic characteristics of vacuolated hepatocytes and the degree of lipid deposition in the parenchyma.
Shen et al[21] found that selenite-induced apoptosis could be evaluated by dUTP nick end labeling (TUNEL) assay and in vitro HepG2 cell morphological changes. The apoptotic cells were observed and analyzed in this study according to the reported methods. After the homogenized liver tissue was filtered through a 40 μm cell strainer, the cells were washed with PBS and then were centrifuged at 1500 r/min for 5 min to remove cell debris. After this procedure was repeated three times, the cells were fixed with 4% paraformaldehyde for 30 min. Apoptotic cells were stained with transferase-mediated TUNEL using a One Step TUNEL Apoptosis Assay Kit (Beyotime, China). The TUNEL reaction took place with the addition of reaction mixture (containing nucleotides and TdT enzyme), which was incubated for 60 min at 37 °C in the dark. After wash with phosphate buffer solution (PBS), cells were finally resuspended in PBS for flow cytometry analysis (XL MCL, Beckman Coulter, United States). The data obtained from flow cytometry were analyzed using Expo32 ADC Analysis software for calculating the percentage of apoptotic cells in each group.
All data were presented as a mean ± SD. Statistical significance was calculated using two-sample relative Wilcoxon test and was accepted at P≤ 0.05. The relationship between the percentage of apoptotic cells and choline concentration was investigated using the linear regression analysis. All analyses were performed using SPSS version 13.0 (SPSS Inc, United States).
T1 and T2 relaxation time of choline was 1129 ms and 236 ms, respectively, while T1 and T2 relaxation time of water was 3172 ms and 206 ms. The fitted data was acceptable for the low SD of choline (9%). The calculated choline concentration was 3.01 mmol/L, which was in agreement with the known choline concentration in the phantom (3 mmol/L).
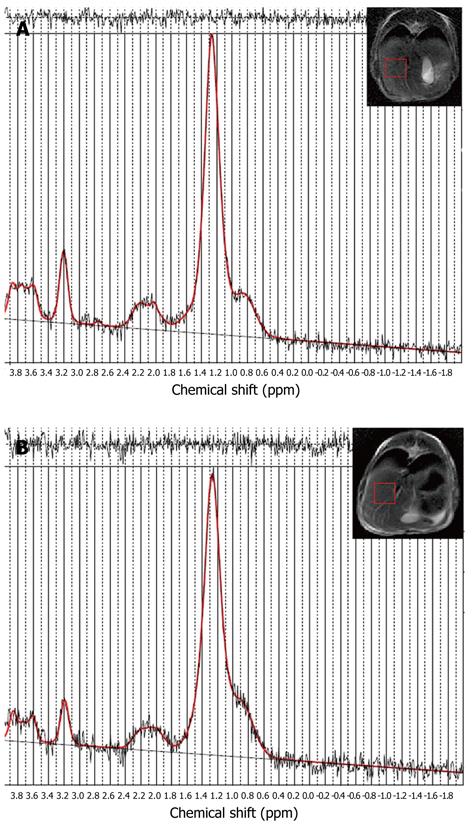
Choline spectra were obtained from all rabbits in the control group and 10/14 selenite-treated rabbits. Among the four failures in experimental group, one rabbit died during the model-making process, two failed in MRS after injection of sodium selenite due to prescan failure, and one spectrum was rejected after the injection of sodium selenite with a full-width at half-maximum (FWHM) > 20 and there was no metabolite resonance peak within a range from 0 ppm to 4.0 ppm. One spectrum of liver cell apoptosis induced by sodium selenite was excluded due to a higher choline SD (> 20%). The spectra from ten rabbits before and after the injection with sodium selenite were finally adopted (Table 1).
| SD of choline (%) | SNR of spectra | FWHM (Hz) | WS (%) | |
| Before injection | 8.5 ± 4.1 | 46.4 ± 19.4 | 12.6 ± 2.0 | 94.3 ± 2.8 |
| After injection | 10.5 ± 4.6 | 26.9 ± 14.3 | 11.8 ± 7.0 | 95.4 ± 2.5 |
The average T1 and T2 relaxation time of choline was 612 ± 15 ms and 74 ± 4 ms in the control group (n = 3) and 670 ± 27 ms and 78 ± 5 ms in apoptotic livers (n = 3). The average T1 and T2 relaxation time of water was 653 ± 23 ms and 48 ± 3 ms in the control group (n = 3), and 719 ± 37 and 50 ± 5 ms in apoptotic livers (n = 3).
