Published online Dec 7, 2011. doi: 10.3748/wjg.v17.i45.5021
Revised: April 26, 2011
Accepted: May 3, 2011
Published online: December 7, 2011
AIM: To investigate which surgical techniques and perioperative regimens yielded the best survival rates for diabetic rats undergoing gastric bypass.
METHODS: We performed Roux-en-Y gastric bypass with reserved gastric volume, a procedure in which gastrointestinal continuity was reestablished while excluding the entire duodenum and proximal jejunal loop. We observed the procedural success rate, long-term survival, and histopathological sequelae associated with a number of technical modifications. These included: use of anatomical markers to precisely identify Treitz’s ligament; careful dissection along surgical planes; careful attention to the choice of regional transection sites; reconstruction using full-thickness anastomoses; use of a minimally invasive procedure with prohemostatic pretreatment and hemorrhage control; prevention of hypothermic damage; reduction in the length of the procedure; and accelerated surgical recovery using fast-track surgical modalities such as perioperative permissive underfeeding and goal-directed volume therapy.
RESULTS: The series of modifications we adopted reduced operation time from 110.02 ± 12.34 min to 78.39 ± 7.26 min (P < 0.01), and the procedural success rate increased from 43.3% (13/30) to 90% (18/20) (P < 0.01), with a long-term survival of 83.3% (15/18) (P < 0.01).
CONCLUSION: Using a number of fast-track and damage control surgical techniques, we have successfully established a stable model of gastric bypass in diabetic rats.
- Citation: Han LO, Zhou LH, Cheng SJ, Song C, Song CF. Key details of the duodenal-jejunal bypass in type 2 diabetes mellitus rats. World J Gastroenterol 2011; 17(45): 5021-5027
- URL: https://www.wjgnet.com/1007-9327/full/v17/i45/5021.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i45.5021
When gastric bypass has been used to treat morbid obesity in patients with concomitant type 2 diabetes mellitus (T2DM), some of these patients have seen their diabetes resolve even before they have lost weight[1-3]. Available data suggest that reduced caloric intake alone is not sufficient to explain the observed short-term metabolic and endocrine effects[4,5]. Recently, Rubino et al[6] demonstrated that a duodenal-jejunal bypass (DJB) procedure that preserved gastric volume could directly benefit T2DM patients, suggesting that duodenal-jejunal exclusion, rather than the restriction or reduction of gastric volume, is the critical factor in surgical treatment of T2DM.
Understanding the mechanisms underlying this surprising phenomenon would enhance our understanding of the pathophysiology of T2DM, and potentially require us to revise our approach to treating this disease[7]. Therefore, as a first step, we chose to apply an existing rat model for DJB in fat-fed/STZ rats. In such a model, a range of surgical, perioperative, and other factors would be expected to influence the effect of bypass surgery on diabetes. However, there are few data regarding the influence of particular surgical modalities or non-surgical aspects of care on diabetic rats. Therefore, in this study, we carefully compared a number of both surgical techniques and perioperative management regimens for their ability to improve the success rate in diabetic rats underlying DJB, including minimally invasive procedural designs and the use of damage control and fast-track surgical modalities.
All animal use was in compliance with the regulations set out by the institutional animal research committee at Harbin Medical University. Male Wistar rats, 8 wk of age and weighing between 200 and 230 g, were purchased from CAAS Harbin Veterinary Research Institute [Approval NO. SCXK (HLJ) 2006-009]. The rats were given a high-fat diet for 8 wk, followed with an intraperitoneal injection of streptozotocin (30 mg/kg). After 72 h of treatment, rats with a non-fasting blood glucose level above 300 mg/dL (16.7 mmol/L), as measured by an electronic glucometer (One Touch Ultra, Lifescan, Johnson and Johnson, Milpitas, CA), were considered diabetic[8,9].
Wistar rats (n = 50) were randomly assigned for either conventional DJB (n = 30) or modified DJB (n = 20). The modification consisted of an improved surgical technique and postoperative care regimen, each of which was based on a prior analysis of the histopathological sequelae in failed cases of treatment with conventional DJB. Rats surviving more than 4 wk postoperatively were considered to constitute an operative success. The operative duration was recorded for rats in both groups, as was the mean plasma glucose level (PGL) both preoperatively and 4 wk postoperatively.
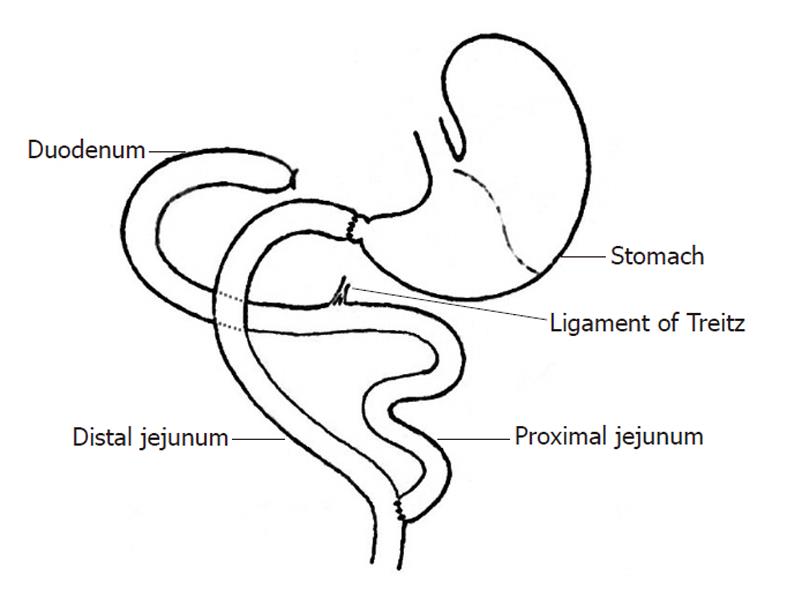
After fasting (12 h) and water deprivation (4 h), rats were anesthetized with 0.5% pelltobarbitalum natricum (30 mg/kg). After a median longitudinal incision was made in the upper abdomen, a Roux-en-Y duodenal-jejunal bypass that preserved the gastric volume while excluding the entire duodenum and proximal jejunal loop was constructed. First, after transecting the pylorus, the distal duodenal end was closed. The jejunum was transected 8 cm distal to Treitz’s ligament, and the distal jejunal loop was connected via an end-to-end anastomosis to the free end of the pylorus[10]. Gastrointestinal continuity was reestablished via an end-to-side anastomosis between the proximal end of the jejunum and the distal jejunal loop, 12 cm distal to the gastrojejunal anastomosis. Consecutive sutures were used for the anastomoses, while full-thickness intermittent sutures were used for the closure of abdominal incisions.
Postoperatively, rats were housed separately in metabolic cages that were warmed for 6 h by a 60-watt incandescent lamp. Fluids were administered daily as subcutaneous injections into the back of the neck, providing a daily total of 18.7 kcal/kg, with a 5:1 ratio of glucose to insulin (Table 1, Formula 1). Enteral nutrition with Nutrison® [Nutricia Pharmaceutical (Wuxi) Co., Ltd., Wuxi, China], given three to four times daily, was begun after the first bowel movements (usually 48 h postoperatively), and provided a total daily caloric intake of 120 kcal/kg and a nitrogen content of 0.8 g/kg. Rats were subsequently given free access to water and semisolid food, and were finally graduated to regular rodent chow (the First Affiliated Hospital of Harbin Medical University Animal Center, Harbin, China). Throughout the postoperative period, rats were carefully monitored for general well-being, urine output, the nature and amount of feces, feeding profile, incision healing, and blood glucose.
