Published online Nov 28, 2011. doi: 10.3748/wjg.v17.i44.4883
Revised: August 25, 2011
Accepted: August 31, 2011
Published online: November 28, 2011
AIM: To investigate whether human acyl-CoA synthetase 5 (ACSL5) is sensitive to the ACSL inhibitor triacsin C.
METHODS: The ACSL isoforms ACSL1 and ACSL5 from rat as well as human ACSL5 were cloned and recombinantly expressed as 6xHis-tagged enzymes. Ni2+-affinity purified recombinant enzymes were assayed at pH 7.5 or pH 9.5 in the presence or absence of triacsin C. In addition, ACSL5 transfected CaCo2 cells and intestinal human mucosa were monitored. ACSL5 expression in cellular systems was verified using Western blot and immunofluorescence. The ACSL assay mix included TrisHCl (pH 7.4), ATP, CoA, EDTA, DTT, MgCl2, [9,10-3H] palmitic acid, and triton X-100. The 200 μL reaction was initiated with the addition of solubilized, purified recombinant proteins or cellular lysates. Reactions were terminated after 10, 30 or 60 min of incubation with Doles medium.
RESULTS: Expression of soluble recombinant ACSL proteins was found after incubation with isopropyl beta-D-1-thiogalactopyranoside and after ultracentrifugation these were further purified to near homogeneity with Ni2+-affinity chromatography. Triacsin C selectively and strongly inhibited recombinant human ACSL5 protein at pH 7.5 and pH 9.5, as well as recombinant rat ACSL1 (sensitive control), but not recombinant rat ACSL5 (insensitive control). The IC50 for human ACSL5 was about 10 μmol/L. The inhibitory triacsin C effect was similar for different incubation times (10, 30 and 60 min) and was not modified by the N- or C-terminal location of the 6xHis-tag. In order to evaluate ACSL5 sensitivity to triacsin C in a cellular environment, stable human ACSL5 CaCo2 transfectants and mechanically dissected normal human intestinal mucosa with high physiological expression of ACSL5 were analyzed. In both models, ACSL5 peak activity was found at pH 7.5 and pH 9.5, corresponding to the properties of recombinant human ACSL5 protein. In the presence of triacsin C (25 μmol/L), total ACSL activity was dramatically diminished in human ACSL5 transfectants as well as in ACSL5-rich human intestinal mucosa.
CONCLUSION: The data strongly indicate that human ACSL5 is sensitive to triacsin C and does not compensate for other triacsin C-sensitive ACSL isoforms.
- Citation: Kaemmerer E, Peuscher A, Reinartz A, Liedtke C, Weiskirchen R, Kopitz J, Gassler N. Human intestinal acyl-CoA synthetase 5 is sensitive to the inhibitor triacsin C. World J Gastroenterol 2011; 17(44): 4883-4889
- URL: https://www.wjgnet.com/1007-9327/full/v17/i44/4883.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i44.4883
Acyl-CoA derivatives play a fundamental role in the lipid metabolism of eukaryotic cells including enterocytes. Several biological processes are influenced by acyl-CoA thioesters (acyl-CoAs), ranging from intermediary and mitochondrial metabolism to nuclear gene transcription[1]. The formation of long-chain acyl-CoA derivatives is catalyzed by acyl-CoA synthetases (ACSLs; E.C. 6.2.1.3.), which convert long-chain fatty acids (FAs) into acyl-CoAs[2]. In humans and rodents, five ACSL isoforms have been identified so far, differing in their substrate preferences, enzyme kinetics, cellular and organelle locations, as well as their expression[3]. Human ACSL5 is strongly expressed by enterocytes of the small and large intestine, and is suggested as a modifier of enterocytic maturation and cell death[4-6]. Impaired ACSL5 expression and synthesis has been found in colorectal carcinogenesis[7]. The diversity of ACSL proteins is of functional interest because recent studies suggest that ACSL proteins may play a role in channelling fatty acids toward diverse and complex lipid functions with high relevance for cellular behaviour[8,9].
Triacsin C [1-hydroxy-3-(E,E,E-2’,4’,7’-undecatrienylidine) triazene], an alkenyl-N-hydroxytriazene fungal metabolite, has been reported to be a potent competitive inhibitor of acyl-CoA synthetase activity[10]. The inhibitory capacity of triacsin C depends on the N-hydroxytriazene moiety of the molecule. In different cellular systems, consequences of ACSL inhibition by triacsin C were found, including a dramatic reduction in cholesterol as well as triglyceride synthesis with non-transition of macrophages to foam cells or enhanced eicosanoid release in leucocytes[11,12]. In endothelial cells, arachidonoyl-CoA synthesis was considerably inhibited by triacsin C[13]. Interference of triacsin C with cellular proliferation via inhibition of hu-ACSLs has been found[14,15]. It has been speculated that the plethora of triacsin C effects results from differences in the triacsin C susceptibility of ACSL isoforms. In accordance with this hypothesis, it has been demonstrated that triacsin C inhibits recombinant rat ACSL1 (r-ACSL1), rat ACSL3 (r-ACSL3), and rat ACSL4 (r-ACSL4), but not ACSL5 (r-ACSL5) enzyme activity and may therefore, be useful for discriminating amongst ACSL functions[16,17]. The activity of recently described rat ACSL6 subtypes (r-ACSL6_v1 and r-ACSL6_v2) was not affected by triacsin C at concentrations as high as 50 µmol/L[17]. However, ACSL6 is not essentially expressed in intestinal tissues and enterocytes are widely negative for ACSL6 species.
