Published online Mar 14, 2012. doi: 10.3748/wjg.v18.i10.1067
Revised: November 17, 2011
Accepted: December 10, 2011
Published online: March 14, 2012
AIM: To investigate the clinical presentations associated with bile acid synthesis defects and to describe identification of individual disorders and diagnostic pitfalls.
METHODS: Authors describe semiquantitative determination of 16 urinary bile acid metabolites by electrospray ionization-tandem mass spectrometry. Sample preparation was performed by solid-phase extraction. The total analysis time was 2 min per sample. Authors determined bile acid metabolites in 363 patients with suspected defects in bile acid metabolism.
RESULTS: Abnormal bile acid metabolites were found in 36 patients. Two patients had bile acid synthesis defects but presented with atypical presentations. In 2 other patients who were later shown to be affected by biliary atresia and cystic fibrosis the profile of bile acid metabolites was initially suggestive of a bile acid synthesis defect. Three adult patients suffered from cerebrotendinous xanthomatosis. Nineteen patients had peroxisomal disorders, and 10 patients had cholestatic hepatopathy of other cause.
CONCLUSION: Screening for urinary cholanoids should be done in every infant with cholestatic hepatopathy as well as in children with progressive neurological disease to provide specific therapy.
- Citation: Haas D, Gan-Schreier H, Langhans CD, Rohrer T, Engelmann G, Heverin M, Russell DW, Clayton PT, Hoffmann GF, Okun JG. Differential diagnosis in patients with suspected bile acid synthesis defects. World J Gastroenterol 2012; 18(10): 1067-1076
- URL: https://www.wjgnet.com/1007-9327/full/v18/i10/1067.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i10.1067
Inborn errors of bile acid synthesis can result in potentially treatable progressive cholestatic liver disease and neurological disease. Liver disease often presents in early childhood with jaundice, deficiency of fat-soluble vitamins, acholic stools and hepatomegaly. Serum aminotransferases and conjugated bilirubin are usually elevated and typically, gamma glutamyl transpeptidase activity is normal. Progressive neurological disease usually occurs later in childhood or early adulthood with pyramidal tract dysfunction, cerebellar signs, sensory-motor neuropathy and cognitive decline.
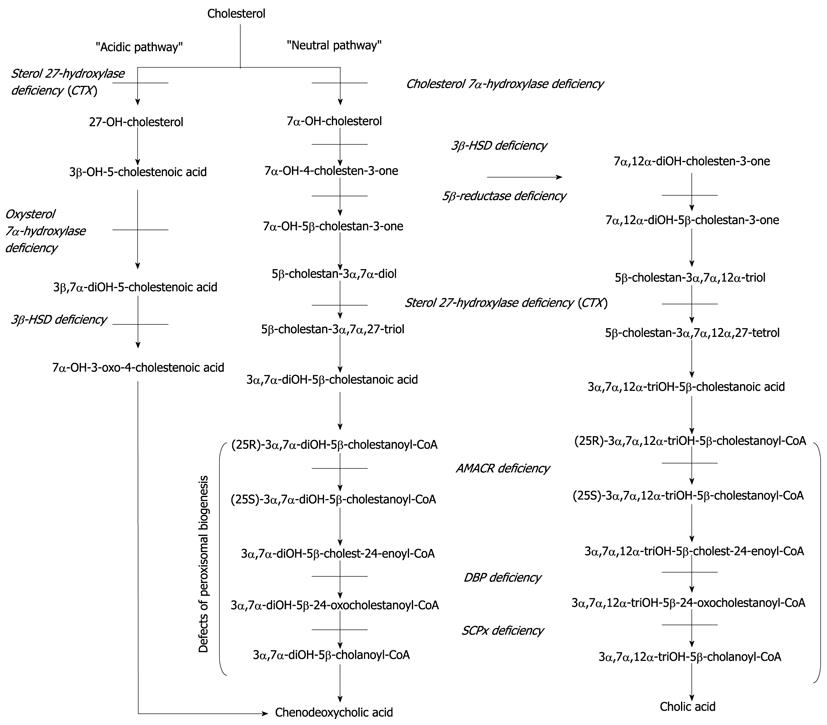
The synthesis of bile acids in the liver is the main pathway of cholesterol degradation. Many enzymes localized in different subcellular compartments of hepatocytes interact in the conversion of cholesterol to bile acids. Thus, defective functioning of one or more of these enzymes results in a deficiency of the final bile acids chenodeoxycholic acid and cholic acid (Figure 1) as well as the formation of atypical sterol intermediates (Table 1) including unusual bile acids and bile alcohols, which can be hepatotoxic or interfere with biliary secretion leading to cholestasis and malabsorption of fat-soluble vitamins. Severe liver disease in turn gives rise to serum aminotransferases and conjugated bilirubin levels while serum gamma glutamyl transpeptidase activity in patients with bile acid synthesis defects remains normal despite severe cholestasis[1]. This contributes to the fact that glutamyl transpeptidase is localized mainly at the luminal plasma membrane of bile duct epithelium cells. It increases if bile acids dissolve the glutamyl transpeptidase from this membrane. In patients with bile acid synthesis defects (and in those with a bile acid transporter defect) these bile acids are not produced. Therefore no increase in glutamyl transpeptidase is seen despite severe cholestasis[2].
| Defect | Metabolite | m/z | Median | Range |
| 3β-hydroxy-Δ5-C27-steroid dehydrogenase deficiency | Glyco-dihydroxy-5-cholenoic acid | 526 | 0.05 | 0.0-0.9 |
| HSD3B7 | Glyco-trihydroxy-5-cholenoic acid | 542 | 0.07 | 0.0-0.4 |
| 16p11.2-12 | Dihydroxy-5-cholenoic acid sulphate | 469 | 0.71 | 0.15-8.1 |
| OMIM: 23110 | Trihydroxy-5-cholenoic acid sulphate | 485 | 0.08 | 0.0-2.0 |
| Δ4-3-oxosteroid 5-β-reductase deficiency | Glyco-hydroxy-oxo-cholenoic acid | 444 | 0.03 | 0.0-0.4 |
| AKR1D1 | Glyco-dihydroxy-oxo-cholenoic acid | 460 | 0.04 | 0.0-2.4 |
| 7q31 | Tauro-hydroxy-oxo-cholenoic acid | 494 | 0.02 | 0.0-0.5 |
| OMIM: 235555 | Tauro-dihydroxy-oxo-cholenoic acid | 510 | 0.04 | 0.0-5.0 |
| Defects of peroxisomal biogenesis | Tauro-tri-hydroxycholestanoic acid | 556 | 0.02 | 0.0-0.3 |
| Multiple | Tauro-tetra-hydroxycholestanoic acid | 572 | 0.02 | 0.0-0.2 |
| OMIM: 214100 | ||||
| Sterol 27-hydroxylase deficiency | Glucuronide-5β-cholestane-tetrol | 611 | 0.24 | 0.0-1.6 |
| (cerebrotendinous xanthomatosis) | Glucuronide-5β-cholestane-pentol | 627 | 0.37 | 0.1-4.3 |
| CYP27A1 | ||||
| 2q33-qter | ||||
| OMIM: 213700 | ||||
| Cholestatic liver disease | Glyco-chenodeoxy-cholic acid | 448 | 0.49 | 0.0-5.2 |
| Multiple | Glyco-cholic acid | 464 | 0.64 | 0.1-14.6 |
| Tauro-chenodeoxy-cholic acid | 498 | 0.05 | 0.0-0.8 | |
| Tauro-cholic acid | 514 | 0.08 | 0.0-8.4 |
At the genetic level, 9 inborn errors in the bile acid biosynthetic pathways have been described to date: cerebrotendinous xanthomatosis, 3β-hydroxy-Δ5-C27-steroid dehydrogenase/isomerase deficiency[3], Δ4-3-oxosteroid 5β-reductase (5β-reductase) deficiency[4], oxysterol 7α-hydroxylase deficiency[5], cholesterol 7α-hydroxylase deficiency[6], as well as the peroxisomally located defects 2-methylacyl-CoA racemase (AMCAR) deficiency[7,8], D-bifunctional protein deficiency[9,10], sterol carrier protein X (SCPx) deficiency[11]. The clinically most important errors are depicted in Table 1.
Several analytical techniques are available for the determination of bile acid profiles in urine and plasma[12-15], but the most useful screening test is analysis of urinary cholanoids (bile acids and bile alcohols) by electrospray ionization-tandem mass spectrometry (ESI-MS/MS)[2].
However, patients with severely compromised liver function of different origin may show patterns of bile acid metabolites resembling those of bile acid synthesis defects and patients with disorders of bile acid synthesis may present with atypical clinical symptoms making a straightforward diagnosis impossible. In this study urinary bile acids of 363 patients with suspected defects in bile acid metabolism were determined and in 36 patients an abnormal bile acid pattern was recognized. This paper focuses on 2 groups of patients: those with proven defects of bile acid synthesis, who present with atypical clinical symptoms and those with cholanoid profiles pointing to a defect of bile acid synthesis who were later shown to have a different disorder. We describe clinical presentations, present differential diagnoses and analyze the diagnostic pitfalls of the ESI-MS/MS method in these patients.
This study has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. Sample collection for this study was approved by the ethics committee of the medical faculty, University of Heidelberg, Germany (No. 071/2005).
The control population consisted of 100 healthy and non-symptomatic children, adolescents and young adults ranging in age from 10 d to 20 years. Urine specimens were collected in our hospital and used to establish the normal ranges of the 16 metabolites assayed. As positive controls, we additionally investigated patient samples collected previously at the UCL Institute of Child Health, London; these samples were obtained from patients with the following confirmed defects: 3β-hydroxy-Δ5-C27-steroid dehydrogenase (3β-HSD) (n = 1), 5β-reductase deficiency (n = 1), peroxisomal biosynthesis disorders (n = 3), and cerebrotendinous xanthomatosis (n = 3).
After establishing reference ranges urine samples of patients with suspected bile acid synthesis defects were investigated in the clinical routine. To date samples of 363 patients have been analyzed. Thirty-six of those showed abnormal results and are further discussed in this study. The remaining 327 patients had normal or non-specific bile acid profiles. In the following, 3 clinically especially instructive histories from patients are briefly summarized.