| Component | Formula 1 | Formula 2 |
| 0.9% sodium chloride | 50 mL | 50 mL |
| 10% potassium chloride | 3 mL | 3 mL |
| 5% glucose | 0 mL | 60 mL |
| 10% glucose | 30 mL | - |
| 25% glucose | 30 mL | - |
| Regular insulin | 2 IU | - |
| Cimetidine | 2 mL (0.2 g) | 2 mL (0.2 g) |
| Cefoperazone sodium/sulbactam sodium | 1.0 g | 1.0 g |
Rats were fasted for 12 h, but were given free access to water bottles containing a 50 g/L solution of glucose in normal saline. Before anesthesia, animals received subdermal injections (2 mL into the flexor side of the hindlimbs) of both sodium chloride (0.9%) and glucose (5%). Twenty minutes after subdermal injection of atropine (0.1 mg/kg), baseline anesthesia was initiated with an intraperitoneal injection of 0.5% pelltobarbitalum natricum (20 mg/kg). Intermittent inhalation of ether was used to maintain anesthetic duration and depth during the operation. Sputum was periodically suctioned throughout anesthesia to maintain airway patency. A median longitudinal incision was begun at the xiphoid and caudally extended approximately 3 cm along the upper abdominal linea alba. The incision was then retracted using a blepharostat. Retraction of the hepatic lobes revealed the gastric pylorus and the initial portion of the duodenum, which was elevated with the notched forceps. The vascular branches running vertically to the pancreatic head, adjacent to the pylorus, were explored to carefully define the transection site. To avoid later damaging the biliary tract, the common bile duct and its point of convergence with the duodenum were also visualized. The first part of the duodenum was retracted caudally to expose the underlying colon, below which Treitz’s ligament was identified. In the Roux-en-Y procedure, the first part of the duodenum was transected 2-3 mm distal to the pylorus and the distal end of the duodenum was closed with a ligature, which both preserved gastric volume and excluded the entire duodenum and proximal jejunal loop. The procedure was otherwise identical to the conventional DJB (Figure 1).
Although the skin incision was extended to the xiphoid so as to fully expose the surgical field, care was taken to avoid pneumothorax. Prehemostasis of the pyloroduodenal transection was achieved by ligating the major pyloric vascular branches of the gastroduodenal arteries with 7-0 minimally invasive sutures (Warwick Medical Supplies Co., Ltd. Hangzhou, China). Treitz’s ligament was carefully identified, and the intended site of jejunal transection, 8.0 cm distal to this structure, was marked with 7-0 sutures. To match the caliber of each side of the anastomosis, the jejunal segment to be transected was exteriorized for 3-5 min and subsequently restored. The duodenal segment adjacent to the intended division line was circled with 5-0 minimally invasive sutures (Warwick Medical Supplies Co., Ltd. Hangzhou China), which were drawn through the vascular branches between the duodenum and the pancreatic head. Before transection of the duodenum, the segment 2-3 mm distal to the pylorus was dissected using a microhemostat. The distal duodenal end was ligated using preplaced 5-0 sutures to close the transection. The jejunum and its mesentery were transected in a similar manner.
Intermittent full-thickness anastomoses were created using 6-0 minimally invasive sutures (Warwick Medical Supplies Co., Ltd. Hangzhou China), with care taken to avoid intestinal distortion. The first stitch of the gastrojejunal anastomosis was made at the mesenteric margin using varus sutures, with 2-3 cm suture tails retained for marking and traction. The second and third stitches were placed to connect the midpoint of the posterior wall and the contramesenteric margin. The first part of duodenum was reversed, using notched forceps, to expose the posterior wall of the anastomosis, which was anastomosed at a stitch interval of 0.5 mm and a margin interval of 1.0 mm. The anterior wall was anastomosed similarly. The jejunojejunal anastomosis was completed in the reverse sequence.
Closure of the mesenteric hole was achieved using end-to-end stitches in the avascular mesentery, with care taken to avoid ligating vessel arches and thereby compromising the anastomotic blood supply. The surgical field was intermittently rinsed with warm saline to compensate for any fluid loss from the laparotomy, as well as to prevent drying damage to the exposed portion of the intestines. Before closing the abdomen, the blood supply of each anastomosis was routinely inspected, and the intestinal loops were appropriately sequenced. The abdominal incision was closed with full-thickness stitches using 2-0 minimally invasive sutures (Yangzhou Huaxia Medical Devices CO., Ltd. Yangzhou, China). Throughout the procedure, the ambient temperature was maintained at 25 °C, using an electric blanket if necessary, to keep the rat body surface dry and prevent intraoperative hypothermia.
Several modifications were made to the standard regimen of postoperative care for DJB in rats[11,12]. First, only half the volume of fluid normally given by infusion was administered on the day of surgery, and it was augmented with insulin-free 5% glucose (12 mL/kg) (Table 1, Formula 2). Routine fluid infusion (50 mL/kg per day) was given on the following day (Table 1, Formula 1). Enteral nutrition using Nutrison® [Nutricia Pharmaceutical (Wuxi) Co., Ltd., Wuxi, China], was begun 48 h postoperatively, when the majority of rats had resumed bowel movements.
All qualitative data were expressed as percent. Fisher’s exact probability test was used to compare the difference in survival rate between rats in the conventional and modified DJB groups. All quantitative data were expressed as mean ± SD. Intragroup PGL values were compared using the paired Student’s t test, while operative duration and mean PGL were compared between the 2 groups using the independent two-sample Student’s t test. A P value less than 0.05 was considered statistically significant.
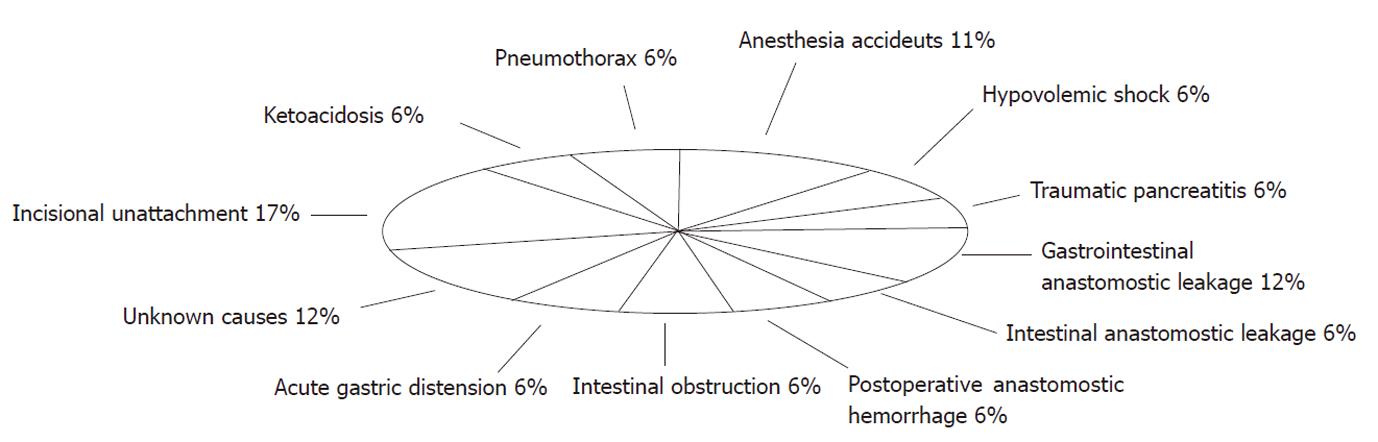
The preliminary study allowed us to become familiar with the anatomical structures involved in the procedure and to become proficient in performing the required microsurgical anastomoses. In the conventional DJB group [mean operative duration time (OT) 110.02 ± 12.34 min], 13 rats survived, representing a success rate of 43.3% (13/30). Judging from the histopathological sequelae, the causes of mortality in this group potentially included anesthetic accidents (n = 2, 11%), hypovolemic shock (n = 1, 6%), pneumothorax (n = 1, 6%), traumatic pancreatitis (n = 1, 6%), ketoacidosis (n = 1, 6%), postoperative anastomotic hemorrhage (n = 1, 6%), acute gastric distension (n = 1, 6%), gastrointestinal anastomotic leakage (n = 2, 12%), intestinal anastomotic leakage (n = 1, 6%), intestinal obstruction (n = 1, 6%), and incisional unattachment (n = 3, 17%). Two rats died of unknown causes within 24 h of surgery (Figure 2). In the modified DJB group, the mean OT was reduced to 78.39 ± 7.26 min (P < 0.01). Of the 20 rats in this group, only 2 died, due to anastomotic leakage or intestinal obstruction. The success rate in the modified DJB group (18/20, 90.0%) was significantly higher than that in the conventional DJB group (P < 0.01).
In the conventional DJB group, the preoperative and 4-wk-postoperative mean PGL levels were 16.69 ± 1.69 mmol/L and 7.46 ± 0.49 mmol/L (P < 0.01), respectively, while the corresponding values in the modified DJB group were 17.02 ± 1.51 mmol/L and 7.23 ± 0.39 mmol/L (P < 0.01). There was no significant difference in mean postoperative PGL between the 2 groups (P > 0.05). However, 4 wk after the operation, the levels of PGL in conventional or modified DJB groups were significantly decreased compared with the preoperative preoperative (P < 0.01).
Obese patients with T2DM have been reported to show an improvement in diabetic symptoms after being treated for obesity with DJB. Although the DJB animal model offers a first step toward understanding the mechanism of this phenomenon, it is technically challenging and associated with high procedure-related mortality. In the current study, we modified a number of technical details of both the procedure and the postoperative care regimen that resulted in a reduced operative duration, while yielding a higher success rate, with postoperative PGL levels comparable to those obtained with the standard procedure.
Anesthetic management is an important consideration when performing animal surgery. The anesthetic pelltobarbitalum natricum has potent effects in suppressing breathing and cardiac function. Unfortunately, its pharmacological effects vary greatly from rat to rat, giving it a very narrow safety range. Rats anesthetized with this drug most often die as a consequence of excessive airway secretion. For this reason, we chose to use a combined general anesthetic protocol, beginning with a low-dose of pelltobarbitalum natricum and then controlling the depth and duration of anesthesia using intermittent administration of inhaled ether[13]. Airway management relied on both premedication with atropine, to inhibit airway secretion, and the intraoperative suction of sputum to maintain airway patency.
The success rate in DJB is critically dependent on choosing the correct site of transection and anastomosis. In the modified DJB procedure, the first part of the duodenum was transected 2-3 mm distal to the pylorus, and the two sides of the prospective gastrojejunostomy were matched in both diameter and layer in order to simplify the anastomotic manipulation and prevent anastomotic stricture. We took care in a number of areas when choosing the jejunal transection site. First, a well-vascularized jejunal loop was transected midway between two main branches of the mesenteric vessel arches, and when necessary, intestinal canals were stitched to reach the major branches of mesenteric vessel arches to ensure that the anastomoses were sufficiently perfused and thus protected from anastomotic leakage or stenosis. In addition, the transected jejunal loop was exteriorized to decrease intestinal tension and allow us to better identify segments that were matched in diameter. The site of anastomosis was chosen based on the mobility of the associated jejunal mesentery, so as to minimize the tension these structures might exert on the subsequent gastrojejunostomy. Although a continuous stitch would have reduced the operative duration, it was also judged likely to increase the risk of postoperative hemorrhage and stenosis at the anastomosis. Supporting this, in the conventional DJB group, hemorrhage from the continuously stitched anastomoses was responsible for one case of postoperative mortality, while intermittent full-thickness sutures had no such sequelae.
We used a number of minimally invasive techniques in an effort to reduce complications from hemorrhage, surgical damage, and infection. First, the anterior pyloric vessels were pre-ligated to provide active hemostasis. Careful identification of anatomical structures and dissection along surgical planes also contributed to preventing excessive hemorrhage and secondary injury[14]. As a relatively constant anatomical marker, Treitz’s ligament was carefully identified, to minimize the surgical invasiveness on the intestines. Simple closure of the distal duodenal end with 5-0 ligature significantly decreased the volume of blood lost, and also reduced operative duration without risking duodenal leakage. To reduce the risk of postoperative traumatic pancreatitis, we took care to avoid contact with the pancreas. For the anastomotic reconstruction, the use of 6-0 minimally invasive sutures and notched forceps lessened intestinal contusion, protected the blood supply to the anastomosis, and significantly lessened anastomotic leakage by minimizing mechanical and ischemic injuries. We took particular care to diminish any action, which could contribute to peritoneal contamination.
Damage Control Surgery philosophy is the key to ensure a successful operation[15,16]. Although frequently ignored as a prognostic factor, hypothermia can initiate a lethal triad of complicating factors, as it aggravates metabolic acidosis and compromises coagulation[17,18]. Intraoperative hypothermia can be caused by heat loss from the open peritoneal cavity as well as by anesthesia and fluid infusion[19]. Hypothermic rats are also susceptible to ventricular arrhythmia, infection, and an elevated catabolic rate[20]. Prolonged hypothermia increases the incidence of multiple organ dysfunction syndrome (MODS) and mortality [21]. In the conventional DJB group, the two deaths that occurred within the first postoperative 24 h might have been due to MODS that was itself secondary to hypothermia. Appropriate damage control techniques in the context of the animal duodenal-jejunal model include reducing both the overall procedure time and the period in which the peritoneal cavity lies open, as well as ensuring postoperative active heat preservation. In this study, the techniques used to shorten the OT included the rapid and accurate identification of Treitz’s ligament, hemorrhage control by careful dissection along anatomical planes, simple closure of the distal duodenal end, and interrupted closure of the abdominal incision in full-thickness.
Fast-track techniques were used in our modified DJB. Preoperative oral administration of glucose-saline solution to fasted rats prevents hypoglycemia and attenuates postoperative insulin resistance[22,23]. Postoperative stress can be addressed by permissive underfeeding of enteral nutrition, with the low-calorie supply best given in the short term to better meet metabolic requirements and optimize blood glucose[11,24]. Stress hyperglycemia on the operative day should be prevented by strict restriction of glucose intake, with 5% glucose given at a dose of 12 mL/kg to prevent postoperative hypoglycemia. To maintain nitrogen balance in the longer term, the number of calories supplied in enteral nutrition can be progressively increased until a state of balanced enteral nutrition is reached.
Another fast-track surgical measure is the strict restriction of fluid and electrolytes, as overdosing of electrolyte solution will delay the resumption of gastrointestinal activity[25-27]. Marjanovic et al[12] reported that restricted rehydration favored the initial healing of intestinal anastomoses, while over-rehydration increased both the severity of peripheral edema and the incidence of complications . In addition to the perioperative use of insulin in T2DM rats, appropriate fluid therapy is also critical to prevent acute metabolic disturbance such as ketosis. Therefore, we suggested using an individualized regimen of goal-directed volume therapy (GDT) to appropriately restrict rehydration[28]. Our results demonstrated that the composition and volume of the fluid infused according to a GDT regimen was able to optimally balance input and output and to effectively decrease the incidence of acute metabolic disturbance. Additionally, in the case of ketoacidosis and hyperosmotic dehydration, adequate rehydration should be given along with insulin, and the animal should be closely monitored.
In conclusion, we successfully established a sound duodenal-jejunal bypass model in diabetic rats that is based on minimally invasive techniques and fast-track surgical management. Our findings suggest that duodenal-jejunal bypass is a potential treatment alternative for diabetic patients who are especially unresponsive to other modalities of medical intervention, provided that surgical invasiveness is similarly minimized and the principles of fast-track and damage control surgery are observed.
When gastric bypass is used to treat morbid obesity in patients with concomitant type 2 diabetes mellitus (T2DM), some of these patients have seen their diabetes resolve even before they have lost weight. Available data suggest that reduced caloric intake alone is not sufficient to explain the observed short-term metabolic and endocrine effects. Recently, Rubino et al demonstrated that a duodenal-jejunal bypass (DJB) procedure that preserved gastric volume could directly benefit T2DM patients, suggesting that duodenal-jejunal exclusion, rather than the restriction or reduction of gastric volume, is the critical factor in surgical treatment of T2DM. Therefore, as a first step, the authors chose to apply an existing rat model for DJB in animals rendered diabetic by administration of streptozotocin. In such a model, a range of surgical, perioperative, and other factors would be expected to influence the effect of bypass surgery on diabetes. However, there is little data regarding the influence of particular surgical modalities or non-surgical aspects of care on diabetic rats.
Obese patients with type 2 diabetes mellitus have been reported to show an improvement in diabetic symptoms after being treated for obesity with DJB. Although the DJB animal model offers a first step toward understanding the mechanism of this phenomenon, it is technically challenging and associated with high procedure-related mortality. In the current study, the authors carefully compared a number of both surgical techniques and perioperative management regimens for their ability to improve the success rate in diabetic rats underlying DJB, including minimally invasive procedural designs and the use of damage control and fast-track surgical modalities.
In the current study, the authors modified a number of technical details of both the procedure and the postoperative care regimen (including anesthetic management, the correct site of transection and anastomosis, a number of minimally invasive techniques, hypothermia and fast-track techniques) yielding a higher success rate, and reduced fasting blood glucose levels in diabetic rats. The authors successfully establish a sound duodenal-jejunal bypass model in diabetic rats that is based on minimally invasive techniques and fast-track surgical management.
In this study the authors successfully establish a sound duodenal-jejunal bypass model in diabetic rats. The findings suggest that duodenal-jejunal bypass is a potential treatment alternative for diabetic patients who are especially unresponsive to other modalities of medical intervention, provided that surgical invasiveness is similarly minimized and the principles of fast-track and damage control surgery are observed.
DJB, the operation consists of a stomach-preserving bypass of a short segment of proximal small intestine. DJB may be associated with a sleeve resection of the stomach to reduce potential for marginal ulcerations and increase the weight loss effect if performed in mildly or severely obese patients. Fast-track surgery, a new method of application of preexisting procedures in pre-intra and post surgical phase pre-written and carried out in a multi-disciplinary way in order to obtain a rapid recovery after operation.
It is a very well conducted animal model study. The results are interesting and very useful for the study of this subject. Morbidly obese patients undergoing gastric by-pass is lost in T2DM can be more easily treated. In this respect, it is an important study.
Peer reviewers: Antonio Basoli, Professor, General Surgery “Paride Stefanini”, Università di Roma – Sapienza, Viale del Policlinico 155, Roma 00161, Italy; Dr. İbrahim Sakçak, 6th Surgery Clinic, Numune Teaching and Education Hospital, Ankara 061000, Turkey
S- Editor Lv S L- Editor O’Neill M E- Editor Li JY
| 1. | Hickey MS, Pories WJ, MacDonald KG, Cory KA, Dohm GL, Swanson MS, Israel RG, Barakat HA, Considine RV, Caro JF. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg. 1998;227:637-643; discussion 643-644. [PubMed] [Cited in This Article: ] |
| 2. | Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467-484; discussion 484-485. [PubMed] [Cited in This Article: ] |
| 3. | Pories WJ. Diabetes: the evolution of a new paradigm. Ann Surg. 2004;239:12-13. [PubMed] [Cited in This Article: ] |
| 4. | Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15-23. [PubMed] [Cited in This Article: ] |
| 5. | Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479-2485. [PubMed] [Cited in This Article: ] |
| 6. | Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741-749. [PubMed] [Cited in This Article: ] |
| 7. | Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 2008;31 Suppl 2:S290-S296. [PubMed] [Cited in This Article: ] |
| 8. | Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313-320. [PubMed] [Cited in This Article: ] |
| 9. | Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49:1390-1394. [PubMed] [Cited in This Article: ] |
| 10. | Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1-11. [PubMed] [Cited in This Article: ] |
| 11. | McCowen KC, Friel C, Sternberg J, Chan S, Forse RA, Burke PA, Bistrian BR. Hypocaloric total parenteral nutrition: effectiveness in prevention of hyperglycemia and infectious complications--a randomized clinical trial. Crit Care Med. 2000;28:3606-3611. [PubMed] [Cited in This Article: ] |
| 12. | Marjanovic G, Villain C, Juettner E, zur Hausen A, Hoeppner J, Hopt UT, Drognitz O, Obermaier R. Impact of different crystalloid volume regimes on intestinal anastomotic stability. Ann Surg. 2009;249:181-185. [PubMed] [Cited in This Article: ] |
| 13. | Flegal MC, Kuhlman SM. Anesthesia monitoring equipment for laboratory animals. Lab Anim (NY). 2004;33:31-36. [PubMed] [Cited in This Article: ] |
| 14. | Modlin IM. Surgical triumvirate of Theodor Kocher, Harvey Cushing, and William Halsted. World J Surg. 1998;22:103-113. [PubMed] [Cited in This Article: ] |
| 15. | Rotondo MF, Schwab CW, McGonigal MD, Phillips GR, Fruchterman TM, Kauder DR, Latenser BA, Angood PA. 'Damage control': an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375-382; discussion 382-383. [PubMed] [Cited in This Article: ] |
| 16. | Finlay IG, Edwards TJ, Lambert AW. Damage control laparotomy. Br J Surg. 2004;91:83-85. [PubMed] [Cited in This Article: ] |
| 17. | Sido B, Grenacher L, Friess H, Büchler MW. [Abdominal trauma]. Orthopade. 2005;34:880-888. [PubMed] [Cited in This Article: ] |
| 18. | Burch JM, Ortiz VB, Richardson RJ, Martin RR, Mattox KL, Jordan GL. Abbreviated laparotomy and planned reoperation for critically injured patients. Ann Surg. 1992;215:476-483; discussion 483-484. [PubMed] [Cited in This Article: ] |
| 19. | Burch JM, Denton JR, Noble RD. Physiologic rationale for abbreviated laparotomy. Surg Clin North Am. 1997;77:779-782. [PubMed] [Cited in This Article: ] |
| 20. | Sessler DI. Mild perioperative hypothermia. N Engl J Med. 1997;336:1730-1737. [PubMed] [Cited in This Article: ] |
| 21. | Ishihara S. [Damage control surgery and perioperative management]. Nihon Geka Gakkai Zasshi. 2002;103:524-528. [PubMed] [Cited in This Article: ] |
| 22. | Nygren J, Soop M, Thorell A, Sree Nair K, Ljungqvist O. Preoperative oral carbohydrates and postoperative insulin resistance. Clin Nutr. 1999;18:117-120. [PubMed] [Cited in This Article: ] |
| 23. | Soop M, Nygren J, Myrenfors P, Thorell A, Ljungqvist O. Preoperative oral carbohydrate treatment attenuates immediate postoperative insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E576-E583. [PubMed] [Cited in This Article: ] |
| 24. | Hoffer LJ. Protein and energy provision in critical illness. Am J Clin Nutr. 2003;78:906-911. [PubMed] [Cited in This Article: ] |
| 25. | Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641-648. [PubMed] [Cited in This Article: ] |
| 26. | Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology. 2005;103:25-32. [PubMed] [Cited in This Article: ] |
| 27. | Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322:473-476. [PubMed] [Cited in This Article: ] |
| 28. | Kehlet H, Bundgaard-Nielsen M. Goal-directed perioperative fluid management: why, when, and how? Anesthesiology. 2009;110:453-455. [PubMed] [Cited in This Article: ] |