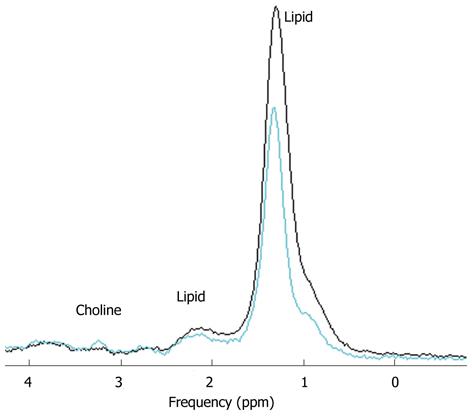
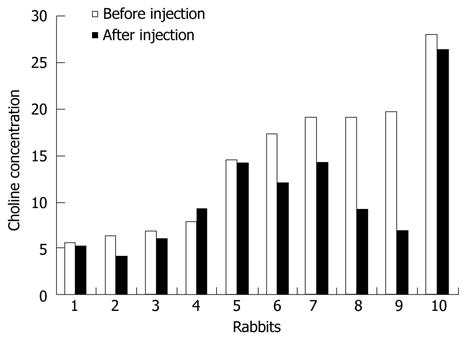
PRESS-localized single-voxel 1H-MR spectra from the liver of one rabbit before and after injection of sodium selenite can be well fitted using LCModel (Figure 1). A decreased choline peak was observed at 3.2 ppm (Figure 2). The calculated average T1 and T2 relaxation time of choline and water in both normal and apoptotic livers were used to calculate the choline concentrations. Decreased choline concentrations ranged from 14.5 ± 7.6 mmol/L (n = 10) before injection of sodium selenite to 10.8 ± 6.6 mmol/L (n = 10) 24 h after that treatment (Z = -2.395, P < 0.05, two-sample relative Wilcoxon test), although increased choline was also found in one rabbit (Figure 3). The calculated concentrations of choline varied from 5.5 mmol/L to 28.1 mmol/L in normal liver and 4.1 mmol/L to 26.4 mmol/L in apoptotic liver after injection of sodium selenite.
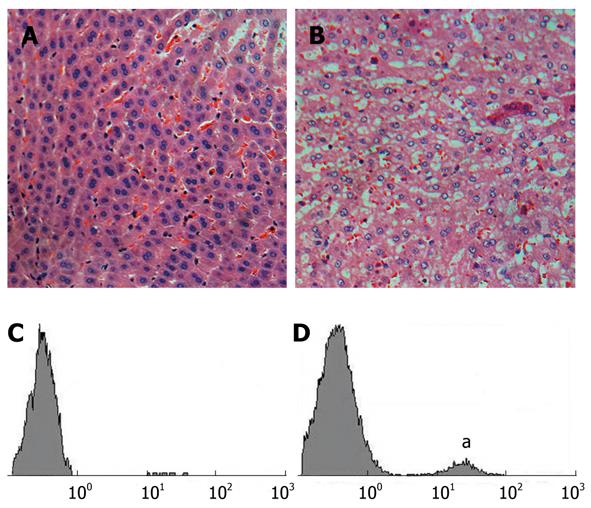
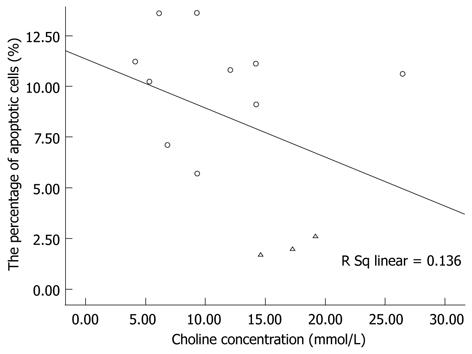
Histological differences between two groups were observed by hematoxylin and eosin staining. Normal liver had no observable effects on the microscopic distribution of vacuolated hepatocytes in liver tissues (Figure 4A and B). Apoptosis of hepatic cells in both groups was confirmed by TUNEL assay and the increased ratio of apoptotic to normal cells was detected by flow cytometry (Figure 4C and D). The average ratio was 2.1% ± 0.5% (n = 3) vs 10.3% ± 7.6% (n = 10), respectively. However, the linear regression analysis revealed no significant linear relationship between choline concentration and the percentage of apoptotic cells (R = 0.369, F = 1.734, P > 0.05) (Figure 5).
Noninvasive quantitative measurement of choline by proton MRS is important for the assessment of tumor characterization, grading, and post-treatment evaluation. The data in this study showed the feasibility of measuring choline concentration in rabbit liver in vivo using 1.5T MR by appropriate acquisition and quantification methods.
Choline spectra were obtained from three healthy rabbits and 10/14 selenite-treated rabbits. Phase and frequency shifts were found in some liver spectral frames. We deduced that this may be due to respiratory motion, which could also lead to inhomogeneous B0 and B1 fields and result in broadening of the spectral resonances. According to the data quality, the FWHM and water suppression of spectra in selenite-treated rabbits were better than those in normal rabbits due to decreased SNR and SD of choline. The possible reason is that rabbits injected with sodium selenite were quieter and therefore had reduced movement of the tissue. However, liver tissue necrosis may cause signal non-uniformity and worsen the effect of shimming, even leading to pleural effusion after injection of sodium selenite.
It is very important to make optimal choices about the spectroscopic sequence and parameters. The PRESS technique was used for its higher SNR and non-sensitivity to movement. The quality of MR spectra also relies on adequate technics, such as effective water suppression and prescan adjustment, such as the position of the VOI and adequate anesthetic dose. The VSS pulse was used to reduce the voxel misregistration leading to outer voxel contamination, and to improve the effect of shimming. Although it is a time-consuming process, it is of importance to obtain good quality spectra that allow for more accuracy of metabolite identification and quantification.
Absolute quantification remains a challenge due to the lack of relatively stable metabolites used as the standard. Therefore, metabolite concentrations were reported in terms of metabolite ratios such as choline/lipid under the assumption of constancy of the reference compound[23,24]. Unfortunately, variations in choline and lipid must be taken into account. The use of the lipid peak as an internal reference was not considered for this study, as the range of lipids is very variable[25].
LCModel software is widely used in the field of quantitative analysis. Compared with other fitting programs, phase and baseline correction can be automatically processed and overlapping spectra can be better resolved. In this study, unsuppressed water was used as an internal reference to acquire the metabolite area ratio of choline to water. However, accurate choline quantification requires additional correction for some factors, such as the concentration and relaxation attenuation of tissue water as well as the relaxation attenuation of choline. Otherwise, the measurement can only be presented as concentrations in arbitrary institutional units (a.u.).
T1 and T2 relaxation time of choline and water in vivo was measured in this study. A number of studies reported the relaxation time of human hepatic tumor metabolites. At 3T, T1 and T2 values of water in the phantom were measured by Li et al[13] to be 3420 ms and 370 ms, respectively, and choline T2 relaxation time in HCC patients ranged from 88 ms to 161 ms. These values are similar to the T1 and T2 relaxation time of water (3172 and 206 ms) and T2 relaxation time of choline (78 ± 5 ms in apoptotic liver) in the present study. In the study by Goldberg et al[26] T1 and T2 relaxation time of water in solid lesions was 1004 ± 234 ms and 80 ± 18 ms at 1.5T by MRI. These values are higher than our data (719 ± 37 ms and 50 ± 5 ms in cell apoptotic livers). The differences of T1 and T2 relaxation time may be attributed to the magnetic field strength, subjects, and measurement methods. Enhanced field strength led to increase in T1 and minor decrease in T2. Moreover, this procedure was subject to failure caused by the movement. Due to individual differences, variable relaxation time should be taken into consideration.
The liver choline concentration in vivo seems not universal. In the report by Fischbach et al[12], it was 7.7 ± 7.3 a.u. in normal liver parenchyma of volunteers and 7.3 ± 4.3 a.u. in normal-appearing liver parenchyma of patients with hepatic tumors using LCModel at 3T. However, Li et al[13] reported that the choline concentrations in four patients ranged from 3.4 mmol/L to 14.0 mmol/L, and in healthy volunteers 1.3 ± 0.9 mmol/L. It was 1.0 ± 0.7 a.u. in the normal liver tissues of rats and 5.6 ± 1.5 a.u. in tumor tissues reported by Chen et al[27]. The choline concentration in our study (14.5 ± 7.6 mmol/L in normal and 10.8 ± 6.6 mmol/L in apoptotic livers) is higher than the above values. In addition to field strength and different subjects as well as the quantification method, the assumed tissue water concentration may be the main reason leading to our higher values. The water concentration was assumed to be 47 778 mmol/L for an 86% water content of liver. This value may be slightly higher for including parts of MR-invisible water. However, the real water concentration of liver is hard to measure in vivo and might vary between individuals.
The basis set is another important issue in quantification with LCModel. It included the metabolites of choline and lipid as well as glycogen in analysis mode of liver-6. Therefore, the above metabolites can be measured in our study. Nevertheless, a choline compound consists of glycerophosphorylcholine, phosphorylcholine and free choline, which can be resolved in vitro at 9.4T[8] . As for the much poorer spectral resolution and SNR of in vivo MRS, only choline is included in the basis set.
Se-induced apoptosis has been studied in a number of cancer cells in vitro and the results generally suggest the involvement of apoptosis in Se-induced cytotoxic and anti-proliferative effects against cancer cells[21]. Choline is a nutrient essential for the normal function of cells and is usually considered as a marker of cell growth[28,29]. The data in our study support that choline concentrations are decreased with the elevated number of apoptotic liver cells. This result is consistent with the results of Blankenberg[30] and our results in vitro[8]. However, it is insufficient to use choline as a possible apoptosis biomarker in this study. High concentrations of selenite can induce oxidative stress and make tissue undergo necrosis, which also leads to a decreased choline concentration. Therefore, it is essential to confine the time window to early apoptosis in future studies. The increased choline after injection of sodium selenite may be caused by the experimental control. This phenomenon might be related to individual diversities.
In conclusion, this work demonstrates the feasibility of noninvasive measurement of liver choline concentrations using in vivo 1.5T clinical MR. Although there are limitations as outlined above, this method has the potential to characterize liver lesions and determine therapeutic responses. Moreover, it lays a foundation for future investigations of cell apoptosis in vivo[8,9,31].
We thank Dr. Stephen Provencher for his useful suggestions about LCModel control parameter settings, and thank Dr. Stanley Lin, Dr. Yan Lin and Dr. Darren Price for their English language editing of this paper.
Through detecting apoptosis, the progression of a tumor can be evaluated and drug effects can be determined. It is, therefore, essential to develop a non-invasive method that can be used to assess apoptosis of tissues in vivo. The liver is a particularly suitable and interesting organ for metabolic studies owing to its rich metabolic activities and location at the body surface. However, the difficulties in measurement in vivo may occur due to respiratory movement.
Changes in choline-containing compounds at 3.2 ppm seem not to be universal, at least in the early stages of apoptosis. In this study, the decreased choline concentrations were observed in the sodium selenite-induced rabbit liver cell apoptosis in vivo.
Several previous in vivo studies focused on the choline-to-lipid ratios in liver tumors. This may be the first study to attempt to quantify the metabolite concentrations of both normal and apoptotic rabbit livers in vivo using single-voxel 1H-magnetic resonance spectroscopy (MRS) with unsuppressed water signal as the internal reference, and to correct the relaxation attenuation.
This study demonstrates the feasibility of noninvasive measure of liver choline concentrations in vivo in a 1.5T clinical MR environment. Moreover, it lays a foundation for future investigations of cell apoptosis in vivo.
The authors have tried to evaluate the feasibility of quantifying liver choline concentrations in both normal and apoptotic rabbit livers in vivo using 1H MRS. Based on the in vitro study using a phantom model and an in vivo study using 18 rabbits, the authors conclude that MRS can be used to quantify choline in rabbit liver in vivo using unsuppressed water as an internal reference. This is an interesting animal study and the authors needed to be lauded for their efforts.
Peer reviewer: Avinash Kambadakone, MD, FRCR, Division of Abdominal Imaging and Intervention, Massachusetts General Hospital, White 270, 55 Fruit Street, Boston, MA 02114, United States
S- Editor Tian L L- Editor Ma JY E- Editor Li JY
| 1. | Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1079] [Cited by in F6Publishing: 1128] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 2. | Ellis PA, Smith IE, McCarthy K, Detre S, Salter J, Dowsett M. Preoperative chemotherapy induces apoptosis in early breast cancer. Lancet. 1997;349:849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Blankenberg FG, Katsikis PD, Storrs RW, Beaulieu C, Spielman D, Chen JY, Naumovski L, Tait JF. Quantitative analysis of apoptotic cell death using proton nuclear magnetic resonance spectroscopy. Blood. 1997;89:3778-3786. [PubMed] [Cited in This Article: ] |
| 4. | Blankenberg FG, Storrs RW, Naumovski L, Goralski T, Spielman D. Detection of apoptotic cell death by proton nuclear magnetic resonance spectroscopy. Blood. 1996;87:1951-1956. [PubMed] [Cited in This Article: ] |
| 5. | Musacchio T, Toniutti M, Kautz R, Torchilin VP. 1H NMR detection of mobile lipids as a marker for apoptosis: the case of anticancer drug-loaded liposomes and polymeric micelles. Mol Pharm. 2009;6:1876-1882. [PubMed] [Cited in This Article: ] |
| 6. | Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol. 2010;16:1560-1566. [PubMed] [Cited in This Article: ] |
| 7. | Brindle KM. Detection of apoptosis in tumors using magnetic resonance imaging and spectroscopy. Adv Enzyme Regul. 2002;42:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Cao Z, Wu LP, Li YX, Guo YB, Chen YW, Wu RH. Change of choline compounds in sodium selenite-induced apoptosis of rats used as quantitative analysis by in vitro 9.4T MR spectroscopy. World J Gastroenterol. 2008;14:3891-3896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Dagnelie PC, Leij-Halfwerk S. Magnetic resonance spectroscopy to study hepatic metabolism in diffuse liver diseases, diabetes and cancer. World J Gastroenterol. 2010;16:1577-1586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Zhao WD, Guan S, Zhou KR, Li H, Peng WJ, Tang F, Chen ZW. In vivo detection of metabolic changes by 1H-MRS in the DEN-induced hepatocellular carcinoma in Wistar rat. J Cancer Res Clin Oncol. 2005;131:597-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kuo YT, Li CW, Chen CY, Jao J, Wu DK, Liu GC. In vivo proton magnetic resonance spectroscopy of large focal hepatic lesions and metabolite change of hepatocellular carcinoma before and after transcatheter arterial chemoembolization using 3.0-T MR scanner. J Magn Reson Imaging. 2004;19:598-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Fischbach F, Schirmer T, Thormann M, Freund T, Ricke J, Bruhn H. Quantitative proton magnetic resonance spectroscopy of the normal liver and malignant hepatic lesions at 3.0 Tesla. Eur Radiol. 2008;18:2549-2558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Li CW, Kuo YC, Chen CY, Kuo YT, Chiu YY, She FO, Liu GC. Quantification of choline compounds in human hepatic tumors by proton MR spectroscopy at 3 T. Magn Reson Med. 2005;53:770-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Cheng XF, Cao Z, Ning LB, Xiao G, Shen ZW, Huo SS, Wu RH. Quantitative proton magnetic resonance spectroscopy of human precentral gyrus and hippocampus. Absolute concentrations of metabolites. Neurosciences (Riyadh). 2011;16:168-169. [PubMed] [Cited in This Article: ] |
| 15. | Fayad LM, Salibi N, Wang X, Machado AJ, Jacobs MA, Bluemke DA, Barker PB. Quantification of muscle choline concentrations by proton MR spectroscopy at 3 T: technical feasibility. AJR Am J Roentgenol. 2010;194:W73-W79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | McLean MA, Priest AN, Joubert I, Lomas DJ, Kataoka MY, Earl H, Crawford R, Brenton JD, Griffiths JR, Sala E. Metabolic characterization of primary and metastatic ovarian cancer by 1H-MRS in vivo at 3T. Magn Reson Med. 2009;62:855-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672-679. [PubMed] [Cited in This Article: ] |
| 18. | Hamilton G, Middleton MS, Bydder M, Yokoo T, Schwimmer JB, Kono Y, Patton HM, Lavine JE, Sirlin CB. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging. 2009;30:145-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Strobel K, van den Hoff J, Pietzsch J. Localized proton magnetic resonance spectroscopy of lipids in adipose tissue at high spatial resolution in mice in vivo. J Lipid Res. 2008;49:473-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med. 2004;51:435-440. [PubMed] [Cited in This Article: ] |
| 21. | Shen HM, Yang CF, Ong CN. Sodium selenite-induced oxidative stress and apoptosis in human hepatoma HepG2 cells. Int J Cancer. 1999;81:820-828. [PubMed] [Cited in This Article: ] |
| 22. | Shen H, Yang C, Liu J, Ong C. Dual role of glutathione in selenite-induced oxidative stress and apoptosis in human hepatoma cells. Free Radic Biol Med. 2000;28:1115-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Fischbach F, Bruhn H. Assessment of in vivo 1H magnetic resonance spectroscopy in the liver: a review. Liver Int. 2008;28:297-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Perman WH, Balci NC, Akduman I. Review of magnetic resonance spectroscopy in the liver and the pancreas. Top Magn Reson Imaging. 2009;20:89-97. [PubMed] [Cited in This Article: ] |
| 25. | van Werven JR, Hoogduin JM, Nederveen AJ, van Vliet AA, Wajs E, Vandenberk P, Stroes ES, Stoker J. Reproducibility of 3.0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J Magn Reson Imaging. 2009;30:444-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Goldberg MA, Hahn PF, Saini S, Cohen MS, Reimer P, Brady TJ, Mueller PR. Value of T1 and T2 relaxation times from echoplanar MR imaging in the characterization of focal hepatic lesions. AJR Am J Roentgenol. 1993;160:1011-1017. [PubMed] [Cited in This Article: ] |
| 27. | Chen F, Sun X, De Keyzer F, Yu J, Peeters R, Coudyzer W, Vandecaveye V, Landuyt W, Bosmans H, Van Hecke P. Liver tumor model with implanted rhabdomyosarcoma in rats: MR imaging, microangiography, and histopathologic analysis. Radiology. 2006;239:554-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Qayyum A. MR spectroscopy of the liver: principles and clinical applications. Radiographics. 2009;29:1653-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Yang ZX, Huo SS, Cheng XF, Xu ZF, Cao Z, Zeng JX, Xiao YY, You KZ, Chen W, Liu YY. Quantitative multivoxel proton MR spectroscopy study of brain metabolites in patients with amnestic mild cognitive impairment: a pilot study. Neuroradiology. 2011;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Blankenberg FG. In vivo detection of apoptosis. J Nucl Med. 2008;49 Suppl 2:81S-95S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Cox IJ, Sharif A, Cobbold JF, Thomas HC, Taylor-Robinson SD. Current and future applications of in vitro magnetic resonance spectroscopy in hepatobiliary disease. World J Gastroenterol. 2006;12:4773-4783. [PubMed] [Cited in This Article: ] |