This girl, the second child of healthy Turkish parents who are first cousins, was born after 35 wk of an otherwise uneventful pregnancy. Shortly after birth a persistent metabolic acidosis was observed, which was treated with bicarbonate supplementation. She also had nephrocalcinosis and failure to thrive. Because phosphate excretion was also elevated DeToni-Debré-Fanconi syndrome was suspected. At age 6 years she developed gait ataxia, muscle weakness, and external ophtalmoplegia. A severe deficiency of fat-soluble vitamins became evident (vitamin A: 0.89 μmol/L, reference range 1.09-3.07, vitamin E: 19.89 μmol/L, reference range 25-42). Aspartate aminotransferase (AST) was 66 U/L (reference range < 39), alanine aminotransferase (ALT) 46 U/L (reference range < 34), gamma glutamyltransferase (GGT) 22 U/L (reference range < 38), total bilirubin 24 μmol/L (reference range < 17), and conjugated bilirubin 12 μmol/L (reference range < 5). Abdominal ultrasound showed normal hepatic echotexture. Because multiple organ systems were involved a defect of mitochondrial energy metabolism was suspected but lactate concentrations in blood and CSF (cerebrospinal fluid), determination of respiratory chain and pyruvate dehydrogenase complexes in frozen muscle as well as mutation analysis for pediatric mitochondrial disorders such as MERRF (myoclonic epilepsy with “ragged red fibres”), MELAS (encephalomyopathy, lactic acidosis, stroke-like episodes) and NARP (neuropathy, ataxia, retinitis pigmentosa) were all normal. After the diagnosis of the bile acid synthesis disorder 3β-hydroxy-Δ5-C27-steroid dehydrogenase deficiency was established by analysis of urinary bile acid metabolites, supplementation with vitamins A, E and K normalized her vitamin A levels but those of vitamin E remained below the normal range. After adding ursodeoxycholic acid and chenodeoxycholic acid, vitamin concentrations normalized quickly and remained so even after discontinuation of vitamin supplementation. Her gait improved significantly and after a short period of treatment her muscle strength returned to normal.
This baby girl was the first child of healthy non-consanguineous Tamil parents. At age 4 wk scleral icterus was observed. Additionally, she developed dark urine and pale stools, but her weight gain was within the normal range. Laboratory investigations at age 13 wk showed elevated AST (681 U/L, reference range < 74), ALT (344 U/L, reference range < 60) and normal GGT (99 U/L, reference range < 160). Total bilirubin was 268 μmol/L (reference range < 21), conjugated bilirubin was 185 μmol/L (reference range < 5). The gall bladder was not detectable in abdominal ultrasound, therefore biliary atresia was suspected. Explorative laparotomy showed a completely cirrhotic liver; there was no excretion of marker dye into the bile ducts. Due to the severe cirrhosis a Kasai procedure was not attempted. A primary liver transplantation was planned. Histological examination of a liver biopsy showed proliferating bile ducts and no giant cells consistent with biliary atresia; however, analysis of bile acids was suggestive of Δ4-3-oxosteroid 5β-reductase deficiency. Subsequent DNA sequencing of the exons of the patient’s AKR1D1 gene failed to reveal mutations, thereby excluding the diagnosis of primary 5β-reductase deficiency. She was treated with cholic acid and ursodeoxycholic acid but her condition deteriorated with progressive ascites, which led to referral for liver transplantation. Segments 2 and 3 of her mother’s liver were transplanted successfully at age 4.5 mo. Examination of the explanted liver and biliary tree confirmed the diagnosis of biliary atresia. Several days after transplantation she developed rejection, which was successfully treated with immunosuppression. At age 10 mo the patient was doing well.
This boy was the first child of non-consanguineous German parents. His mother suffered from a stroke at the age of 20 years. Follow-up investigations were not performed. At the age of 6 years attention deficit hyperactivity disorder (ADHD) was diagnosed in patient KS and treatment with methylphenidate was initiated. After primary school he attended a secondary school for children with learning difficulties. His parents noted recurrent diarrhea, especially after consuming sweets. At the age of 14 years bilateral cataracts were diagnosed. After surgery he was referred for metabolic workup. Galactose metabolites, homocysteine and urinary oligosaccharides were all normal. Cranial magnetic resonance imaging (MRI) showed increased signal intensity in cerebellar white matter in T2. Analysis of urinary bile acid metabolites was suggestive of cerebrotendinous xanthomatosis. Sterol analysis of plasma showed a clearly elevated concentration of cholestanol (0.24 μmol/L, reference range < 0.01), together with positive results of mutation analysis confirming the diagnosis. Treatment with chenodeoxycholic acid normalized bowel movements and the sterol profile. The patient has not developed ataxia or xanthomata. At the age of 19 years he is an apprentice landscaper.
Urine samples were stored at -20 °C until analysis. Sample preparation was performed by solid-phase extraction (SPE) using a OasisTM HLB cartridge as described previously[16]. In brief, the cartridge was preconditioned with 2 × 1 mL dichlormethane/methanol (2:1, v/v) and 1 mL purified water. To a fixed volume of 300 μL urine 10 μL internal standard solution (100 μmol/L D4-glycocholic acid) was added. The urine sample was then applied onto the preconditioned cartridge and washed with 2 mL each of purified water and n-hexane. The bile acid conjugates were eluted with 300 μL of 700 mL/L aqueous methanol (v/v). The eluate was directly used for injection to tandem mass spectrometer. The sample preparation time for a series of 20 samples takes about 1.5 h.
ESI-MS/MS measurement was carried out using a triple quadrupole IONICS EP 10+ upgraded Perkin Elmer Sciex API 365 mass spectrometer equipped with a turbo spray ion source. The mobile phase contained acetonitrile/purified water (1:1, v/v). 10 μL eluate of the SPE cartridge was directly injected. The flow rate was 80 μL/min over a total run time of 2 min.
The 16 urinary bile acid metabolites (glycine and taurine conjugates, sulfates and glucuronides) were identified with negative electrospray ionisation. Characteristic fragment ions used to determine the bile acid metabolites according to[17-22] are given in Table 1 as their mass-to-charge ratio (m/z). ESI-MS/MS operating parameters for the four precursor ion scans are given in Table 2.
| Glycine conjugates (IS included) | Taurine conjugates | Sulfatides | Glucuronides | |
| Nebulizer gas (au) | 11 | 11 | 11 | 11 |
| Curtain gas (au) | 9 | 9 | 9 | 9 |
| Collision gas (au) | 5 | 5 | 5 | 5 |
| Ion spray voltage (V) | -4100 | -4100 | -4100 | -4100 |
| Temperature (°C) | 200 | 200 | 200 | 200 |
| Declustering potential (V) | -35 | -35 | -35 | -35 |
| Focusing potential (V) | -31 | -31 | -31 | -31 |
| Entrance potential (V) | -10 | -10 | -10 | -10 |
| Prefilter (V) | 23 | 23 | 23 | 23 |
| Collision energy (V) | -55 | -129 | -60 | -75 |
| Collision cell exit potential (V) | -9 | -9 | -9 | -9 |
| Daughter ion (m/z) | 74 | 80 | 97 | 85 |
| Scan range of precursor ions (m/z) | 430 to 550 | 490 to 590 | 460 to 490 | 600 to 630 |
Data acquisition was carried out by Analysis 1.4.1 software. The determination of the 16 relevant analytes in urine was semi-quantitative since only one internal standard, D4-glycocholic acid, was used as a reference quantity. Therefore the resulting values were given as analyte/internal standard peak-area ratios normalized to mol creatinine. Creatinine determination in urine was performed on an Olympus AU 400 analyzer using the creatinine kit (Beckman Coulter, Krefeld, Germany).
Patient and control fibroblasts were cultured in Dulbecco's modified eagle medium with 10% bovine serum, and antibiotic supplements. After 5-10 passages cells were trypsinized, washed with PBS and stored as pellets at -70 °C until required.
The activity of 3β-hydroxy-Δ5-C27-steroid dehydrogenase was determined as described previously[17,18]. Briefly, fibroblasts were thawed on ice, resuspended in potassium phosphate buffer (0.1 mol/L, pH 7.5) and sonicated. Cell suspensions containing 1 mg protein were preincubated with 14C-labelled 7α-hydroxycholesterol (0.05 mmol/L in 5 μL acetone) and buffer for 5 min in a 37 °C shaking water bath. The reaction was initiated with the addition of 50 μL of nicotinamide adenine nucleotide (NAD) (1.5 mmol/L), with a final reaction volume of 0.5 mL. After 1 hour the reaction was stopped with the addition of 2 mL of ethanol, followed by 3 mL of water. Extractions were performed twice with n-hexane/ethyl acetate (1:1 v/v). Negative controls included a reaction with extract from control fibroblasts without the addition of NAD, and an assay with no cellular extract added.
The extracts were dried under argon, re-dissolved in toluene/ethyl acetate (1:1 v/v) and spotted onto a thin layer chromatography (TLC) plate, which was developed using a mobile phase of toluene/ethyl acetate (1:1 v/v).
Visualization of sterol products was performed using a phosphoimager according to the manufacturer’s recommendations (Fuji Medical Systems, Stamford, United States).
Genomic DNA was extracted from cultured fibroblasts or whole blood collected into EDTA-containing tubes by standard methods. Individual exons together with an average of 25 base pairs of 5’- and 3’-flanking intronic DNA from the 3β-hydroxy-Δ5-C27-steroid dehydrogenase gene (HSD3B7) and Δ4-3-oxosteroid 5β-reductase gene (AKR1D1) were amplified and subjected to DNA sequence analysis as described previously[18,19].
Bile acid conjugates were extracted from urine and 16 metabolites (6 glycine conjugates, 6 taurine conjugates, 2 sulfates and 2 glucuronides) were determined semi-quantitatively by ESI-MS/MS. We established reference ranges for these 16 metabolites studied here in 100 subjects ranging in age from 10 d to 20 years (Table 1); no age-dependency for any of these bile acid conjugates was found (data not shown).
Cholestatic liver disease and cholanoid profiles mimicking 5β-reductase deficiency: In patients with cholestatic liver disease analysis of urinary bile acid metabolites showed clearly increased excretion of glycine and taurine conjugates of cholic acid and chenodeoxycholic acid (m/z 448, 464, 498 and 514). In a subgroup of patients with non-specified cholestatic liver disease (n = 10) we additionally detected elevated excretion of glycine and taurine conjugates of dihydroxy-oxo-cholenoic acid (m/z 460, 510) and hydroxy-oxo-cholenoic acid (m/z 444, 494), however the elevation of cholic and chenodeoxycholic conjugates always exceeded the excretion of (di)-hydroxy-oxo-cholenoic acid conjugates (Table 3).
| Metabolite | m/z | Controls(n = 100) | Cholestasis(n = 10) | AS | MK | MK1 |
| Glyco-hydroxy-oxo-cholenoic acid | 444 | 0-0.4 | 1-210 | 34 | 104 | 14 |
| Glyco-dihydroxy-oxo-cholenoic acid | 460 | 0-2.4 | 8-251 | 15 | 1331 | 1 |
| Tauro-hydroxy-oxo-cholenoic acid | 494 | 0-0.5 | 1-26 | 39 | 6 | 17 |
| Tauro-dihydroxy-oxo-cholenoic acid | 510 | 0-5 | 8-133 | 19 | 779 | 0 |
| Glyco-chenodeoxy-cholic acid | 448 | 0-5.2 | 2-10 754 | 4 | 9 | 25 |
| Glyco-cholic acid | 464 | 0.1-14.6 | 36-2814 | 17 | 49 | 47 |
| Tauro-chenodeoxy-cholic acid | 498 | 0-0.8 | 1-718 | 9 | 45 | 10 |
| Tauro-cholic acid | 514 | 0-8.4 | 6-749 | 9 | 10 | 1 |
In patient AS with suspected biliary atresia excretion of glyco-(di)-hydroxy-oxo-cholenoic acid conjugates was elevated (Table 3). However, conjugates of cholic acid and chenodeoxycholic acid were very low for an infant with cholestasis. As some aspects of biliary atresia such as absent gall bladder upon ultrasound and missing excretion of bile in the gut have been described previously in patients with 5β-reductase deficiency, we performed mutation analysis of the AKR1D1 gene, which was normal, excluding 5β-reductase deficiency. Similarly, no mutations were detected in the 3β-hydroxy-Δ5-C27-steroid-dehydrogenase gene (HSD3B7).
A quite similar cholanoid profile with low amounts of cholic and chenodeoxycholic acid conjugates was found in another patient, MK, a 7 mo old patient with failure to thrive and severe cholestatic liver disease (Table 3). In this patient cystic fibrosis was confirmed by an abnormal sweat test, abnormal ion transport in rectal epithelial cells and heterozygosity for the mutation ΔF508 in the CFTR gene. Supplementation with ursodeoxycholic acid, pancreatic enzymes and fat-soluble vitamins completely normalized the cholanoid profile.
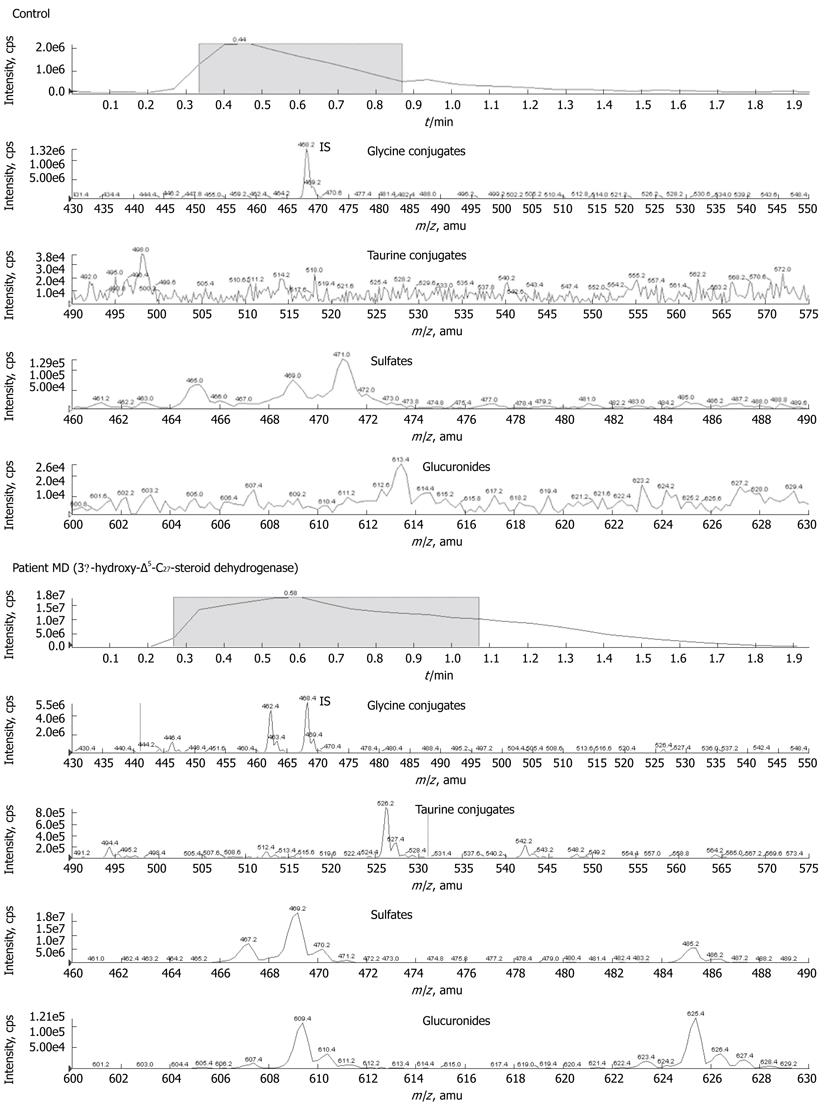
3β-hydroxy-Δ5-C27-steroid dehydrogenase deficiency-patient MD: Analysis of urinary bile acid metabolites in patient MD revealed large quantities of sulphate conjugates of di- and trihydroxy-5-cholenoic acid metabolites characterized by m/z 469 and 485, respectively. Glycine conjugates of di- and trihydroxy-5-cholenoic acid (m/z 526 and 542) were less pronounced, but clearly increased compared to controls (Figure 2). Peaks attributable to the glycine and taurine conjugates of chenodeoxycholic acid and cholic acid (m/z 448, 464, 498 and 514, respectively) were small or present at near baseline levels. This constellation of metabolites is characteristic of 3β-hydroxy-Δ5-C27-steroid dehydrogenase deficiency.
Mutation analysis of the HSD3B7 gene showed homozygosity for a single base pair mutation (c.605G > C, GenBank Accession #AF277719)) in exon 5 resulting in an amino acid substitution (p.G196A). This mutation has not been described before but has not been found in > 50 control DNAs.
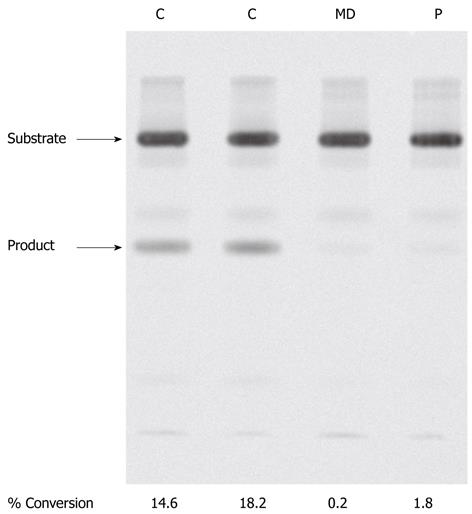
To examine if this mutation affected enzyme activity we determined 3β-hydroxy-Δ5-C27-steroid dehydrogenase activity in the patient’s fibroblasts (Figure 3) as described in materials and methods. In control fibroblasts (C) two clear sterols were visualised after TLC developed under standard conditions. These sterols were identified by gas chromatography mass spectrometry as the substrate, 7α-hydroxycholesterol and the product, 7α-hydroxy-4-cholesten-3-one of the 3β-HSD enzyme. In fibroblasts of patient MD, little or no product was formed, indicative of severely reduced 3β-hydroxy-Δ5-C27-steroid dehydrogenase activity. As a negative control, unaffected fibroblasts were incubated with substrate in the absence of NAD and the reaction subjected to TLC. In this case no product was observed on the silica plate (results not shown).
Cerebrotendinous xanthomatosis-patient KS and others: In patient KS analysis of urinary bile acid metabolites showed increased excretion of glucuronide-5β-cholestane-pentole (m/z 627) and less pronounced excretion of glucuronide-5β-cholestane-tetrole (m/z 611). Mutation analysis of the CYP27A1 gene revealed compound heterozygosity for c.IVS6-1A and c.1213C > T (p.R405W) (University Medical Center Nijmegen, Department of Human Genetics, DNA-Diagnostics). These are known pathogenic mutations for cerebrotendinous xanthomatosis[20].
Additionally we found elevated concentrations of 5β-cholestane-tetrol glucuronides (mean excretion 4, normal < 1.6) and -pentol glucuronides (mean excretion 28, normal < 4.3) in three adults unrelated to KS and to each other with previously confirmed cerebrotendinous xanthomatosis.
We analyzed urinary bile acid metabolites in 21 infants with suspected peroxisome biogenesis defects. All patients had profound neurological abnormalities and clearly elevated concentrations of hexacosanoic acid (C26:0). In 16 patients, tauro-tetra-hydroxycholestanoic (m/z 572) was elevated, consistent with a peroxisome biogenesis defect. Of these patients 12 additionally showed an elevation of tauro-tri-hydroxycholestanoic acid (m/z 556), but this increase was always lower than that measured for tauro-tetra-hydroxycholestanoic acid. In two patients there was no elevation of tauro-tetra-hydroxycholestanoic acid but increases of tauro-tetra-hydroxy-24-ene-cholestanoic acid (m/z 570) and tauro-penta-hydroxy-24-ene-cholestanoic acid (m/z 586) were detected, pointing to D-bifunctional protein deficiency (HSD17B4 gene).
In a previously described 45-year old patient (HPH)[11] with dystonic head tremor, spasmodic torticollis and leukencephalopathy in MR imaging we found increased excretion of 27-nor-5β-cholestanepentol-glucuronide (m/z 613) and 27-nor-5β-cholestanehexol-glucuronide (m/z 629). This patient was found to suffer from a defect of peroxisomal sterol carrier protein X (SCPx)[11].
Defects of bile acid synthesis can present with a wide variety of clinical symptoms from early infancy to adulthood. Cholestatic liver disease, which is often described as the leading symptom, may not be evident as in patient MD in the present cohort. Instead impaired absorption of fat-soluble vitamins may result in a multisystem disorder with predominantly neurological symptoms in early childhood. It is likely that vitamin E deficiency contributed to the ataxia seen in MD; spinocerebellar ataxia is a common feature of vitamin E deficiency (as seen in untreated abetalipoproteinemia). Myopathy has also been reported in vitamin E deficiency in both man and experimental animals.
Cataracts are often the first sign of cerebrotendinous xanthomatosis in school children and adolescents, with xanthomatas and ataxia usually occurring later in life. Treatment with chenodeoxycholic acid positively influences neurological disease and avoids development of xanthomata and arteriosclerosis[21], therefore determination of bile acid metabolites and sterols should be included in the diagnostic workup of cataract in this age group.
In patients with peroxisomal disorders determination of bile acid metabolites is helpful in distinguishing between defects of peroxisome biogenesis versus single enzyme deficiencies. An elevated excretion of tauro-tetrahydroxy-cholestanoic acid is highly suggestive of a defect in peroxisome biogenesis whereas increased excretion of tauro-tetra-hydroxy-24-ene-cholestanoic acid and tauro-penta-hydroxy-24-ene-cholestanoic acid point to a D-bifunctional protein defect. It is important to note that a normal bile acid profile does not exclude peroxisome biogenesis disorders as some patients may present without urinary bile acid abnormalities. Importantly bile acid analysis may elucidate peroxisomal defects with predominantly neurological presentations as in peroxisomal SCPx or α-methyl-acyl-CoA racemase deficiencies, in which analysis of very long-chain fatty acids is not of diagnostic value[11].
Earlier studies[22] have indicated that non-specific cholestatic liver disease may result in elevated excretion of hydroxy- and dihydroxy-oxo-cholenoic acids mimicking 5β-reductase deficiency. However, non-specific cholestatic liver disease can usually be distinguished from 5β-reductase deficiency by the finding of elevated urinary concentrations of the primary bile acids cholic acid and chenodeoxycholic acid. From our study cohort 2 examples of patients with elevated hydroxy- and dihydroxy-oxo-cholenic acids and low primary bile acids are described, one presenting with biliary atresia (patient AS) and the other (patient MK) with cystic fibrosis, cholestasis and severe malabsorption.
Since one patient with 5β-reductase deficiency had a clinical presentation resembling biliary atresia 5β-reductase deficiency should be considered in the differential diagnosis of biliary atresia[19]. Although liver transplantation is curative for both biliary atresia and 5β-reductase deficiency, it is crucial to define the molecular basis of the diseases in these individuals prior to counselling of the parents regarding recurrence risk in further pregnancies. In our patient AS, mutation analysis of the 5β-reductase gene (AKR1D1) excluded a deficiency in bile acid synthesis leaving biliary atresia as the most likely diagnosis, so that she subsequently underwent successful liver transplantation.
In patient MK, the common ΔF508 mutation was detected in the CFTR gene, and this individual experienced a normalization of her urinary cholanoid profile after supplementation with pancreatic enzymes, vitamins and ursodesoxycholic acid.
The mechanism underlying the aberrant cholanoid profiles in patients AS and MK is not clear. There may be secondary inhibition of 5β-reductase by an as yet undefined substance occurring in both conditions.
Because of the clinical variability associated with atypical presentations, as in the patients described in this article, the availability of a quick and reliable method for determination of bile acid metabolites is essential in the diagnostic work-up. The ESI-MS/MS method described is fast and reliable. However, in some patients cholanoid profiles suggesting 5β-reductase deficiency are obtained. In these cases mutation analysis of the AKR1D1 gene is necessary. If 5β-reductase deficiency is excluded cystic fibrosis or biliary atresia should be considered in the differential diagnosis.
We thank Davis DL, Treiber DK, and Schmidt KV for their excellent technical assistance and the Department of Human Genetics, DNA-Diagnostics, University Medical Center Nijmegen for mutation analysis of the CYP27A1 gene.
Bile acid synthesis defects can result in progressive cholestatic liver disease and neurological disease. They can be treated effectively with bile acid supplementation when determined early but result in permanent liver failure requiring liver transplantation or irreversible neurological damage when diagnosed at a late stage. Therefore knowledge of clinical symptoms and early diagnosis are important.
Analysis of urinary bile acids by electrospray ionization-tandem mass spectrometry (ESI-MS/MS) is the method of choice to screen for bile acid synthesis defects. However, patients with liver disease of other origin may show similar patterns of bile acid metabolites. Moreover patients with disorders of bile acid synthesis may present with atypical clinical symptoms.
In the present study authors analyzed urinary bile acid metabolites in a large number of patients, in whom bile acid synthesis defects were suspected. In patients with abnormal bile acid profiles authors performed complete biochemical and molecular diagnostic workup. The pitfalls of the ESI-MS/MS method were evaluated and differential diagnoses discussed.
To expand the knowledge about clinical presentation of bile acid synthesis defects and the differential diagnoses. To be aware of pitfalls of the ESI-MS/MS method and further diagnostic procedures to confirm or exclude defects of bile acid synthesis.
This work is well written. Although the group of patients with bile acid synthesis defects is small, it is important to make the clinical diagnosis. This paper will be particularly interesting for pediatricians.
Peer reviewers: Frank G Schaap, PhD, Tytgat Institute for Liver and Intestinal Research, Academic Medical Center, Meibergdreef 69-71, Amsterdam 1105 BK, The Netherlands; Karel van Erpecum, Dr., Department of Gastroenterology and Hepatology, University Hospital Utrecht, Utrecht 855003508 GA, The Netherlands; Vasiliy I Reshetnyak, MD, PhD, Professor, Scientist Secretary of the Scientific Research Institute of General Reanimatology, 25-2, Petrovka str., Moscow 107031, Russia
S- Editor Gou SX L- Editor O’Neill M E- Editor Zhang DN
| 1. | Bove KE, Daugherty CC, Tyson W, Mierau G, Heubi JE, Balistreri WF, Setchell KD. Bile acid synthetic defects and liver disease. Pediatr Dev Pathol. 2000;3:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Clayton PT. Disorders of bile acid synthesis. J Inherit Metab Dis. 2011;34:593-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Clayton PT, Leonard JV, Lawson AM, Setchell KD, Andersson S, Egestad B, Sjövall J. Familial giant cell hepatitis associated with synthesis of 3 beta, 7 alpha-dihydroxy-and 3 beta,7 alpha, 12 alpha-trihydroxy-5-cholenoic acids. J Clin Invest. 1987;79:1031-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 124] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Setchell KD, Suchy FJ, Welsh MB, Zimmer-Nechemias L, Heubi J, Balistreri WF. Delta 4-3-oxosteroid 5 beta-reductase deficiency described in identical twins with neonatal hepatitis. A new inborn error in bile acid synthesis. J Clin Invest. 1988;82:2148-2157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 167] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Setchell KD, Schwarz M, O'Connell NC, Lund EG, Davis DL, Lathe R, Thompson HR, Weslie Tyson R, Sokol RJ, Russell DW. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J Clin Invest. 1998;102:1690-1703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 285] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 142] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Ferdinandusse S, Overmars H, Denis S, Waterham HR, Wanders RJ, Vreken P. Plasma analysis of di- and trihydroxycholestanoic acid diastereoisomers in peroxisomal alpha-methylacyl-CoA racemase deficiency. J Lipid Res. 2001;42:137-141. [PubMed] [Cited in This Article: ] |
| 8. | Setchell KD, Heubi JE, Bove KE, O'Connell NC, Brewsaugh T, Steinberg SJ, Moser A, Squires RH. Liver disease caused by failure to racemize trihydroxycholestanoic acid: gene mutation and effect of bile acid therapy. Gastroenterology. 2003;124:217-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Une M, Konishi M, Suzuki Y, Akaboshi S, Yoshii M, Kuramoto T, Fujimura K. Bile acid profiles in a peroxisomal D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein deficiency. J Biochem. 1997;122:655-658. [PubMed] [Cited in This Article: ] |
| 10. | Ferdinandusse S, Ylianttila MS, Gloerich J, Koski MK, Oostheim W, Waterham HR, Hiltunen JK, Wanders RJ, Glumoff T. Mutational spectrum of D-bifunctional protein deficiency and structure-based genotype-phenotype analysis. Am J Hum Genet. 2006;78:112-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Ferdinandusse S, Kostopoulos P, Denis S, Rusch H, Overmars H, Dillmann U, Reith W, Haas D, Wanders RJ, Duran M. Mutations in the gene encoding peroxisomal sterol carrier protein X (SCPx) cause leukencephalopathy with dystonia and motor neuropathy. Am J Hum Genet. 2006;78:1046-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Niwa T. Mass spectrometry in hepatic diseases. Clin Chim Acta. 1995;241-242:253-289. [PubMed] [Cited in This Article: ] |
| 13. | Scalia S. Bile acid separation. J Chromatogr B Biomed Appl. 1995;671:299-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Roda A, Piazza F, Baraldini M. Separation techniques for bile salts analysis. J Chromatogr B Biomed Sci Appl. 1998;717:263-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Setchell KD, Lawson AM, Tanida N, Sjövall J. General methods for the analysis of metabolic profiles of bile acids and related compounds in feces. J Lipid Res. 1983;24:1085-1100. [PubMed] [Cited in This Article: ] |
| 16. | Gan-Schreier H, Okun JG, Kohlmueller D, Langhans CD, Peters V, Ten Brink HJ, Verhoeven NM, Jakobs C, Voelkl A, Hoffmann GF. Measurement of bile acid CoA esters by high-performance liquid chromatography-electrospray ionisation tandem mass spectrometry (HPLC-ESI-MS/MS). J Mass Spectrom. 2005;40:882-889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Buchmann MS, Kvittingen EA, Nazer H, Gunasekaran T, Clayton PT, Sjövall J, Björkhem I. Lack of 3 beta-hydroxy-delta 5-C27-steroid dehydrogenase/isomerase in fibroblasts from a child with urinary excretion of 3 beta-hydroxy-delta 5-bile acids. A new inborn error of metabolism. J Clin Invest. 1990;86:2034-2037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Cheng JB, Jacquemin E, Gerhardt M, Nazer H, Cresteil D, Heubi JE, Setchell KD, Russell DW. Molecular genetics of 3beta-hydroxy-Delta5-C27-steroid oxidoreductase deficiency in 16 patients with loss of bile acid synthesis and liver disease. J Clin Endocrinol Metab. 2003;88:1833-1841. [PubMed] [Cited in This Article: ] |
| 19. | Lemonde HA, Custard EJ, Bouquet J, Duran M, Overmars H, Scambler PJ, Clayton PT. Mutations in SRD5B1 (AKR1D1), the gene encoding delta(4)-3-oxosteroid 5beta-reductase, in hepatitis and liver failure in infancy. Gut. 2003;52:1494-1499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Verrips A, Hoefsloot LH, Steenbergen GC, Theelen JP, Wevers RA, Gabreëls FJ, van Engelen BG, van den Heuvel LP. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain. 2000;123:908-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 183] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Berginer VM, Salen G, Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med. 1984;311:1649-1652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 254] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Clayton PT, Patel E, Lawson AM, Carruthers RA, Tanner MS, Strandvik B, Egestad B, Sjövall J. 3-Oxo-delta 4 bile acids in liver disease. Lancet. 1988;1:1283-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 1.9] [Reference Citation Analysis (0)] |