The aim of the present study was to characterize the effect of triacsin C on human ACSL5 protein in vitro and in the intestinal cellular environment. Our findings show that human ACSL5 is, unlike rat ACSL5, sensitive to triacsin C.
The rat anti-human ACSL5 antibody KD7 was prepared as previously described[5]. Additional antibodies and substances were anti-beta-actin (Sigma, Deisenhofen, Germany), anti-histidine antibodies (Roche, Mannheim, Germany), HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Heidelberg, Germany; Dianova, Hamburg, Germany), enhanced chemiluminescence (PIERCE, Rockfort, United States), rainbow protein standard (Amersham Bioscience, United Kingdom), PVDF Immobilon-P membrane (Millipore, Bedford, United States), MitoTracker RedCMXRos (Molecular probes, Eugen, United States), and DAPI (Vysis Inc., Downer’s Grove, United States). The alkenyl-N-hydroxytriazene fungal metabolite triacsin C (Biomol, Hamburg, Germany) and other materials were obtained from commercial sources. Standard cloning techniques were used for cloning hu-ACSL5 sequences (GeneBank accession no. AB033899) into the pENTRY vector of the GATEWAY cloning system (Invitrogen Life Technologies, Karlsruhe, Germany). Cytomegalovirus expression constructs were synthesized by recombination into the pcDNA_DEST40 vector. CaCo2 cell lines, stably transfected with hu-ACSL5 in pcDNA_DEST40 or empty pcDNA_DEST40, were cultured under appropriate culture conditions. In one approach, T5 promoter expression constructs with N-terminal fusion of hu-ACSL5 sequences to 6xHis-tag and factor Xa protease recognition sites were synthesized by recombination into the pQE-30Xa vector (Qiagen, Hilden, Germany). In a second approach, sequences of r-ACSL1 (EST clone IMAGp998O0814978Q in pExpress-1) and r-ACSL5 (I.M.A.G.E. full length cDNA clone IRBPp993B014D in pExpress-1), both delivered from RZPD Berlin, Germany, as well as the hu-ACSL5 sequence were cloned into pET22b(+) (Merck, Darmstadt, Germany) in Escherichia coli (E. coli) without a pelB leader sequence for cytosolic expression of C-terminal 6xHis-tagged proteins. Specimens of human normal intestinal mucosa (n = 10) were mechanically dissected from surgical resections and used fresh or immediately frozen in liquid nitrogen and stored at -80 °C. The use of human tissues for study purposes was approved by each patient and by the local ethics committee at the University Hospital of the RWTH Aachen (EK019/06).
For cloning of hu-ACSL5 into appropriate vectors, sequences were generated from human intestine by long distance RT-PCR using the following set of primers: 5’-GGGGACAAGTTTGTACAAAAAAGCAGGCTCTACCATGCTTTTTATCTTTAACTTTTTGTTTTCCCCACTTCC-3’ (sense) and 5’-GGGGACCACTTTGTACAAGAAAGCTGGGTCATCCTGGATGTGCTCATACAGGCTGT-3’ (antisense). Sequences of r-ACSL1 and r-ACSL5 were amplified for recombinant expression cloning. Correctness of all ACSL target sequences (accession nos.: AB033899, AM262166, NM_012820, NM_053607) were controlled by full length sequencing. Recombinant expression of different ACSL proteins in E. coli M15 or E. coli BL21 was induced with 1 mmol/L isopropyl beta-D-1-thiogalactopyranoside (IPTG) in LB medium when an OD600 of 0.6-0.9 was reached. In E. coli M15, formation of inclusion bodies was highly diminished by an incubation period of 45 min at 20 °C. An incubation period of 24 h at 16 °C was used for r-ACSL1 and r-ACSL5 expression in E. coli BL21, whereas 6 h at 28 °C was optimal for expressing hu-ACSL5 in E. coli BL21. Expression of soluble ACSL proteins was verified by Western or Dot blotting of ultracentrifuged fractions and anti-ACSL5 or anti-His antibody probes. In control experiments, bacteria transformed with the empty vector were used.
Samples of IPTG-treated bacteria were sonicated in ice-cooled buffer (buffer A) containing 20 mmol/L Tris-HCl (pH 7.5), 1 mmol/L EDTA, 1% triton X-100, and 0.1% sodium cholate, and further processed by ultracentrifugation. The resulting supernatants were applied to a Ni2+-affinity column (5 mL HisTrap HP; Amersham Pharmacia GE Healthcare, Freiburg, Germany). Elution was performed at a flow rate of 1 mL/min at 20 °C; a linear gradient from buffer A to A/B (50:50, buffer B: buffer A + 500 mmol/L imidazole); 10 min with A/B (50:50); a linear gradient from A/B (50:50) to solvent B; finally with buffer B. Purification of ACSL proteins was controlled by SDS gel electrophoresis with subsequent silver staining, Western blotting, and ACSL activity assays of the purified proteins. ACSL proteins were purified to near homogeneity, migrating as single bands. The Lowry procedure was used for measurement of total protein, and bacteria transformed with the empty vector was used as a negative control.
ACSL activity assays were performed as previously described[6]. The standard ACSL assay mix contained 150 mmol/L TrisHCl (pH 7.4), 40 mmol/L ATP, 1.2 mmol/L CoA, 2 mmol/L EDTA, 2 mmol/L DTT, 0.1 mol/L MgCl2, 0.5 μmol/L [9,10-3H] palmitic acid, and 0.2% triton X-100. The 200 μL reaction was initiated by adding solubilized purified recombinant hu- or r-ACSL proteins (ca. 0.1-2.0 μg) or cellular lysates in 0.1% sodium deoxycholate and 1% triton X-100 in 20 mmol/L Tris (pH 7.4). Reactions were terminated after 10, 30 or 60 min incubation with Doles medium. After phase separation, the watery phase was washed twice with palmitic acid-enriched n-heptane. Radioactivity was measured using Ultima Gold cocktail in a Tri-Carb liquid scintillation (2900TR) counter equipped with QuantaSmart software (PerkinElmer, Rodgau, Germany). The studies were repeated three times, and virtually identical results were obtained from each experiment. Enzyme activity was always demonstrated in percent of the related peak activity. Error bars are SEM.
Cellular proteins were separated by one-dimensional SDS-PAGE and transferred to a PVDF Immobilon-P membrane. Immunodetection was performed with primary antibodies [mAB KD7, specific for ACSL5 (undiluted); anti-beta-actin (1:1000)], probed with HRP-conjugated secondary antibodies (1:10 000) and developed with enhanced chemiluminescence as suggested by the provider. Rainbow protein standard was used for molecular weight estimation.
Cells were incubated with anti-histidine antibodies, specific for His-tagged proteins, followed by Cy2-labeled anti-mouse antibodies. For negative controls, the primary antibody was replaced by normal serum. MitoTracker RedCMXRos was used as a mitochondrial marker following the manufacturer’s recommendations. Cells were incubated with 25 nmol/L MitoTracker for 15 min, washed, fixed (methanol at -20 °C for 5 min, followed by acetone at 4 °C for 2 min), and permeabilized with 0.2% Triton X-100. After incubation of primary and secondary antibodies, DAPI was applied for nuclear staining. Images were visualized using a confocal laser microscope (Nicon, Düsseldorf, Germany).
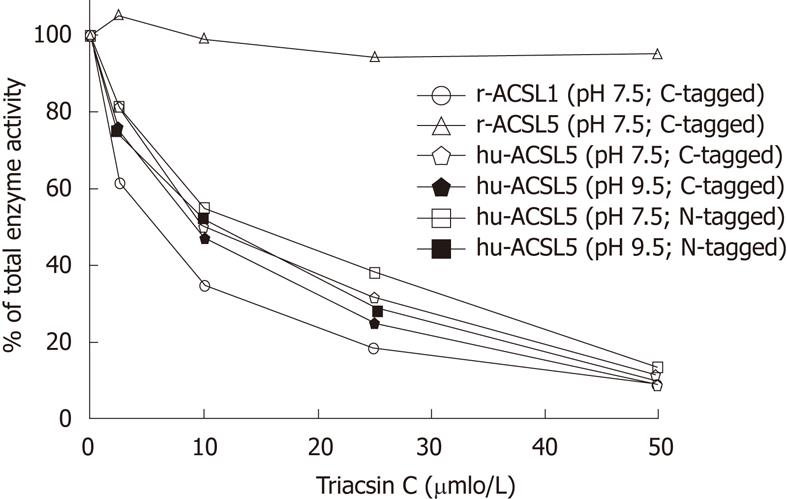
Expression of soluble recombinant hu-ACSL5 protein in E. coli M15 was found after incubation with IPTG for 45 min at 20 °C. Soluble activity in supernatants after ultracentrifugation (about 10% of total ACSL activity) was further purified to near homogeneity using Ni2+-affinity chromatography. The specific activity of recombinant hu-ACSL5 proteins from different preparations were in the range of 1.08-2.31 nmol/min per mg and independent of the epitope and its location at the N- or C-terminus (Figure 1).
Since hu-ACSL5 displayed activity peaks at pH 7.5[6,16] and pH 9.5[6], conditions favouring maximal enzyme activity were chosen when the inhibitory effects of triacsin C to ACSL proteins were tested. To test the effects of triacsin C on purified rat and human ACSL proteins in simultaneous reactions, the triacsin C substance dissolved in DMSO (2.5% of final assay reaction) was directly added to the reaction mixture (0-50 µmol/L) following the procedures by Kim et al[16]. Triacsin C selectively and strongly inhibited recombinant human ACSL5 protein (pH 7.5 and pH 9.5), as well as recombinant r-ACSL1 (sensitive control), but not recombinant r-ACSL5 (insensitive control) in a dose dependent manner (Figure 1). The IC50 for human ACSL5 proteins was about 10 μmol/L, and the inhibitory triacsin C effect was similar for different incubation times (10, 30 and 60 min) and was not modified by the N- or C-terminal location of the 6xHis-tag.
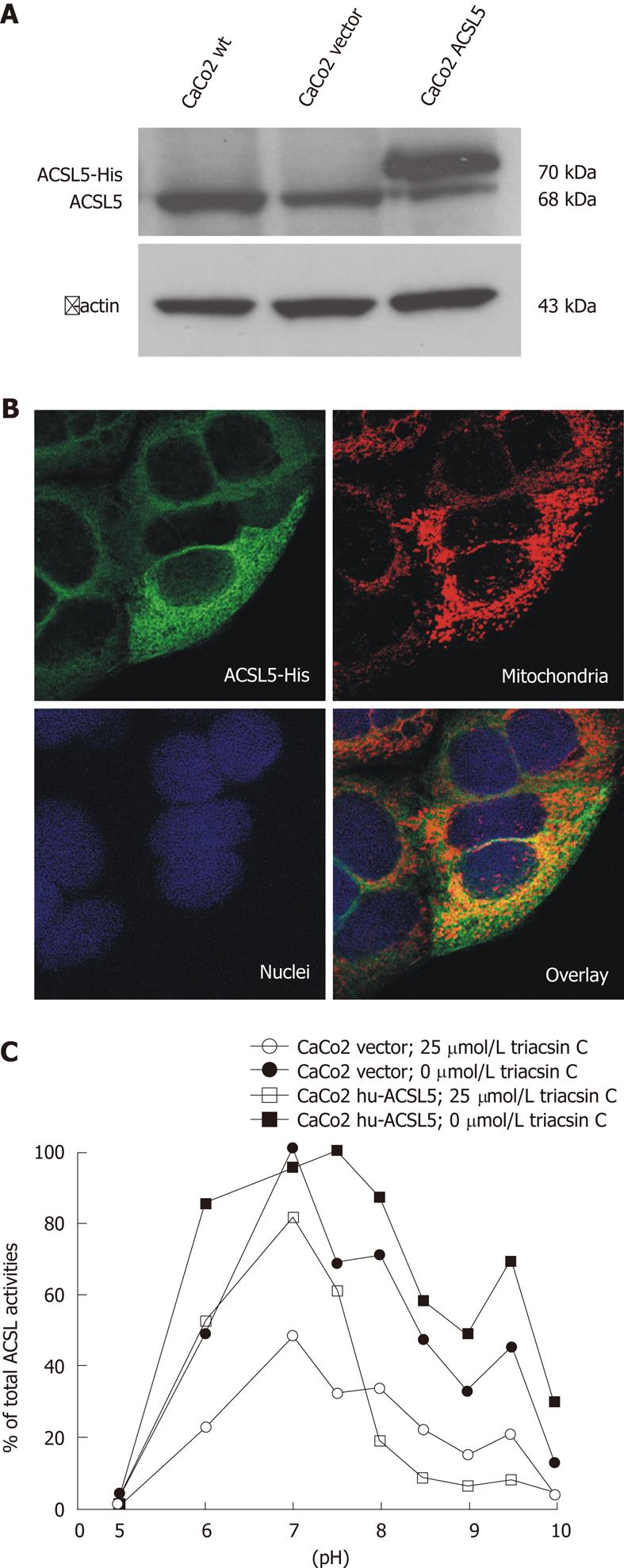
In order to further characterize the inhibitory potency of triacsin C on hu-ACSL5 protein in a cellular environment, stable hu-ACSL5 transfectants in CaCo2 cells (ATCC No. HTB-37) were used as previously published[6]. Transgenic and endogenous hu-ACSL5 protein expressions were controlled with Western blotting and indirect immunofluorescence (Figure 2). The shift between both ACSL5 proteins in Western blotting is due to the 6xHis-tag and a linker sequence, which result in a higher molecular weight of the transgenic ACSL5 protein (Figure 2A). N-terminal 6xHis-tagged hu-ACSL5 proteins are strongly co-localized with mitochondria. Some extramitochondrial signalling is found, including ER/ribosomes (Figure 2B). Monitoring of ACSL-activity in stable hu-ACSL5 N-terminal 6xHis-tagged transfectants and controls (wild type CaCo2 cells and CaCo2 transfectants with the empty vector) was performed between pH 5 and pH 10 (Figure 2C). In stable hu-ACSL5 CaCo2 transfectants, peak activity was found at pH 7.5 and pH 9.5, corresponding to the properties of recombinant hu-ACSL5 protein. This bimodal curve of ACSL activity was not observed in control cells, where the main ACSL activity was detectable at pH 7. The data further indicate that the bimodal distribution of ACSL activity was mainly due to hu-ACSL5 activity in the CaCo2 cellular environment.
In the presence of triacsin C (25 μmol/L), total ACSL activity was dramatically diminished in hu-ACSL5 transfectants as well as in controls. Importantly, the bimodal curve of ACSL activity with peak values at pH 7.5 and pH 9.5, due to hu-ACSL5 transgenic over-expression, was essentially smoothed in the presence of triacsin C (Figure 2C).
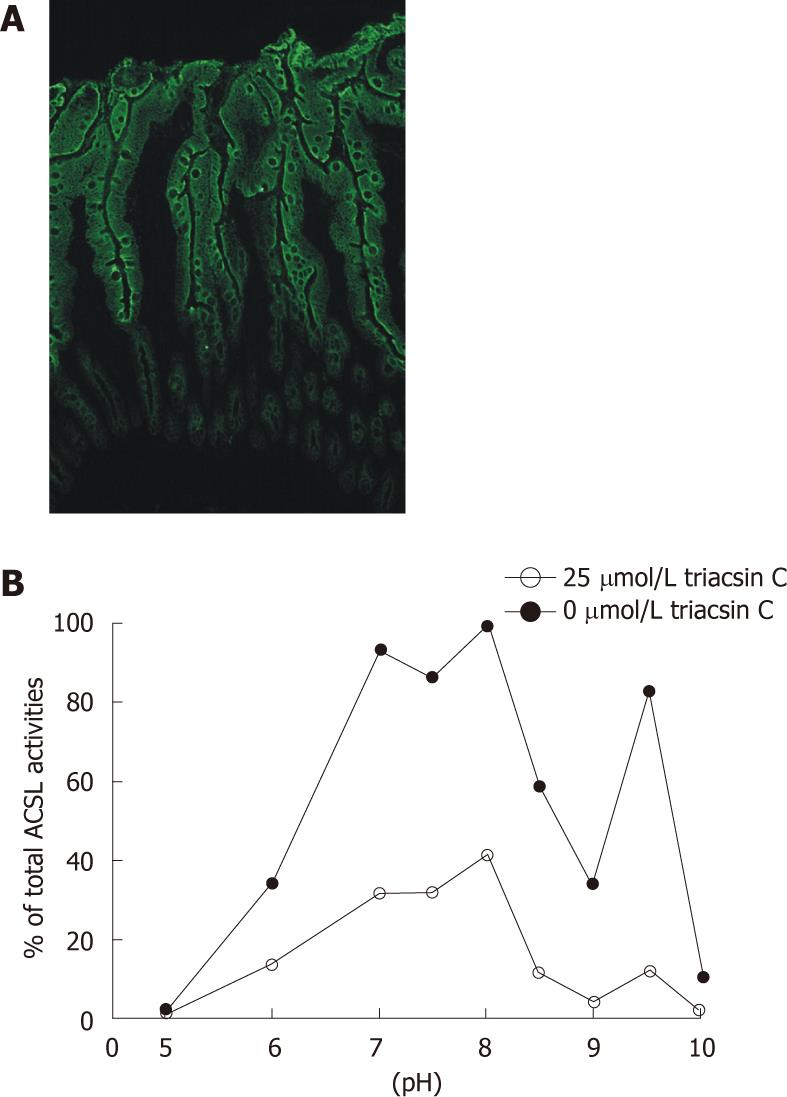
Next, mechanically dissected normal human intestinal mucosa with high ACSL5 expression (Figure 3A) was monitored for ACSL activity in the pH range of 5 to 10 (Figure 3B). Strong ACSL activity was found at pH 7, pH 8, and pH 9.5, partly reflecting the characteristic bimodal activity of human ACSL5 proteins. In the presence of triacsin C (25 μmol/L), ACSL activity was dramatically inhibited; the pH 9.5 peak was especially diminished (Figure 3B). The resulting smooth curve paralleled the findings with triacsin C treated purified hu-ACSL5 recombinant proteins and stable hu-ACSL5 transfected CaCo2 cells.
A considerable amount of data indicates that long chain fatty acids are essential in intestinal physiology and pathophysiology. In the modifier concept of intestinal carcinogenesis, the activity of intestinal long chain fatty acids is suggested as an important cell cycle modifier[18]. The function of ACSL mediated metabolic channelling of fatty acids in the regulation of intestinal cell behaviour includes the lipidation of proteins and translation of long chain fatty acid modifiers in several signalling cascades and receptor structures[19]. Specific inhibitors of enzyme activity are well established and powerful tools for determining enzymatic functions in cellular and non-cellular systems. Both sensitivity and specificity of inhibitors are essential prerequisites for a stringent functional analysis of target enzymes. Several molecular mechanisms in enzyme inhibition have been characterized so far, including covalent and non-covalent binding of substrate-like or non-substrate compounds. Competitive binding with the substitution of a characteristic substrate frequently underlies non-covalent enzyme inhibition. Competitive inhibition is the mechanism behind the triacsin C-mediated biochemical effects on ACSL isoforms[10,16,20].
The overwhelming number of studies concerning triacsin C mediated effects on ACSL molecules have been performed in rat models. There is convincing experimental data demonstrating the triacsin C sensitivity of rat ACSL isoforms 1, 3, 4 and 6, but insensitivity of the rat isoform 5[16,17]. However, many ACSL isoforms have splice variants, most of which have not been tested and characterized for triacsin C sensitivity. In addition, species-related differences of ACSL protein sensitivity to triacsin C have not been systematically examined up-to-now. The present study was, therefore, designed to systematically analyze triacsin C effects on human ACSL5 proteins, which are predominantly found in enterocytes of the human small intestinal mucosa.
In order to analyze the biochemical behaviour of hu-ACSL5, we cloned the respective sequences from an intestinal or plasmid cDNA resource, expressed the 6xHis-tagged recombinant proteins, and further purified the enzymes to near homogeneity using Ni2+-affinity chromatography. As demonstrated by digestion experiments and the subsequent analysis of ACSL-activity, the N- or C-terminal 6xHis-tag did not alter the enzymatic activity of ACSL5 at 30 °C or 37 °C in a broad range of pH values (pH 5-10). Monitoring ACSL-activity of recombinant hu-ACSL5 protein revealed triacsin C-sensitivity with an IC50 about 10 μmol/L. In this experimental setting, insensitivity of recombinant r-ACSL5 and sensitivity of recombinant r-ACSL1 was found, identical to previously described data by Kim et al[16].
Additional experiments ruled out the possibility that triacsin C-sensitivity of purified recombinant hu-ACSL5 protein could be a result of the absence of a cellular environment. Stable hu-ACSL5 transfectants and stable controls were established from CaCo2 cells, and in another set of experiments, human intestinal mucosa was investigated. The ACSL activity of cellular systems and tissues was monitored at different pH values in the presence or absence of triacsin C (25 μmol/L), and similar results to those with recombinant proteins were seen. Especially at pH 9.5, a pH value highly characteristic for hu-ACSL5 activity[6], ACSL activity was significantly decreased in cultured cells as well as intestinal mucosa. The background ACSL activity was probably due to enzymes other than ACSL5, including triacsin C insensitive ACSL splice forms, ACSL isoforms, and fatty acid binding proteins. In conclusion, we demonstrate experimental evidence that hu-ACSL5 is triacsin C sensitive as a purified recombinant protein, in hu-ACSL5 over-expressing epithelial cell lines, and in human small intestinal mucosa, a tissue with high ACSL5 expression levels. These findings imply that human ACSL5 is not able to compensate for triacsin C-inhibited ACSL isoforms, and triacsin C cannot be used to differentiate functions of different ACSL enzymes in human cells or tissues.
The insensitivity of human ACSL5 to triacsin C has been postulated by the observation that recombinant rat ACSL5 was not inhibited by this fungal metabolite[16]. This has been addressed in several studies[15,21-24]. In all of these studies, triacsin C was preferentially used to incubate cultured cells in a concentration clearly below the IC50hu-ACSL5 of approximately 10 μmol/L, and the substance was not directly added to the acyl-CoA activity assay mixture. In our study triacsin C was always used as a competitive inhibitor. Identical to the experimental approach of Kim et al[16], triacsin C was directly added to the acyl-CoA activity assay mixture.
We hypothesize that the divergent triacsin C effects on ACSL activity are species-related and determined by human and rat ACSL5 protein sequences. A sequence analysis of the proteins revealed that these were only 81% identical, and that discrepancies existed in exon 20 splicing as well as in the organization and length of functional domains, like the ATP-binding domain or the FA activation domain (NCBI data base: http//http://www.nlm.nih.gov/nlmhome.html). Species-related differences in ACSL5 activity are further suggested by expression experiments. Over-expression of r-ACSL5 in a rat cellular environment increases fatty acid incorporation into diacylglycerol and triacylglycerol but does not affect fatty acids used for beta-oxidation[25], whereas r-ACSL5 in a human cellular environment increases palmitate oxidation[26].
In the present study, sensitivity of human ACSL5 protein to triacsin C was demonstrated using purified recombinant protein, CaCo2 cells, and human intestinal mucosa. The divergent inhibitory effect of triacsin C with sensitivity of hu-ACSL5 and insensitivity of rat-ACSL5 is most likely species-related. Our findings indicate that human ACSL5 does not compensate for other triacsin C sensitive ACSL isoforms.
The authors are grateful to Petra Akens for typing and proofreading the manuscript.
Strong expression of acyl-CoA synthetase 5 (ACSL5) is found in surface lining epithelia of the large and small intestine. ACSL5 enzyme activity is probably related to enterocytic maturation and intestinal carcinogenesis. Triacsin C is an inhibitor of several ACSL isoforms and is used to differentiate amongst ACSL functions. In rat, ACSL5 is insensitive to triacsin C.
Analysis of human ACSL5 in vitro as well as in a cellular environment revealed sensitivity to the inhibitor triacsin C, which is in contrast to rat ACSL5. The data indicate that a species-related difference in triacsin C inhibition of ACSL5 exists, and human ACSL5 does not compensate for other triacsin C-sensitive human ACSL isoforms.
In previous triacsin C related studies of ACSL activity, cultured cells were preferentially incubated with triacsin C in a concentration clearly below the IC50hu-ACSL5 of approximately 10 μmol/L. Moreover, triacsin C was not used as a competitive inhibitor. In particular, species-related differences of ACSL protein sensitivity to triacsin C have not been systematically addressed up to now.
The recent finding that human ACSL5 is sensitive to the inhibitor triacsin C should be considered in related experiments. In human tissues, the differentiation of ACSL activities and the characterization of ACSL5 function with triacsin C are limited. The current finding suggests that human ACSL5 does not compensate for other triacsin C-sensitive ACSL isoforms.
Acyl-CoA synthetase 5 is an enzyme that catalyzes formation of long-chain acyl-CoA derivatives and belongs to the family of acyl-CoA synthetases (ACSLs; E.C. 6.2.1.3.). Five ACSL isoforms differing in their enzyme kinetics, substrate preferences, and cellular expression have been identified so far in humans and rodents. Triacsin C [1-hydroxy-3-(E,E,E-2’,4’,7’-undecatrienylidine) triazene], an alkenyl-N-hydroxytriazene fungal metabolite, has been reported to be a potent competitive inhibitor of acyl-CoA synthetase activity.
Kaemmerer et al showed that human intestinal ACSL5 is sensitive to triacsin C using purified recombinant protein, CaCo2 cells, and human intestinal mucosa. The experimental design is good and interpretation of results was conducted appropriately.
Peer reviewers: Hitoshi Tsuda, MD, PhD, Diagnostic Pathology Section, Clinical Laboratory Division, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan; Haruhiko Sugimura, MD, PhD, Professor, Department of Pathology, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu, 431-3192, Japan
S- Editor Lv S L- Editor O’Neill M E- Editor Xiong L
| 1. | Shrago E. Long-chain acyl-CoA as a multi-effector ligand in cellular metabolism. J Nutr. 2000;130:290S-293S. [PubMed] [Cited in This Article: ] |
| 2. | Bar-Tana J, Rose G, Brandes R, Shapiro B. Palmitoyl-coenzyme A synthetase. Mechanism of reaction. Biochem J. 1973;131:199-209. [PubMed] [Cited in This Article: ] |
| 3. | Mashek DG, Bornfeldt KE, Coleman RA, Berger J, Bernlohr DA, Black P, DiRusso CC, Farber SA, Guo W, Hashimoto N. Revised nomenclature for the mammalian long-chain acyl-CoA synthetase gene family. J Lipid Res. 2004;45:1958-1961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Yamashita Y, Kumabe T, Cho YY, Watanabe M, Kawagishi J, Yoshimoto T, Fujino T, Kang MJ, Yamamoto TT. Fatty acid induced glioma cell growth is mediated by the acyl-CoA synthetase 5 gene located on chromosome 10q25.1-q25.2, a region frequently deleted in malignant gliomas. Oncogene. 2000;19:5919-5925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Gassler N, Kopitz J, Tehrani A, Ottenwälder B, Schnölzer M, Kartenbeck J, Lyer S, Autschbach F, Poustka A, Otto HF. Expression of acyl-CoA synthetase 5 reflects the state of villus architecture in human small intestine. J Pathol. 2004;202:188-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Gassler N, Roth W, Funke B, Schneider A, Herzog F, Tischendorf JJ, Grund K, Penzel R, Bravo IG, Mariadason J. Regulation of enterocyte apoptosis by acyl-CoA synthetase 5 splicing. Gastroenterology. 2007;133:587-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Gassler N, Herr I, Schneider A, Penzel R, Langbein L, Schirmacher P, Kopitz J. Impaired expression of acyl-CoA synthetase 5 in sporadic colorectal adenocarcinomas. J Pathol. 2005;207:295-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Coleman RA, Lewin TM, Van Horn CG, Gonzalez-Baró MR. Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J Nutr. 2002;132:2123-2126. [PubMed] [Cited in This Article: ] |
| 9. | Caviglia JM, Li LO, Wang S, DiRusso CC, Coleman RA, Lewin TM. Rat long chain acyl-CoA synthetase 5, but not 1, 2, 3, or 4, complements Escherichia coli fadD. J Biol Chem. 2004;279:11163-11169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Tomoda H, Igarashi K, Omura S. Inhibition of acyl-CoA synthetase by triacsins. Biochim Biophys Acta. 1987;921:595-598. [PubMed] [Cited in This Article: ] |
| 11. | Namatame I, Tomoda H, Arai H, Inoue K, Omura S. Complete inhibition of mouse macrophage-derived foam cell formation by triacsin C. J Biochem. 1999;125:319-327. [PubMed] [Cited in This Article: ] |
| 12. | Oh-ishi S, Yamaki K, Abe M, Tomoda H, Omura S. The acyl-CoA synthetase inhibitor triacsin C enhanced eicosanoid release in leukocytes. Jpn J Pharmacol. 1992;59:417-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Weis MT, Brady M, Moore M, Crumley J, Stallone JN. Inhibiting long-chain fatty acyl CoA synthetase does not increase agonist-induced release of arachidonate metabolites from human endothelial cells. J Vasc Res. 2005;42:275-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Tomoda H, Igarashi K, Cyong JC, Omura S. Evidence for an essential role of long chain acyl-CoA synthetase in animal cell proliferation. Inhibition of long chain acyl-CoA synthetase by triacsins caused inhibition of Raji cell proliferation. J Biol Chem. 1991;266:4214-4219. [PubMed] [Cited in This Article: ] |
| 15. | Mashima T, Oh-hara T, Sato S, Mochizuki M, Sugimoto Y, Yamazaki K, Hamada J, Tada M, Moriuchi T, Ishikawa Y. p53-defective tumors with a functional apoptosome-mediated pathway: a new therapeutic target. J Natl Cancer Inst. 2005;97:765-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Kim JH, Lewin TM, Coleman RA. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J Biol Chem. 2001;276:24667-24673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Van Horn CG, Caviglia JM, Li LO, Wang S, Granger DA, Coleman RA. Characterization of recombinant long-chain rat acyl-CoA synthetase isoforms 3 and 6: identification of a novel variant of isoform 6. Biochemistry. 2005;44:1635-1642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Gassler N, Klaus C, Kaemmerer E, Reinartz A. Modifier-concept of colorectal carcinogenesis: lipidomics as a technical tool in pathway analysis. World J Gastroenterol. 2010;16:1820-1827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006:re14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 293] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Lewin TM, Kim JH, Granger DA, Vance JE, Coleman RA. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J Biol Chem. 2001;276:24674-24679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol. 2010;21:212-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Fujimoto Y, Onoduka J, Homma KJ, Yamaguchi S, Mori M, Higashi Y, Makita M, Kinoshita T, Noda J, Itabe H. Long-chain fatty acids induce lipid droplet formation in a cultured human hepatocyte in a manner dependent of Acyl-CoA synthetase. Biol Pharm Bull. 2006;29:2174-2180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Fujimoto Y, Itabe H, Kinoshita T, Homma KJ, Onoduka J, Mori M, Yamaguchi S, Makita M, Higashi Y, Yamashita A. Involvement of ACSL in local synthesis of neutral lipids in cytoplasmic lipid droplets in human hepatocyte HuH7. J Lipid Res. 2007;48:1280-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Mashima T, Sato S, Okabe S, Miyata S, Matsuura M, Sugimoto Y, Tsuruo T, Seimiya H. Acyl-CoA synthetase as a cancer survival factor: its inhibition enhances the efficacy of etoposide. Cancer Sci. 2009;100:1556-1562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Mashek DG, McKenzie MA, Van Horn CG, Coleman RA. Rat long chain acyl-CoA synthetase 5 increases fatty acid uptake and partitioning to cellular triacylglycerol in McArdle-RH7777 cells. J Biol Chem. 2006;281:945-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Zhou Y, Abidi P, Kim A, Chen W, Huang TT, Kraemer FB, Liu J. Transcriptional activation of hepatic ACSL3 and ACSL5 by oncostatin m reduces hypertriglyceridemia through enhanced beta-oxidation. Arterioscler Thromb Vasc Biol. 2007;27:2198-2205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |