Published online Dec 14, 2011. doi: 10.3748/wjg.v17.i46.5049
Revised: July 14, 2011
Accepted: July 21, 2011
Published online: December 14, 2011
Adhesions are the most frequent complication of abdominopelvic surgery, yet the extent of the problem, and its serious consequences, has not been adequately recognized. Adhesions evolved as a life-saving mechanism to limit the spread of intraperitoneal inflammatory conditions. Three different pathophysiological mechanisms can independently trigger adhesion formation. Mesothelial cell injury and loss during operations, tissue hypoxia and inflammation each promotes adhesion formation separately, and potentiate the effect of each other. Studies have repeatedly demonstrated that interruption of a single pathway does not completely prevent adhesion formation. This review summarizes the pathogenesis of adhesion formation and the results of single gene therapy interventions. It explores the promising role of combinatorial gene therapy and vector modifications for the prevention of adhesion formation in order to stimulate new ideas and encourage rapid advancements in this field.
- Citation: Atta HM. Prevention of peritoneal adhesions: A promising role for gene therapy. World J Gastroenterol 2011; 17(46): 5049-5058
- URL: https://www.wjgnet.com/1007-9327/full/v17/i46/5049.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i46.5049
Peritoneal adhesions are abnormal deposits of fibrous tissue that occur in the peritoneal cavity as a result of surgery or peritonitis, or their combination. Adhesions occur in more than 90% of patients following abdominal or pelvic surgery[1-3], and are only moderately reduced after laparoscopic surgical procedures compared to open surgery[4-8]. Adhesion reformation occurs postoperatively in 85% of patients, regardless of whether the adhesiolysis is performed via laparotomy or laparoscopy[9].
Intraperitoneal adhesions are a major source of morbidity, being the commonest cause of intestinal obstruction[10,11], secondary female infertility, and ectopic gestation[12,13]. They may also cause chronic abdominal and pelvic pain[14,15]. Small bowel obstruction is the most serious consequence of intra-abdominal adhesions. Retrospective studies have shown that 32%-75% of patients who require abdominal re-operation have adhesion-related intestinal obstruction[16,17].
Adhesions result in a large surgical workload and cost to health care systems. An epidemiological study in the United States showed that 282 000 hospital admissions in 1988 were due to adhesion-related disorders, and the cost of in-patient adhesiolysis was $1.18 billion[18]. In 1994, 1% of all United States admissions involved adhesiolysis treatment, resulting in $1.33 billion in health care expenditure[19].
Adhesions and their associated complications are of rising medico-legal interest. Physicians worldwide need to be aware of the increasing burden of medico-legal claims arising from the complications of intra-abdominal adhesions. Successful medico-legal claims include cases of bowel perforation after laparoscopic division of adhesions, delays in the diagnosis of adhesion obstruction of the small bowel, infertility as a result of adhesions, and pain[20].
Currently, there is no effective method for preventing adhesion formation or reformation[21]. A better understanding of the pathogenesis of adhesion formation at the cellular and molecular level would undoubtedly help to develop more effective treatment strategies[3].
The peritoneum is lined by mesothelial cells loosely attached to the basement membrane, which can readily be detached by the slightest trauma[22]. After injury to the peritoneum, a local inflammatory reaction causes increased vascular permeability in blood vessels supplying the damaged area, followed by an exudation of serosanguinous fluid rich in fibrin and inflammatory cells, ultimately leading to the formation of a fibrin matrix. Normally, the plasminogen activator activity (PAA), which resides in the mesothelial cells and submesothelial fibroblasts, degrades the fibrinous mass, resulting in healing of peritoneal surfaces (within three to five days) without adhesions. However, if the level of PAA is diminished, the fibrinous mass persists and the underlying fibroblasts migrate into the fibrinous mass. The fibroblasts then deposit extracellular matrix, including collagen and fibronectin, leading to adhesion formation. Over time, the adhesion may provide the framework for vascular ingrowth, during the process of angiogenesis[3,23,24].
The pathogenesis of adhesions involves three important trauma-induced processes (Figure 1): (1) trauma induces inhibition of the fibrinolytic and extracellular matrix (ECM) degradation systems[25,26]; (2) trauma, as well as foreign bodies, incites an inflammatory response with the production of cytokines, mainly transforming growth factor-β (TGF-β1), a key regulator of tissue fibrosis[27-29]; and (3) trauma also induces tissue hypoxia as a result of interruption of the blood supply to mesothelial cells and submesothelial fibroblasts, leading to increased expression of hypoxia inducible factor-1 α (HIF-1α)[30,31] and vascular endothelial growth factor (VEGF), responsible for collagen formation and angiogenesis[32].
Molecular pathways involved in fibrinolysis inhibition, inflammation, and tissue hypoxia crosstalk and potentiate the effect of each. The principal molecular aberrations included in this crosstalk are the reduction of tissue plasminogen activator (tPA) and upregulation of TGF-β1 and HIF-1α.
The role of fibrinolysis in adhesion formation/reformation is to breakdown the fibrin clots that are formed during the healing process. The inactive proenzyme, plasminogen, is converted to plasmin by the action of tPA. Plasmin degrades fibrin and thus limits adhesion formation. Experimental and clinical studies have identified the presence of PAA in the mesothelium[33,34] and that tPA is the major (95%) physiological mediator of PAA[35,36]. Both mechanical and chemical injury reduce peritoneal PAA, with a progressive reduction in PAA in the first hours following a surgical operation, followed by complete loss of fibrinolytic activity up to 72 h after the operation[37,38]. Laparoscopic surgery also decreases peritoneal tPA[39]. This reduction in PAA is the result of reduced tPA production and increased release of plasminogen activator inhibitors 1 and 2 (PAI-1, PAI-2) by mesothelial, endothelial, and inflammatory cells[3,40]. Extensive human and animal studies confirmed the central role of altered tPA/PAI-1 balance in adhesion formation and demonstrated that this imbalance is more exaggerated in severe adhesions[25,26,34,40].
Plasmin also activates latent matrix metalloproteinases (MMPs) involved in extracellular matrix (ECM) degradation. The proteolytic activity of MMPs is regulated in part by their physiological inhibitors, tissue inhibitors of MMPs (TIMPs). It has been shown that MMPs and TIMPs are expressed in the human peritoneum, in adhesion fibroblasts, and in serosal layers of several intraperitoneal organs with and without adhesions[3,41,42]. Chegini et al[43] demonstrated that serosal tissue of intraperitoneal organs obtained during open surgery expresses more tissue inhibitor metalloproteinase-1 (TIMP-1) than matrix metalloproteinase-1 (MMP-1), and that adhesions express elevated levels of TIMP-1 and a lower ratio of MMP-1 to TIMP-1 compared with intact parietal peritoneum. The association between the imbalance of MMP/TIMP production and adhesion formation has been confirmed in another study in women undergoing laparoscopy[29].
Several studies have demonstrated that during the acute phase of the inflammatory response, mesothelial cells and peritoneal macrophages produce a variety of cytokines, including TGF-β1, tumor necrosis factor α (TNF-α), interleukin-1 (IL-1), and IL-6. These pro-inflammatory cytokines, individually and synergistically, stimulate the production of PAI-1 and reduce the synthesis of tPA by human mesothelial cells (Figure 1)[3,44-46]. TGF-β not only interacts with the fibrinolytic system and ECM, but also with many other cellular mediators involved in the process of adhesion formation. TGF-β1 overexpression by the peritoneum, as well as increased concentrations of TGF-β in the peritoneal fluid, has been associated with increased incidence of adhesion formation in both humans and animals[3,27,47,48].
Several studies demonstrated that increased TGF-β1 is associated with a reduction of tPA and an increase of PAI-1 release[27,49,50], an excess of TGF-β1 leads to an increase in the severity of adhesions formed[27,51], whereas an inhibitory antibody to TGF-β1 decreased adhesion formation[52]. TGF-β1 contributes to the synthesis of the ECM by stimulating fibroblastic cell production of collagen and fibronectin[53-55]. TGF-β also antagonizes ECM resorption by decreasing the activity of MMPs through decreasing MMP-1 and increasing TIMP-1 expression from mesothelial cells[3,56,57]. This impairment of MMP activity prevents the ECM deposition that occurs early in wound healing from being adequately remodeled and degraded when necessary, as healing progresses.
Several lines of evidence have demonstrated that peritoneal tissue hypoxia plays a key role in adhesion formation[58-61]. During laparotomy, tissue injury including trauma, desiccation, and vascular disruption (due to ligatures and other vascular hemostatic methods, including cauterization) reduces oxygen supply to the peritoneum. Laparoscopic surgery was shown to be less adhesiogenic, not only because it is less traumatic, but also due to elevated peritoneal tissue oxygen tension levels compared to those during laparotomy[58]. However, adhesions during laparoscopic surgery increase with duration of pneumoperitoneum and with insufflation pressure. These effects were attributed to desiccation and compression of the capillary flow in the superficial peritoneal layers by the pneumoperitoneum. The addition of oxygen to the insufflation gas decreases adhesion formation[59]. Moreover, supplemental perioperative oxygen was found to increase peritoneal tissue oxygen tension and to reduce the severity of adhesions[61].
Hypoxia negatively modulates all pathways involved in adhesion formation. Hypoxia decreases tPA and increases PAI expression in human peritoneal fibroblasts in vitro[62] and in peritoneal tissues in vivo[61], thereby decreasing plasmin, inhibiting lysis of fibrin, and increasing adhesion formation. The PAI-1 gene contains oxygen responsive promoter sequences, namely hypoxia response element (HRE-1 and HRE-2), to which HIF-1α binds and induces gene expression[63]. Hypoxia was found to increase expression of TIMP-1, but not MMP-1, in both peritoneal and adhesion fibroblasts[55], thus decreasing matrix degradation. Hypoxic conditions in cultured human mesothelial cells and peritoneal fibroblasts also increased the expression of TGF-β1[55,64,65]. Hypoxia resulted in increased expression of collagen 1 mRNA in both peritoneal and adhesion fibroblasts[55,66], probably through the production of superoxide[66]. Moreover, hypoxia induces proliferation while inhibiting apoptosis in fibroblasts from adhesion, thus favoring adhesion formation[67]. Finally, hypoxia increases VEGF production through activation of HIF-1α in normal and adhesion fibroblasts in vitro[68] and in vivo in human adhesion mesothelial cells[69] and in animal adhesion tissues[30,70]. VEGF plays a central role in angiogenesis and its role in adhesion blood vessel development has been established[71]. Furthermore, TGF-β1 stimulates VEGF and connective tissue growth factor (CTGF) expression[72]. CTGF stimulates increased expression of ECM fibronectin, collagen 1, and laminin, while CTGF knockdown inhibits ECM production induced by TGF-β1 in human mesothelial cells[73].
Single therapeutic strategies have failed to completely prevent peritoneal adhesions because of the multifactorial nature of adhesion pathogenesis[74]. As these multifactorial etiologies act independently and synergisctically in adhesion formation, it is imperative to simultaneously address the major molecular aberrations, including reduction of tPA and upregulation of TGF-β1 and HIF-1α, for any therapeutic strategy to be successful. The current preventive approaches of reducing surgical trauma, use of physical barriers or administration of single pharmacological agent or gene therapy have all failed to achieve satisfactory results.
The general surgical precautions aiming at minimizing surgical trauma include meticulous surgical techniques, delicate purposeful tissue handling, achieving optimal hemostasis, minimizing the risk of infection, and avoiding contaminants (e.g., fecal matter) and the use of foreign materials (e.g., talcum powder) when possible[74,75]. However, these surgical techniques alone are not effective.
Physical barriers work by separating surgically injured tissues during the initial postoperative time period while remesothelization is occurring, a process that is usually expected to take three to five days[74]. Currently, three barriers, Interceed® (Johnson and Johnson, Gynecare, Somerville, NJ), Seprafilm® (Genzyme, Cambridge, MA), and ADEPT® (Baxter, Deerfield, IL), are approved by the Food and Drug Administration (FDA) for clinical use in the United States[74]. Although barriers have shown some success[74,76], this experience is not universally confirmed[77]. In fact, the FDA warns surgeons that when Interceed is used laparoscopically, patients have more adhesions than patients in the control group[76]. In the United States, ADEPT is only approved for laparoscopic gynecological surgery, and is contraindicated for patients with infection or allergies to cornstarch, as well as procedures involving laparotomy incision, bowel resection, or appendectomy. If used in these contraindicated procedures, patient may experience dehiscence, cutaneous fistula formation, anastomotic failure, ileus, and/or peritonitis. Thus, application and adoption of this product have been very limited[76]. Furthermore, the surgeon must predict the potential sites of adhesion formation in order to determine the placement site and to optimize barrier function[78].
A multitude of pharmacological agents, including recombinant proteins and antibodies, have demonstrated moderate success in reducing adhesion formation in different experimental adhesion models. These agents are applied locally into the peritoneal cavity, and work by correcting aberrant molecular pathways operative during adhesion development. One of the most extensively studied pharmacological agents that has demonstrated consistent success is recombinant tPA (reviewed in Ref 92)[79-92]. Experimental studies have reported reduction in adhesion formation and reformation using intraperitoneal recombinant human tPA in a variety of delivery methods and preparations, without impairing the healing of bowel anastomosis and without reduction in wound strength or causing hemorrhagic complications[79,80,92,93]. The action of tPA is localized to fibrin deposits; therefore, fibrinolytic activity is limited to this site, which prevents indiscriminate fibrinolysis[90]. Similar experiences were obtained in studies using neutralizing antibodies for PAI-1[94], TGFβ-1[45,52,95], TNF-α and IL-1[96], IL-6[97], and for VEGF[98,99]. However, these agents have short half-lives (few minutes) limiting their fibrinolytic effect for a sufficient duration of time (three to five days) until complete healing of peritoneal surfaces[92,100].
Local molecular therapy is inherently limited; therefore, an alternative strategy using gene therapy has been recently employed to correct molecular aberrations induced by surgical trauma in a regulated manner during the period of remesothelialization. Postoperative peritoneal adhesion is an attractive target for gene therapy because of several inherent biological features. The disease is localized to the site of peritoneal trauma and develops over a short period of time, extending for the first few days following surgical trauma. These characteristics lend themselves perfectly to gene therapy using non-integrating vectors. The vector can be applied locally following completion of the operation, and the short duration of gene expression would cover the period of altered molecular aberrations (e.g., depressed tPA, elevated PAI-1, TGF-β1, HIF-1α, etc.) following surgery. Nevertheless, gene therapy for peritoneal adhesions is still in its infancy, with very few in vivo studies reported in the literature (Table 1).
| Ref. | Vector/dose | Nucleic acid | Adhesion reduction (%) |
| Atta et al[101] | Adenovirus, 5 × 107 pfu | tPA gene | 34 |
| Guo et al[102] | Plasmid, 100 μg, sonoporation | Smad7 gene | 37 |
| Guo et al[103] | Adenovirus | Sphingosine kinase-1 gene | 62 |
| Segura et al[31] | Polyethylenimine cationic polymer, 2-4 nmol | siRNA HIF-1α | 36 to 52 |
| siRNA PAI-1 | |||
| Liu et al[104] | Adenovirus, 1 × 109 pfu | HGF gene | 56 |
Using different vectors, the five gene therapy studies reported in the literature were able to express therapeutic nucleic acids (transgenes or small interfering RNA) in the peritoneal tissues after intra-peritoneal administration in a rat adhesion model for at least seven days post-administration[31,101-104]. This duration of expression is enough to cover the time required for complete healing of the mesothelial cell layer of the peritoneum. The mechanism of adhesion reduction differed among these studies. Two studies showed that an adenovirus encoding the genes of hepatocyte growth factor (HGF) itself or its downstream signaling molecule sphingosine kinase 1 (SK-1) could achieve adhesion reduction via a stimulatory effect on proliferation and migration of mesothelial cells[103,104]. The altered tPA/PAI-1 balance occupies a central role in adhesion formation and two studies tackled this molecular imbalance. Atta et al[101] used an adenovirus vector encoding human tPA, while Segura et al[31] employed a cationic polymer containing siRNAs to PAI-1 and HIF-1α to downregulate PAI-1, either directly or through its gene inducer, HIF-1α. The expression of TGF-β1 was attenuated by overexpressing its down-stream Smad2/3 natural inhibitor Smad7 using plasmid vector[102].
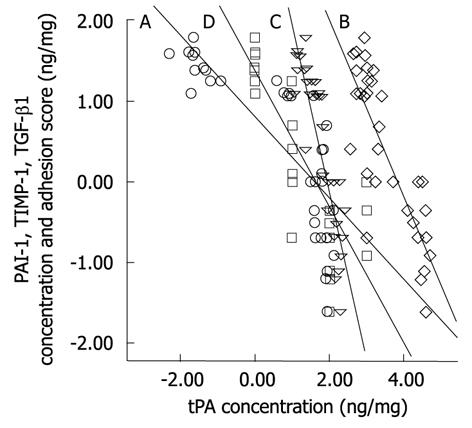
The moderate success in adhesion reduction in these gene therapy studies supports the concept that the multifactorial nature of molecular aberration during adhesion formation should be collectively and simultaneously addressed for any therapeutic strategy to be effective. This concept does not contradict, but reinforces, the established hypothesis of the central role of tPA reduction following surgical trauma in adhesion formation. In a recent study of adhesion prevention from our laboratory using an adenovirus vector encoding human tPA, we verified that overexpression of human tPA resulted in abrogation of the elevated fibrogenic molecules PAI-1, TIMP-1, and TGF-β1. The study showed that the reduction of these molecules depends on the concentration of the expressed human tPA protein[101]. Further analysis showed that there are significant negative correlations (at 0.01 level, 2-tailed) between human tPA and either of PAI-1 (Spearman’s r = -0.89), TIMP-1 (r = -0.73), TGF-β1 (r = -0.87), or adhesion score (r = -0.87) (Figure 2).
Gene therapy for the prevention of peritoneal adhesions has not been fully explored. Two potential developments for safe and effective gene therapy studies for adhesion prevention include combinatorial gene therapy and vector modifications.
The multifactorial nature of adhesion formation proposes that a combinatorial gene therapy would be more efficacious than a single gene therapy approach. For example, this could include overexpression of the fibrinolytic tPA gene together with downregulation of fibrogenic genes, such as TGF-β1 and/or HIF-1α. Overexpression is achieved through delivery of an exogenous gene, while downregulation is accomplished by the delivery of small interfering RNA (siRNA) molecules. Upon delivery, siRNAs complement with specific mRNAs resulting in their degradation, thus enabling the specific silencing of a single gene at the cellular level. As discussed above, single gene overexpression or silencing was moderately successful in reducing experimental adhesions[31,101]. The synergistic effects from the combined fibrinolysis stimulation and fibrogenesis inhibition, however, remain to be confirmed.
Delivery of therapeutic nucleic acid molecules to target tissues is accomplished using either viral vectors or nonviral carrier systems. Replication-deficient recombinant adenovirus vectors have become the most widely used viral vectors for in vivo gene transfer[101]. Adenovirus vectors have many positive attributes, including their ability to provide efficient in vivo gene transfer to both dividing and non-dividing cells, their high in vivo stability, and their non integrating nature into the host genome. These merits make adenoviral vectors suitable for proof-of-principle experimental studies. However, the clinical application of virus-mediated gene delivery in vivo is hampered by virus-induced acute inflammation, which could be fatal, high immunogenicity, and low tissue specificity. The broad tropism of adenovirus allows the virus to infect many cell types and is responsible for virus dissemination to distant organs. Various modifications of adenoviral vectors are underway to enhance the targeting of adenoviral vectors towards adhesion fibroblasts, which will provide effective and safe methods for localized treatment of postoperative peritoneal adhesions.
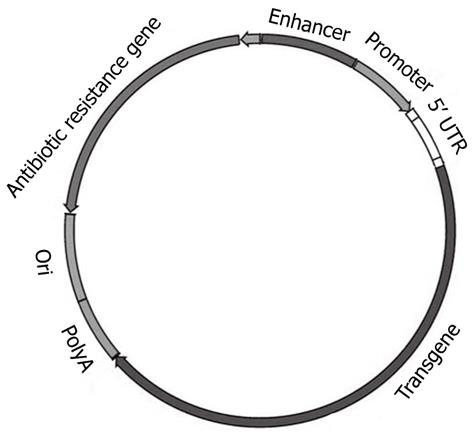
Given the unresolved safety limitations of viral vectors, significant research efforts have been directed towards the development of non-viral (plasmid-based) delivery systems. Plasmids are extrachromosomal genetic elements able to replicate autonomously and to be maintained in a host cell. Plasmids, the most basic forms of non-viral gene therapy, carry two main units: a eukaryotic transcription cassette and the bacterial amplification unit (Figure 3). The first bears genetic elements intended for gene expression in mammalian (eukaryotic) cells, such as the enhancer/promoter sequences for gene expression; 5′ untranslated region (5′ UTR), the gene of interest and polyadenylation (polyA) sequence. The bacterial amplification unit commonly contains an origin for plasmid DNA replication (ori) in bacteria and, generally, an antibiotic resistance selection marker[105]. Plasmids do not enter cells efficiently because of their large size, hydrophilic nature (due to negatively charged phosphate groups), and their susceptibility to nuclease-mediated degradation. Plasmid DNA and siRNA are stable for only 0.5 and 2 h, respectively in human serum[106]. Plasmids can be delivered to cells either naked (carrier-free) by direct injection, electroporation, ultrasound etc., or complexed with cationic lipids (lipoplexes), cationic polymers (polyplexes), peptides or inorganic nanoparticles[107]. Two promising recent modifications of plasmid vectors, minicircles and CpG-depleted vectors, are briefly discussed below. Evaluating the rapid progress in the field of cationic liposomes and polymers is, however, beyond the scope of this brief review, and the reader is referred to excellent recent reviews[107,108].
Limitations of conventional DNA plasmid vectors are related to their size (> 3 kb). Moreover, bacterial sequences contain immunotoxic cytidine-phosphate-guanosine (CpG) dinucleotides motifs, which are approximately four times more prevalent in bacterial than mammalian DNA. Bacterial CpG dinucleotides have been identified to be major contributors to the low and short-lived transgene expression (transgene silencing) in vertebrates after non-viral gene delivery. These bacterial sequences can also interfere with short hairpin RNA (shRNA, precursor of siRNA) expression[106,109]. To overcome these limitations, highly safe and efficient vector systems for gene transfer in eukaryotic cells called minivectors (minicircles) were developed[110]. Minicircles are supercoiled minimal expression cassettes, derived from conventional plasmid DNA by site-specific recombination in vivo in Escherichia coli. As a result, two well-defined circular molecules are generated from the parent conventional plasmid, termed minicircle (mammalian expression cassette) and miniplasmid (bacterial backbone elements). Further purification of the minicircle renders it therapeutically applicable[105]. Thus, minicircle DNA lacks the bacterial backbone sequence consisting of an antibiotic resistance gene and an origin of replication. Minicircle DNA is low in immunogenicity due to its lower content of bacterial unmethylated CpG dinucleotides. In addition to their improved safety profile, minicircles have been shown to greatly increase the efficiency of transgene expression in various in vitro and in vivo studies, compared to the conventional plasmid with the same transgene expression cassette. It has been reported that a minivector incorporating short hairpin RNA efficiently transfected adhesion fibroblasts and was shown to be stable in human serum for > 48 h[107].
Bacterial DNA is rich in unmethylated CpG dinucleotides, in contrast to mammalian DNA, which contains a low frequency of CpG dinucleotide, which are mostly methylated. Recognition of unmethylated CpGs present in the bacterial backbone could trigger an innate immune response following detection in the endosome by toll-like receptor 9 (TLR9)[109] and initiate a signaling cascade, leading to the production of proinflammatory cytokines. As plasmids used in in vivo gene therapy studies are produced in Escherichia coli (E. coli), their CpGs are unmethylated and induce immune responses through this host defense mechanism. Recently, plasmids that are completely devoid of CpG dinucleotides have been developed. These plasmids yield high levels of transgene expression both in vitro and in vivo, and, in contrast to CMV-based plasmids, allow sustained expression in vivo[111,112]. In these CpG-free plasmids, all elements required for replication and selection of the plasmid in E. coli and for gene expression in mammalian cells (e.g., promoter, polyadenylation signal, reporter gene, etc.) either naturally lack CpG dinucleotides, were modified to remove all CpGs, or are entirely synthesized.
Gene therapy for the prevention of postoperative peritoneal adhesions is still in its infancy. The potential applications of this strategy have not been fully explored. The recent explosive progress in advanced nonviral gene delivery systems, coupled with the newly developed less immunogenic and more efficient expression plasmids, will undoubtedly accelerate research studies in gene therapy for peritoneal adhesions.
Peer reviewer: Michael E Zenilman, MD, Clarence and Mary Dennis Professor and Chairman, Department of Surgery, SUNY Downstate Medical Center, PO Box 40, 450 Clarkson Avenue, Brooklyn, NY 11202, United States
S- Editor Sun H L- Editor Stewart GJ E- Editor Zhang DN
| 1. | Parker MC, Wilson MS, van Goor H, Moran BJ, Jeekel J, Duron JJ, Menzies D, Wexner SD, Ellis H. Adhesions and colorectal surgery - call for action. Colorectal Dis. 2007;9 Suppl 2:66-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 443] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 3. | Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001;7:556-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 228] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Gutt CN, Oniu T, Schemmer P, Mehrabi A, Büchler MW. Fewer adhesions induced by laparoscopic surgery? Surg Endosc. 2004;18:898-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Krähenbühl L, Schäfer M, Kuzinkovas V, Renzulli P, Baer HU, Büchler MW. Experimental study of adhesion formation in open and laparoscopic fundoplication. Br J Surg. 1998;85:826-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Garrard CL, Clements RH, Nanney L, Davidson JM, Richards WO. Adhesion formation is reduced after laparoscopic surgery. Surg Endosc. 1999;13:10-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Polymeneas G, Theodosopoulos T, Stamatiadis A, Kourias E. A comparative study of postoperative adhesion formation after laparoscopic vs open cholecystectomy. Surg Endosc. 2001;15:41-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Molinaro F, Kaselas C, Lacreuse I, Moog R, Becmeur F. Postoperative intestinal obstruction after laparoscopic versus open surgery in the pediatric population: A 15-year review. Eur J Pediatr Surg. 2009;19:160-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod Update. 2001;7:567-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Dijkstra FR, Nieuwenhuijzen M, Reijnen MM, van Goor H. Recent clinical developments in pathophysiology, epidemiology, diagnosis and treatment of intra-abdominal adhesions. Scand J Gastroenterol Suppl. 2000;52-59. [PubMed] [Cited in This Article: ] |
| 11. | Al-Jaroudi D, Tulandi T. Adhesion prevention in gynecologic surgery. Obstet Gynecol Surv. 2004;59:360-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Alpay Z, Saed GM, Diamond MP. Female infertility and free radicals: potential role in adhesions and endometriosis. J Soc Gynecol Investig. 2006;13:390-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Trimbos-Kemper TC, Trimbos JB, van Hall EV. Adhesion formation after tubal surgery: results of the eighth-day laparoscopy in 188 patients. Fertil Steril. 1985;43:395-400. [PubMed] [Cited in This Article: ] |
| 14. | Kresch AJ, Seifer DB, Sachs LB, Barrese I. Laparoscopy in 100 women with chronic pelvic pain. Obstet Gynecol. 1984;64:672-674. [PubMed] [Cited in This Article: ] |
| 15. | Sutton C, MacDonald R. Laser laparoscopic adhesiolysis. J Gynecol Surg. 1990;6:155-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D, McGuire A, Lower AM, Hawthorn RJ, O'Brien F. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353:1476-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 651] [Cited by in F6Publishing: 617] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 17. | McEntee G, Pender D, Mulvin D, McCullough M, Naeeder S, Farah S, Badurdeen MS, Ferraro V, Cham C, Gillham N. Current spectrum of intestinal obstruction. Br J Surg. 1987;74:976-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 120] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Ray NF, Larsen JW, Stillman RJ, Jacobs RJ. Economic impact of hospitalizations for lower abdominal adhesiolysis in the United States in 1988. Surg Gynecol Obstet. 1993;176:271-276. [PubMed] [Cited in This Article: ] |
| 19. | Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 390] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Ellis H, Crowe A. Medico-legal consequences of post-operative intra-abdominal adhesions. Int J Surg. 2009;7:187-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Wiseman DM, Trout JR, Diamond MP. The rates of adhesion development and the effects of crystalloid solutions on adhesion development in pelvic surgery. Fertil Steril. 1998;70:702-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Raftery AT. Regeneration of parietal and visceral peritoneum: an electron microscopical study. J Anat. 1973;115:375-392. [PubMed] [Cited in This Article: ] |
| 23. | diZerega GS, Campeau JD. Peritoneal repair and post-surgical adhesion formation. Hum Reprod Update. 2001;7:547-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 243] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Alpay Z, Saed GM, Diamond MP. Postoperative adhesions: from formation to prevention. Semin Reprod Med. 2008;26:313-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Holmdahl L, Eriksson E, Eriksson BI, Risberg B. Depression of peritoneal fibrinolysis during operation is a local response to trauma. Surgery. 1998;123:539-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Ivarsson ML, Bergström M, Eriksson E, Risberg B, Holmdahl L. Tissue markers as predictors of postoperative adhesions. Br J Surg. 1998;85:1549-1554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Holmdahl L, Kotseos K, Bergström M, Falk P, Ivarsson ML, Chegini N. Overproduction of transforming growth factor-beta1 (TGF-beta1) is associated with adhesion formation and peritoneal fibrinolytic impairment. Surgery. 2001;129:626-632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Chegini N, Kotseos K, Zhao Y, Bennett B, McLean FW, Diamond MP, Holmdahl L, Burns J. Differential expression of TGF-beta1 and TGF-beta3 in serosal tissues of human intraperitoneal organs and peritoneal adhesions. Hum Reprod. 2001;16:1291-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Cheong YC, Shelton JB, Laird SM, Li TC, Ledger WL, Cooke ID. Peritoneal fluid concentrations of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1, and transforming growth factor-beta in women with pelvic adhesions. Fertil Steril. 2003;79:1168-1175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Molinas CR, Campo R, Elkelani OA, Binda MM, Carmeliet P, Koninckx PR. Role of hypoxia inducible factors 1alpha and 2alpha in basal adhesion formation and in carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in transgenic mice. Fertil Steril. 2003;80 Suppl 2:795-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Segura T, Schmokel H, Hubbell JA. RNA interference targeting hypoxia inducible factor 1alpha reduces post-operative adhesions in rats. J Surg Res. 2007;141:162-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Cahill RA, Wang JH, Soohkai S, Redmond HP. Mast cells facilitate local VEGF release as an early event in the pathogenesis of postoperative peritoneal adhesions. Surgery. 2006;140:108-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Thompson JN, Paterson-Brown S, Harbourne T, Whawell SA, Kalodiki E, Dudley HA. Reduced human peritoneal plasminogen activating activity: possible mechanism of adhesion formation. Br J Surg. 1989;76:382-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 110] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Holmdahl L, Falkenberg M, Ivarsson ML, Risberg B. Plasminogen activators and inhibitors in peritoneal tissue. APMIS. 1997;105:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Holmdahl L, Eriksson E, al-Jabreen M, Risberg B. Fibrinolysis in human peritoneum during operation. Surgery. 1996;119:701-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 110] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Scott-Coombes D, Whawell S, Vipond MN, Thompson J. Human intraperitoneal fibrinolytic response to elective surgery. Br J Surg. 1995;82:414-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Sulaiman H, Dawson L, Laurent GJ, Bellingan GJ, Herrick SE. Role of plasminogen activators in peritoneal adhesion formation. Biochem Soc Trans. 2002;30:126-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Rout UK, Diamond MP. Role of plasminogen activators during healing after uterine serosal lesioning in the rat. Fertil Steril. 2003;79:138-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Brokelman WJ, Holmdahl L, Janssen IM, Falk P, Bergström M, Klinkenbijl JH, Reijnen MM. Decreased peritoneal tissue plasminogen activator during prolonged laparoscopic surgery. J Surg Res. 2009;151:89-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Molinas CR, Elkelani O, Campo R, Luttun A, Carmeliet P, Koninckx PR. Role of the plasminogen system in basal adhesion formation and carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in transgenic mice. Fertil Steril. 2003;80:184-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Chegini N, Kotseos K, Bennett B, Diamond MP, Holmdahl L, Burns J. Matrix metalloproteinase (MMP-1) and tissue inhibitor of MMP in peritoneal fluids and sera and correlation with peritoneal adhesions. Fertil Steril. 2001;76:1207-1211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Martin J, Yung S, Robson RL, Steadman R, Davies M. Production and regulation of matrix metalloproteinases and their inhibitors by human peritoneal mesothelial cells. Perit Dial Int. 2000;20:524-533. [PubMed] [Cited in This Article: ] |
| 43. | Chegini N, Kotseos K, Zhao Y, Ma C, McLean F, Diamond MP, Holmdahl L, Burns J. Expression of matrix metalloproteinase (MMP-1) and tissue inhibitor of MMP in serosal tissue of intraperitoneal organs and adhesions. Fertil Steril. 2001;76:1212-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Haney AF. Identification of macrophages at the site of peritoneal injury: evidence supporting a direct role for peritoneal macrophages in healing injured peritoneum. Fertil Steril. 2000;73:988-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Gorvy DA, Herrick SE, Shah M, Ferguson MW. Experimental manipulation of transforming growth factor-beta isoforms significantly affects adhesion formation in a murine surgical model. Am J Pathol. 2005;167:1005-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Whawell SA, Thompson JN. Cytokine-induced release of plasminogen activator inhibitor-1 by human mesothelial cells. Eur J Surg. 1995;161:315-318. [PubMed] [Cited in This Article: ] |
| 47. | Chegini N, Rong H, Bennett B, Stone IK. Peritoneal fluid cytokine and eicosanoid levels and their relation to the incidence of peritoneal adhesion. J Soc Gynecol Investig. 1999;6:153-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Ghellai AM, Stucchi AF, Chegini N, Ma C, Andry CD, Kaseta JM, Burns JW, Skinner KC, Becker JM. Role of transforming growth factor beta-1 in peritonitis-induced adhesions. J Gastrointest Surg. 2000;4:316-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Falk P, Ma C, Chegini N, Holmdahl L. Differential regulation of mesothelial cell fibrinolysis by transforming growth factor beta 1. Scand J Clin Lab Invest. 2000;60:439-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Tietze L, Elbrecht A, Schauerte C, Klosterhalfen B, Amo-Takyi B, Gehlen J, Winkeltau G, Mittermayer C, Handt S. Modulation of pro- and antifibrinolytic properties of human peritoneal mesothelial cells by transforming growth factor beta1 (TGF-beta1), tumor necrosis factor alpha (TNF-alpha) and interleukin 1beta (IL-1beta). Thromb Haemost. 1998;79:362-370. [PubMed] [Cited in This Article: ] |
| 51. | Williams RS, Rossi AM, Chegini N, Schultz G. Effect of transforming growth factor beta on postoperative adhesion formation and intact peritoneum. J Surg Res. 1992;52:65-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Lucas PA, Warejcka DJ, Young HE, Lee BY. Formation of abdominal adhesions is inhibited by antibodies to transforming growth factor-beta1. J Surg Res. 1996;65:135-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Freeman ML, Saed GM, Elhammady EF, Diamond MP. Expression of transforming growth factor beta isoform mRNA in injured peritoneum that healed with adhesions and without adhesions and in uninjured peritoneum. Fertil Steril. 2003;80 Suppl 2:708-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Saed GM, Zhang W, Chegini N, Holmdahl L, Diamond MP. Alteration of type I and III collagen expression in human peritoneal mesothelial cells in response to hypoxia and transforming growth factor-beta1. Wound Repair Regen. 1999;7:504-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75:763-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Ma C, Tarnuzzer RW, Chegini N. Expression of matrix metalloproteinases and tissue inhibitor of matrix metalloproteinases in mesothelial cells and their regulation by transforming growth factor-beta1. Wound Repair Regen. 1999;7:477-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Chegini N. The role of growth factors in peritoneal healing: transforming growth factor beta (TGF-beta). Eur J Surg Suppl. 1997;17-23. [PubMed] [Cited in This Article: ] |
| 58. | Bourdel N, Matsuzaki S, Bazin JE, Pouly JL, Mage G, Canis M. Peritoneal tissue-oxygen tension during a carbon dioxide pneumoperitoneum in a mouse laparoscopic model with controlled respiratory support. Hum Reprod. 2007;22:1149-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Molinas CR, Mynbaev O, Pauwels A, Novak P, Koninckx PR. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril. 2001;76:560-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Saed GM, Galijasevic S, Diamond MP, Abu-Soud HM. Measurement of oxygen and nitric oxide levels in vitro and in vivo: relationship to postoperative adhesions. Fertil Steril. 2005;84:235-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Matsuzaki S, Canis M, Bazin JE, Darcha C, Pouly JL, Mage G. Effects of supplemental perioperative oxygen on post-operative abdominal wound adhesions in a mouse laparotomy model with controlled respiratory support. Hum Reprod. 2007;22:2702-2706. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Saed GM, Diamond MP. Modulation of the expression of tissue plasminogen activator and its inhibitor by hypoxia in human peritoneal and adhesion fibroblasts. Fertil Steril. 2003;79:164-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Kietzmann T, Samoylenko A, Roth U, Jungermann K. Hypoxia-inducible factor-1 and hypoxia response elements mediate the induction of plasminogen activator inhibitor-1 gene expression by insulin in primary rat hepatocytes. Blood. 2003;101:907-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Saed GM, Zhang W, Chegini N, Holmdahl L, Diamond MP. Transforming growth factor beta isoforms production by human peritoneal mesothelial cells after exposure to hypoxia. Am J Reprod Immunol. 2000;43:285-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Saed GM, Diamond MP. Hypoxia-induced irreversible up-regulation of type I collagen and transforming growth factor-beta1 in human peritoneal fibroblasts. Fertil Steril. 2002;78:144-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Fletcher NM, Jiang ZL, Diamond MP, Abu-Soud HM, Saed GM. Hypoxia-generated superoxide induces the development of the adhesion phenotype. Free Radic Biol Med. 2008;45:530-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Saed GM, Diamond MP. Apoptosis and proliferation of human peritoneal fibroblasts in response to hypoxia. Fertil Steril. 2002;78:137-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Diamond MP, El-Hammady E, Munkarah A, Bieber EJ, Saed G. Modulation of the expression of vascular endothelial growth factor in human fibroblasts. Fertil Steril. 2005;83:405-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Wiczyk HP, Grow DR, Adams LA, O'Shea DL, Reece MT. Pelvic adhesions contain sex steroid receptors and produce angiogenesis growth factors. Fertil Steril. 1998;69:511-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Molinas CR, Campo R, Dewerchin M, Eriksson U, Carmeliet P, Koninckx PR. Role of vascular endothelial growth factor and placental growth factor in basal adhesion formation and in carbon dioxide pneumoperitoneum-enhanced adhesion formation after laparoscopic surgery in transgenic mice. Fertil Steril. 2003;80 Suppl 2:803-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Epstein JC, Wilson MS, Wilkosz S, Ireland G, O'Dwyer ST, Herrick SE. Human peritoneal adhesions show evidence of tissue remodeling and markers of angiogenesis. Dis Colon Rectum. 2006;49:1885-1892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Szeto CC, Lai KB, Chow KM, Szeto CY, Wong TY, Li PK. Differential effects of transforming growth factor-beta on the synthesis of connective tissue growth factor and vascular endothelial growth factor by peritoneal mesothelial cell. Nephron Exp Nephrol. 2005;99:e95-e104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Xiao L, Sun L, Liu FY, Peng YM, Duan SB. Connective tissue growth factor knockdown attenuated matrix protein production and vascular endothelial growth factor expression induced by transforming growth factor-beta1 in cultured human peritoneal mesothelial cells. Ther Apher Dial. 2010;14:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Diamond MP, Wexner SD, diZereg GS, Korell M, Zmora O, Van Goor H, Kamar M. Adhesion prevention and reduction: current status and future recommendations of a multinational interdisciplinary consensus conference. Surg Innov. 2010;17:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | Robertson D, Lefebvre G, Leyland N, Wolfman W, Allaire C, Awadalla A, Best C, Contestabile E, Dunn S, Heywood M. Adhesion prevention in gynaecological surgery. J Obstet Gynaecol Can. 2010;32:598-608. [PubMed] [Cited in This Article: ] |
| 76. | Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J Surg Res. 2011;165:91-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 77. | Wallwiener D, Meyer A, Bastert G. Adhesion formation of the parietal and visceral peritoneum: an explanation for the controversy on the use of autologous and alloplastic barriers? Fertil Steril. 1998;69:132-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Cohen Z, Senagore AJ, Dayton MT, Koruda MJ, Beck DE, Wolff BG, Fleshner PR, Thirlby RC, Ludwig KA, Larach SW. Prevention of postoperative abdominal adhesions by a novel, glycerol/sodium hyaluronate/carboxymethylcellulose-based bioresorbable membrane: a prospective, randomized, evaluator-blinded multicenter study. Dis Colon Rectum. 2005;48:1130-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Buckenmaier CC, Summers MA, Hetz SP. Effect of the antiadhesive treatments, carboxymethylcellulose combined with recombinant tissue plasminogen activator and Seprafilm, on bowel anastomosis in the rat. Am Surg. 2000;66:1041-1045. [PubMed] [Cited in This Article: ] |
| 80. | Doody KJ, Dunn RC, Buttram VC. Recombinant tissue plasminogen activator reduces adhesion formation in a rabbit uterine horn model. Fertil Steril. 1989;51:509-512. [PubMed] [Cited in This Article: ] |
| 81. | Dörr PJ, Vemer HM, Brommer EJ, Willemsen WN, Veldhuizen RW, Rolland R. Prevention of postoperative adhesions by tissue-type plasminogen activator (t-PA) in the rabbit. Eur J Obstet Gynecol Reprod Biol. 1990;37:287-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Dunn RC, Buttram VC. Tissue-type plasminogen activator as an adjuvant for post surgical adhesions. Prog Clin Biol Res. 1990;358:113-118. [PubMed] [Cited in This Article: ] |
| 83. | Dunn RC, Steinleitner AJ, Lambert H. Synergistic effect of intraperitoneally administered calcium channel blockade and recombinant tissue plasminogen activator to prevent adhesion formation in an animal model. Am J Obstet Gynecol. 1991;164:1327-1330. [PubMed] [Cited in This Article: ] |
| 84. | Evans DM, McAree K, Guyton DP, Hawkins N, Stakleff K. Dose dependency and wound healing aspects of the use of tissue plasminogen activator in the prevention of intra-abdominal adhesions. Am J Surg. 1993;165:229-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Gehlbach DL, O'Hair KC, Parks AL, Rosa C. Combined effects of tissue plasminogen activator and carboxymethylcellulose on adhesion reformation in rabbits. Int J Fertil Menopausal Stud. 1994;39:172-176. [PubMed] [Cited in This Article: ] |
| 86. | Lai HS, Chen Y, Chang KJ, Chen WJ. Tissue plasminogen activator reduces intraperitoneal adhesion after intestinal resection in rats. J Formos Med Assoc. 1998;97:323-327. [PubMed] [Cited in This Article: ] |
| 87. | Menzies D, Ellis H. Intra-abdominal adhesions and their prevention by topical tissue plasminogen activator. J R Soc Med. 1989;82:534-535. [PubMed] [Cited in This Article: ] |
| 88. | Montz FJ, Fowler JM, Wolff AJ, Lacey SM, Mohler M. The ability of recombinant tissue plasminogen activator to inhibit post-radical pelvic surgery adhesions in the dog model. Am J Obstet Gynecol. 1991;165:1539-1542. [PubMed] [Cited in This Article: ] |
| 89. | Orita H, Fukasawa M, Girgis W, diZerega GS. Inhibition of postsurgical adhesions in a standardized rabbit model: intraperitoneal treatment with tissue plasminogen activator. Int J Fertil. 1991;36:172-177. [PubMed] [Cited in This Article: ] |
| 90. | Vipond MN, Whawell SA, Scott-Coombes DM, Thompson JN, Dudley HA. Experimental adhesion prophylaxis with recombinant tissue plasminogen activator. Ann R Coll Surg Engl. 1994;76:412-415. [PubMed] [Cited in This Article: ] |
| 91. | Yeo Y, Bellas E, Highley CB, Langer R, Kohane DS. Peritoneal adhesion prevention with an in situ cross-linkable hyaluronan gel containing tissue-type plasminogen activator in a rabbit repeated-injury model. Biomaterials. 2007;28:3704-3713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Hellebrekers BW, Trimbos-Kemper TC, Trimbos JB, Emeis JJ, Kooistra T. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil Steril. 2000;74:203-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Menzies D, Ellis H. The role of plasminogen activator in adhesion prevention. Surg Gynecol Obstet. 1991;172:362-366. [PubMed] [Cited in This Article: ] |
| 94. | Falk K, Björquist P, Strömqvist M, Holmdahl L. Reduction of experimental adhesion formation by inhibition of plasminogen activator inhibitor type 1. Br J Surg. 2001;88:286-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 95. | Zhang Z, Garron TM, Li XJ, Liu Y, Zhang X, Li YY, Xu WS. Recombinant human decorin inhibits TGF-beta1-induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns. 2009;35:527-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 96. | Kaidi AA, Nazzal M, Gurchumelidze T, Ali MA, Dawe EJ, Silva YJ. Preoperative administration of antibodies against tumor necrosis factor-alpha (TNF-alpha) and interleukin-1 (IL-1) and their impact on peritoneal adhesion formation. Am Surg. 1995;61:569-572. [PubMed] [Cited in This Article: ] |
| 97. | Saba AA, Kaidi AA, Godziachvili V, Dombi GW, Dawe EJ, Libcke JH, Silva YJ. Effects of interleukin-6 and its neutralizing antibodies on peritoneal adhesion formation and wound healing. Am Surg. 1996;62:569-572. [PubMed] [Cited in This Article: ] |
| 98. | Ignjatovic D, Aasland K, Pettersen M, Sund S, Chen Y, Spasojevic M, Nesgaard JM. Intra-abdominal administration of bevacizumab diminishes intra-peritoneal adhesions. Am J Surg. 2010;200:270-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Saltzman AK, Olson TA, Mohanraj D, Carson LF, Ramakrishnan S. Prevention of postoperative adhesions by an antibody to vascular permeability factor/vascular endothelial growth factor in a murine model. Am J Obstet Gynecol. 1996;174:1502-1506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | Hellebrekers BW, Trimbos-Kemper TC, Boesten L, Jansen FW, Kolkman W, Trimbos JB, Press RR, van Poelgeest MI, Emeis SJ, Kooistra T. Preoperative predictors of postsurgical adhesion formation and the Prevention of Adhesions with Plasminogen Activator (PAPA-study): results of a clinical pilot study. Fertil Steril. 2009;91:1204-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Atta HM, Al-Hendy A, El-Rehany MA, Dewerchin M, Abdel Raheim SR, Abdel Ghany H, Fouad R. Adenovirus-mediated overexpression of human tissue plasminogen activator prevents peritoneal adhesion formation/reformation in rats. Surgery. 2009;146:12-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 102. | Guo H, Leung JC, Cheung JS, Chan LY, Wu EX, Lai KN. Non-viral Smad7 gene delivery and attenuation of postoperative peritoneal adhesion in an experimental model. Br J Surg. 2009;96:1323-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Guo Q, Li QF, Liu HJ, Li R, Wu CT, Wang LS. Sphingosine kinase 1 gene transfer reduces postoperative peritoneal adhesion in an experimental model. Br J Surg. 2008;95:252-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Liu HJ, Wu CT, Duan HF, Wu B, Lu ZZ, Wang L. Adenoviral-mediated gene expression of hepatocyte growth factor prevents postoperative peritoneal adhesion in a rat model. Surgery. 2006;140:441-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 105. | Rodríguez EG. Nonviral DNA vectors for immunization and therapy: design and methods for their obtention. J Mol Med (Berl). 2004;82:500-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 106. | Zhao N, Fogg JM, Zechiedrich L, Zu Y. Transfection of shRNA-encoding Minivector DNA of a few hundred base pairs to regulate gene expression in lymphoma cells. Gene Ther. 2011;18:220-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 107. | Al-Dosari MS, Gao X. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 2009;11:671-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 428] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 108. | Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2000;29:3477-3496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 627] [Cited by in F6Publishing: 568] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 109. | Gill DR, Pringle IA, Hyde SC. Progress and prospects: the design and production of plasmid vectors. Gene Ther. 2009;16:165-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 110. | Mayrhofer P, Schleef M, Jechlinger W. Use of minicircle plasmids for gene therapy. Methods Mol Biol. 2009;542:87-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 111. | Hattori K, Nishikawa M, Watcharanurak K, Ikoma A, Kabashima K, Toyota H, Takahashi Y, Takahashi R, Watanabe Y, Takakura Y. Sustained exogenous expression of therapeutic levels of IFN-gamma ameliorates atopic dermatitis in NC/Nga mice via Th1 polarization. J Immunol. 2010;184:2729-2735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 112. | Lesina E, Dames P, Rudolph C. The effect of CpG motifs on gene expression and clearance kinetics of aerosol administered polyethylenimine (PEI)-plasmid DNA complexes in the lung. J Control Release. 2010;143:243-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |