Published online Oct 21, 2011. doi: 10.3748/wjg.v17.i39.4408
Revised: March 14, 2011
Accepted: March 21, 2011
Published online: October 21, 2011
AIM: To compare narrow-band imaging (NBI) without image magnification, and chromoendoscopy with Lugol’s solution for detecting high-grade dysplasia and intramucosal esophageal squamous cell carcinoma (SCC) in patients with head and neck cancer.
METHODS: This was a prospective observational study of 129 patients with primary head and neck tumors consecutively referred to the Gastrointestinal Endoscopy Unit of Hospital das Clínicas, São Paulo University Medical School, Brazil, between August 2006 and February 2007. Conventional examinations with NBI and Lugol chromoendoscopy were consecutively performed, and the discovered lesions were mapped, recorded and sent for biopsy. The results of the three methods were compared regarding sensitivity, specificity, accuracy, positive predictive value, negative predictive value, positive likelihood value and negative likelihood value.
RESULTS: Of the 129 patients, nine (7%) were diagnosed with SCC, 5 of which were in situ and 4 which were intramucosal. All carcinomas were detected through NBI and Lugol chromoendoscopy. Only 4 lesions were diagnosed through conventional examination, all of which were larger than 10 mm.
CONCLUSION: NBI technology with optical filters has high sensitivity and high negative predictive value for detecting superficial esophageal SCC, and produces results comparable to those obtained with 2.5% Lugol chromoendoscopy.
- Citation: Ide E, Maluf-Filho F, Chaves DM, Matuguma SE, Sakai P. Narrow-band imaging without magnification for detecting early esophageal squamous cell carcinoma. World J Gastroenterol 2011; 17(39): 4408-4413
- URL: https://www.wjgnet.com/1007-9327/full/v17/i39/4408.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i39.4408
In recent studies, narrow-band imaging (NBI) technology was found to be useful for detecting squamous cell carcinomas (SCC) of the pharynx and esophagus. Morphologic patterns in the changes of intrapapillary capillary loops were found by Muto et al[1], Arima et al[2] and Yoshida et al[3], and can be useful for diagnosing SCC and even predicting the extent of this type of lesion. Accordingly, various reports on early-stage pharyngeal and esophageal SCC diagnosed through the use of NBI technology can be found in the literature. A small, superficial SCC of the pharynx with a small, well-defined brown area was diagnosed by Muto et al[1], without the use of magnification. Using NBI technology without image magnification, Watanabe et al[4], in a prospective study, found six pharyngeal squamous cell carcinomas and Goda et al[5] found an esophageal SCC not identified by conventional endoscopy (obscure lesion).
Despite encouraging results with this new technology, esophageal Lugol staining remains the gold standard for detecting mucosal superficial neoplasias formed by glycogen-poor cells, including epidermoid carcinoma of the esophagus[6,7]. Although Lugol staining is a simple and low-cost method, instillation of its solution may lead to complications, namely hypersensitivity to iodine, laryngitis, pneumonitis, as well as frequent painful sensations and nausea[8-11]. A significant reduction in retrosternal discomfort was demonstrated by Kondo et al[11] with the use of sodium thiosulphate costing $0.15. However, Lugol’s solution is not used in the pharynx or larynx. Therefore, the evaluation of alternative methods for “optical staining” such as NBI is desirable because these methods are potentially simpler, with no complications. The groups most likely to benefit from these methods are those at high risk of developing esophageal SCC, namely patients with malignant squamous cell neoplasias of the head and neck[7,12-14], because these patients routinely undergo endoscopic surveillance for this type of cancer.
The aim of this study was to compare NBI technology with Lugol staining during endoscopic examination of the esophagus for the detection of high-grade intraepithelial neoplasia and superficial SCC in this organ in patients with head and neck cancer.
From August 2006 to February 2007, 136 consecutive patients with head and neck tumors were referred to the Gastrointestinal Endoscopy Unit of Hospital das Clínicas, São Paulo University Medical School, Brazil, for detection of esophageal SCC. Patients with head and neck SCC undergo annual upper gastrointestinal (GI) endoscopy and associated chromoendoscopy of the esophageal mucosa with Lugol’s solution.
The inclusion criteria were indication of upper GI endoscopy examination for patients with head and neck SCC under surveillance for the detection of synchronous or metachronous lesions.
Exclusion criteria for patients were as follows: (1) clinical conditions precluding upper GI endoscopy examination and 2.0% Lugol staining; (2) history of allergic reaction to iodine; and (3) diagnosis of esophageal neoplasia of advanced endoscopic appearance defined as an ulcerated, infiltrative or stenotic lesion easily detected on conventional examination
All participants provided written informed consent. This study was approved by the Ethics Committee of the Gastroenterology Department of São Paulo University Medical School, under research protocol No. 1083/06.
An Exera II Evis 180 GIF180 (Olympus, Tokyo, Japan) videoendoscope with high resolution (1080 dpi), 1.5-fold magnification and NBI technology was used. Examinations were performed conventionally under conscious sedation with midazolam and fentanyl chlorhydrate. A 2.0% Lugol’s solution was used for staining. NBI and Lugol staining procedures were followed up by a single physician, and both methods were performed in one single procedure. First the organ was conventionally assessed with white light, and adhered residues or exudates were removed through potable water instillation. Assessment with NBI and 2.0% Lugol staining was subsequently performed. Upon mucosal analysis and biopsy, 0.5% sodium thiosulfate solution was instilled to remove the Lugol’s solution from the mucosa to reduce spasm and pain. Therefore, the examination was divided into three phases. The first phase was the white light analysis, the second phase was the analysis of the mucosa with NBI, and the final phase was the assessment following Lugol staining. At the end of each phase, changes were documented in dynamic and static images and mapped using the anterior, posterior, and right and left lateral walls of the organ and the distance of the lesion from the anterior incisors as references. A biopsy was always performed after staining was completed.
For patients with malignant or actinic stenosis of the pharyngeal-esophageal tract, a smaller-diameter endoscope, GIF 180N model (Olympus, Tokyo, Japan), with a 4.9-mm caliber and 2.1-mm biopsy channel was used. Some of the possible complications resulting from Lugol’s solution, such as laryngitis, chemical pneumonitis, hypersensitivity, and anaphylactic shock were registered.
When NBI was used, brown-stained areas of the mucosa were considered lesions suspected to be neoplasia (compared to “normal” mucosa, which is green independently of changes in surface or vascular texture). As for the Lugol dye solution, areas clearly not stained were suspected to be neoplasia, which is characterized by a white color in contrast with brown or brownish “normal” areas. The size and macroscopic shape were evaluated according to the Paris Classification[15] for superficial esophageal lesions and their topography (cervical up to 5 cm of cricopharyngeal, thoracic and abdominal esophagus).
Histopathology was performed by a senior pathologist from the Department of Pathology of Hospital das Clínicas, São Paulo. The pathologist was aware of the endoscopic suspicion of esophageal SCC. Biopsy specimens were immersed in formaldehyde for fixation and stained using hematoxylin and eosin. The lesions were classified according to the Revised Vienna Classification. In the absence of lamina propria invasion, noninvasive neoplastic lesions were divided into two groups based on the degree of intraepithelial neoplasia: low grade and high grade. High-grade dysplasia, intraepithelial carcinoma and carcinoma in situ were considered equivalent entities[15]. Whenever the lamina propria of the mucosa was invaded, the lesion was referred to as a microinvasive or intramucosal carcinoma.
In this study, only the findings of high-grade intraepithelial neoplasia (carcinoma in situ) and intramucosal carcinoma of squamous cells were considered true-positives for esophageal epidermoid carcinoma[15].
Values were calculated for sensitivity, specificity, positive predictive value, negative predictive value, accuracy, positive and negative likelihood ratio, and their respective 95% confidence intervals (CIs).
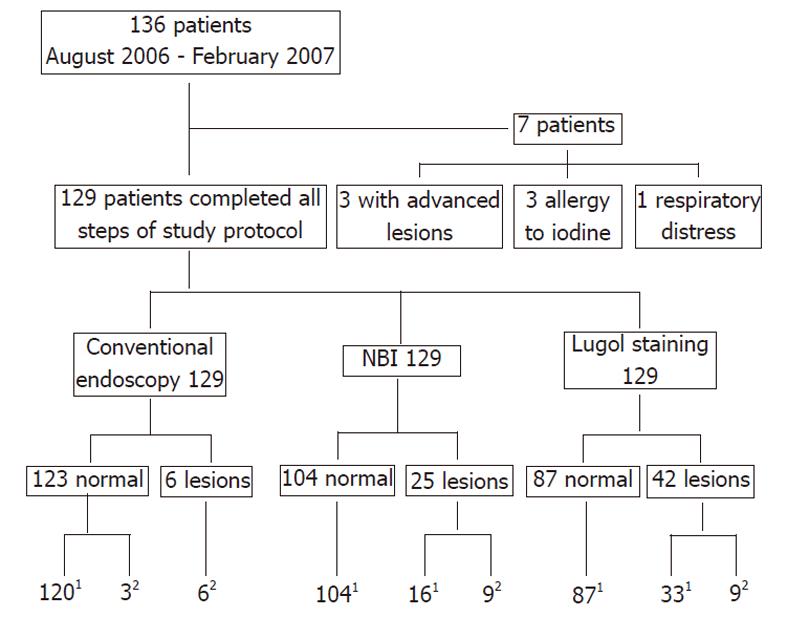
Of the 136 patients, three were excluded as they had malignant esophageal lesions that were easily detected by conventional endoscopy, three as they had a prior history of allergy to iodine, and one had respiratory distress that prevented Lugol staining. Of the remaining 129 patients, there were 103 males and 26 females, aged 33 to 89 years (mean, 59 years).
One hundred and twenty-nine patients underwent all stages of the protocol of investigation for this study (Figure 1). For 42 patients in whom lesions were detected by NBI or Lugol dye, 9 (24.5%) proved to have esophageal neoplasias (four intramucosal neoplasias and five carcinomas in situ), and the remaining 33 patients exhibiting unstained lesions with Lugol’s solution had inflammatory disease only (Table 1).
| Method | No. of endoscopic findings | SCC | Esophagitis | |
| In situ | Intramucosal | |||
| Conventional | 6 | 3 | 3 | 0 |
| NBI | 25 | 5 | 4 | 16 |
| Lugol | 42 | 5 | 4 | 33 |
Macroscopic classification, size, location, and histopathologic findings of the samples of the endoscopic or surgical resection are presented in Table 2. All carcinomas were found in the thoracic esophagus. The incidence was 4% for females and 8% for males.
| Case | Macroscopic classification by conventional endoscopy | Size(mm) | Location at esophagus | Histopathologic examination |
| 161 | n/i | 10 | Thoracic | Intramucosal |
| 175 | 0-IIc | 50 | Thoracic | In situ |
| 72 | 0-IIc | 30 | Thoracic | Intramucosal |
| 15 | 0-IIb | 20 | Thoracic | In situ |
| 94 | 0-IIc | 50 | Thoracic | Intramucosal |
| 64 | 0-IIb | 20 | Thoracic | In situ |
| 115 | n/i | 20 | Thoracic | In situ |
| 119 | n/i | 10 | Thoracic | In situ |
| 74 | 0-IIb | 25 | Thoracic | Intramucosal |
Conventional endoscopy was able to identify most of the 10 mm lesions, and the sensitivity was 85.7% (95% CI, 59.8%-100%). However, this method failed in the diagnosis of smaller lesions (< 10 mm), where the sensitivity was 0%.
The performance of NBI was similar to that obtained by Lugol staining. Sensitivity and negative predictive value were 100% for both methods, and the specificity was 86.7% for NBI (95% CI, 80.6%-92.7%) and 72.5% (95% CI, 64.5%-80.5%) for Lugol’s solution.
Diagnostic performances for conventional endoscopic examinations, NBI and Lugol staining are presented in Table 3, and the performance results by lesion size are presented in Table 4.
| Conventional endoscopic examination | NBI | Lugol’s solution | |
| Sensitivity | 66.7 (35.9-97.5) | 100 (100-100) | 100 (100-100) |
| Specificity | 100 (100-100) | 86.7 (80.6-92.7) | 72.5 (64.5-80.5) |
| PPV | 100 (100-100) | 36 (17.2-54.8) | 21.4 (9.0-33.8) |
| NPV | 97.6 (98.4-100) | 100 (100-100) | 100 (100-100) |
| Accuracy | 97.7 (95.1-100) | 87.6 (81.9-93.3) | 74.4 (66.9-81.9) |
| PLR | n/c | 7.5 | 3.6 |
| NLR | 0.33 | 0 | 0 |
| Conventional examination | NBI | Lugol’s solution | ||||
| <10 mm | >10 mm | <10 mm | >10 mm | <10 mm | >10 mm | |
| Sensitivity (95% CI) | 0 (0-0) | 85.7 (59.8-100) | 100 (100-100) | 100 (100-100) | 100 (100-100) | 100 (100-100) |
| Specificity (95% CI) | 100 (100-100) | 100 (100-100) | 90 (84.6-95.4) | 96.7 (93.5-100) | 75.8 (68.2-83.5) | 96.7 (93.5-100) |
| PPV (95% CI) | n/c | 100 (100-100) | 14.3 (4.0-32.6) | 63.6 (35.2-92.1) | 6.5 (2.2-15.1) | 63.6 (35.2-92.1) |
| NPV (95% CI) | 98.4 (96.1-100) | 99.20 (97.6-100) | 100 (100-100) | 100 (100-100) | 100 (100-100) | 100 (100-100) |
| Accuracy (95% CI) | 98.4 (96.1- 100) | 99.2 (97.6-100) | 90.2 (84.9-95.4) | 96.9 (93.8-100) | 76.2 (68.7-83.8) | 96.9 (93.8-99.9) |
| PLR | n/c | n/c | 10 | 30 | 4.1 | 30 |
| NLR | 1 | 0.1 | 0 | 0 | 0 | 0 |
In the Lugol’s solution group, there were 3 cases of chemical laryngitis and one of hypersensitivity to iodine (3% complication rate). No complications were reported with the conventional or NBI procedures.
Upper GI endoscopy with Lugol’s solution staining is still considered the best method for tracking, diagnosing and delimiting superficial neoplasias of the esophagus[16-18]. From 1965 to 1984, no intraepithelial and/or intramucosal lesion was diagnosed in the University Hospital of Kyushu, Japan[19]. These lesions have only been observed since 1985, when Lugol’s solution was initially used routinely for endoscopic evaluation of the mucosal surface. From 1985 to 1988, Sugimachi et al[19] reported an increase in the number of patients with early stages of the disease receiving surgical treatment, ranging from 7% to 23% of patients undergoing surgical treatment. The results for the surgical treatment of esophageal-thoracic SCC in two major referral centers in Japan and China were compared by Fang et al[20], and demonstrated a 2-year survival rate of 70.9% in Japan vs 56.2% in China. According to these authors, the use of Lugol chromoendoscopy to detect early lesions could explain the better results obtained at the Japanese center.
Lugol chromoendoscopy also proved useful for the detection of early esophageal SCC in patients with head and neck cancer, a recognized high-risk group for the disease. In more than half of the head and neck cancer patients exhibiting the minimal mucosal changes associated with a lack of iodine impregnation (negative Lugol staining area), biopsies confirmed the presence of malignant neoplasia (sensitivity of 81.96% vs 59.1% when only the negative Lugol staining area was evaluated separately). Furthermore, no cases of neoplasia in normally-stained areas were found by Hashimoto et al[7].
However, Lugol’s solution irritates the mucosa and may lead to retrosternal chest pain and discomfort because of its alcoholic nature. Its use is limited by other factors, namely hypersensitivity to iodine and the risks of chemical esophagitis, laryngitis and bronchopneumonia. Several authors have reported necrosis and injury to esophageal and gastric mucosa caused by hypersensitivity to Lugol’s solution[8-10,12]. Furthermore, Lugol chromoendoscopy significantly increases the examination period[11].
Slightly over 50% of early lesions are detected on conventional endoscopy, and the use of Lugol staining is still restricted to patients considered at high risk of developing this neoplasia. Furthermore, because of the previously mentioned risks and difficulties, a large portion of the population does not have access to efficient and safe examinations for the detection of early lesions, including those that are locally resectable. NBI does not have the limitations of Lugol chromoendoscopy and should therefore be considered as a replacement if it is equally efficient in detecting esophageal SCC.
A few studies have evaluated the capacity of NBI without the use of image magnification for detecting esophageal SCC. A 2-fold capacity for detecting pharyngeal SCC compared with conventional white-light evaluation was found by Watanabe et al[4]. In a multicenter study comparing the evaluation by narrow-band technology vs conventional white-light evaluation, the accuracies were 90.2% and 55.3%, respectively, (P < 0.0001)[22]. Comparing Lugol chromoendoscopy to NBI technology with image magnification, equal results were found in the sensitivity of the two methods (92.3% vs 92.3%), but NBI had a better specificity (91.7% vs 72.2%)[24].
The present study compared use of NBI technology with Lugol chromoendoscopy (a method considered the gold standard) for the detection of esophageal epidermoid carcinoma. This study was conducted in patients with head and neck cancer and without the use of image magnification. Many medical services do not have the resources for magnification; therefore, the aim of this study was to analyze whether NBI alone would suffice to detect small, superficial neoplasias of the esophagus. Of nine esophageal neoplasias, conventional white light could not detect three neoplasias, whereas both NBI and Lugol chromoendoscopy detected all neoplasias. The elevated likelihood ratio certifies the equivalence and high sensitivity of both methods. On the other hand, NBI esophagoscopy without image magnification, similar to Lugol chromoendoscopy, has a lower specificity for detecting early squamous cell neoplasias in the esophagus. Although Ponchon et al[25] reported a 75% specificity for NBI, this may have been related to false detection of nonspecific inflammation. In two recent studies by Takenaka et al[26] and Lee et al[27], similar results were found: the sensitivity of NBI endoscopy for detecting esophageal SCC and high-grade intraepithelial neoplasia was 90.9% (95% CI, 58.7%-99.8%), specificity was 95.4% (95% CI, 90.3%-98.3%), and accuracy was 95.1% (95% CI, 90.1%-98.0%). Recently, transnasal endoscopy with NBI and Lugol staining were employed to screen patients with head and neck cancer whose condition prevented oral intubation with a standard endoscope and, as in our study, NBI and Lugol staining had the same sensitivity (88.9%) for the detection of high-grade mucosal lesions and performed far better than standard endoscopy (27.3% sensitivity)[26,27]. The equivalent performance of NBI esophagoscopy and Lugol chromoendoscopy indicate that NBI esophagoscopy, without image magnification, is a potential surveillance method for patients at risk of esophageal squamous cell neoplasia. Therefore, this technology should be tested in other groups that are at risk of SCC (e.g., chronic corrosive esophagitis patients, tobacco smokers and alcohol users), and should be compared to Lugol chromoendoscopy.
The current study has some limitations. First, a sequential approach was adopted in which, in order, standard endoscopy, NBI and Lugol staining were employed by the same operator. This approach has the potential bias of the same operator entering an examination phase having already detected any lesion in the prior phase. However, the sequential approach seems to be the best strategy for daily practice. Furthermore, the same methodology was used in similar studies[22-25].
In conclusion, narrow-band technology with optical filters has a high sensitivity and high negative predictive value for detecting superficial squamous cell carcinomas of the esophagus (intramucosal carcinoma and carcinoma in situ). These results are comparable to those obtained with 2.5% Lugol chromoendoscopy but without the risks and technical difficulties related to this method. NBI could replace Lugol chromoendoscopy as a screening tool for detecting esophageal squamous cell carcinoma in patients with head and neck cancer.
Squamous cell carcinoma (SCC) of the esophagus is aggressive with high mortality. Early diagnosis has a major impact on survival and treatment costs. Narrow-band imaging (NBI) technology without magnification is simple to use and increases the rate of diagnosis of early lesions.
In this study, the authors showed that NBI without magnification can replace Lugol staining in early diagnosis of lesions of the esophagus SCC.
The device used in Japanese studies are different from those used in Western countries (Lucera system), and led to the first use of NBI technology without magnification in detection of SCC of the esophagus.
NBI technology is easy to learn and use, increasing the diagnosis rate of early lesions in patients considered at risk of SCC.
This is an interesting study comparing NBI to Lugo’s staining and white light endoscopy for detection of early esophageal cancer in a Brazil medical center over 6 mo involving 129 patients. This study evaluated the usefulness of NBI in daily practice for screening of esophageal cancer. The study shows NBI is as effective as Lugol’s chromoendoscopy for detecting early esophageal cancer.
Peer reviewers: Dr. Hsu-Heng Yen, MD, Department of Gastroenterology, Changhua Christian Hospital, 135 Nanhsiao Street, Changhua 500, Taiwan, China; Dr. Shinji Tanaka, Director, Department of Endoscopy, Hiroshima University Hospital, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8551, Japan
S- Editor Tian L L- Editor Cant MR E- Editor Xiong L
| 1. | Muto M, Nakane M, Katada C, Sano Y, Ohtsu A, Esumi H, Ebihara S, Yoshida S. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375-1381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 265] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Arima M, Tada M, Arima H. Evaluation of microvascular patterns of superficial oesophageal cancers by magnifying endoscopy. Esophagus. 2005;2:191-197. [DOI] [Cited in This Article: ] |
| 3. | Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288-295. [PubMed] [Cited in This Article: ] |
| 4. | Watanabe A, Tsujie H, Taniguchi M, Hosokawa M, Fujita M, Sasaki S. Laryngoscopic detection of pharyngeal carcinoma in situ with narrowband imaging. Laryngoscope. 2006;116:650-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Goda KI, Tajiri H, Kaise M, Kato M, Takubo K. Flat and small squamous cell carcinoma of the oesophagus detected and diagnosed by endoscopy with narrow-band imaging system. Dig Endosc. 2006;18:S9-S12. [Cited in This Article: ] |
| 6. | Nabeya K, Hanaoka T, Onozawa K, Ri S, Nyumura T, Kaku C. Early diagnosis of esophageal cancer. Hepatogastroenterology. 1990;37:368-370. [PubMed] [Cited in This Article: ] |
| 7. | Hashimoto CL, Iriya K, Baba ER, Navarro-Rodriguez T, Zerbini MC, Eisig JN, Barbuti R, Chinzon D, Moraes-Filho JP. Lugol's dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Sreedharan A, Rembacken BJ, Rotimi O. Acute toxic gastric mucosal damage induced by Lugol's iodine spray during chromoendoscopy. Gut. 2005;54:886-887. [PubMed] [Cited in This Article: ] |
| 9. | Myung Park J, Seok Lee I, Young Kang J, Nyol Paik C, Kyung Cho Y, Woo Kim S, Choi MG, Chung IS. Acute esophageal and gastric injury: complication of Lugol's solution. Scand J Gastroenterol. 2007;42:135-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Thuler FP, de Paulo GA, Ferrari AP. Chemical esophagitis after chromoendoscopy with Lugol's solution for esophageal cancer: case report. Gastrointest Endosc. 2004;59:925-926. [PubMed] [Cited in This Article: ] |
| 11. | Kondo H, Fukuda H, Ono H, Gotoda T, Saito D, Takahiro K, Shirao K, Yamaguchi H, Yoshida S. Sodium thiosulfate solution spray for relief of irritation caused by Lugol’s stain in staining. Gastroint Endosc. 2001;53:199-202. [Cited in This Article: ] |
| 12. | Shiozaki H, Tahara H, Kobayashi K, Yano H, Tamura S, Imamoto H, Yano T, Oku K, Miyata M, Nishiyama K. Endoscopic screening of early esophageal cancer with the Lugol dye method in patients with head and neck cancers. Cancer. 1990;66:2068-2071. [PubMed] [Cited in This Article: ] |
| 13. | Tincani AJ, Brandalise N, Altemani A, Scanavini RC, Valério JB, Lage HT, Molina G, Martins AS. Diagnosis of superficial esophageal cancer and dysplasia using endoscopic screening with a 2% lugol dye solution in patients with head and neck cancer. Head Neck. 2000;22:170-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 14. | Goldstein HM, Zornoza J. Association of squamous cell carcinoma of the head and neck with cancer of the esophagus. AJR Am J Roentgenol. 1978;131:791-794. [PubMed] [Cited in This Article: ] |
| 15. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58 suppl 6:S3-S43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1117] [Cited by in F6Publishing: 1197] [Article Influence: 57.0] [Reference Citation Analysis (3)] |
| 16. | Dubuc J, Legoux JL, Winnock M, Seyrig JA, Barbier JP, Barrioz T, Laugier R, Boulay G, Grasset D, Sautereau D. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy. 2006;38:690-695. [PubMed] [Cited in This Article: ] |
| 17. | Rossini AR, Hashimoto CL, Iriya K, Zerbini C, Baba ER, Moraes-Filho JP. Dietary habits, ethanol and tobacco consumption as predictive factors in the development of esophageal carcinoma in patients with head and neck neoplasms. Dis Esophagus. 2008;21:316-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Muto M, Takahashi M, Ohtsu A, Ebihara S, Yoshida S, Esumi H. Risk of multiple squamous cell carcinomas both in the esophagus and the head and neck region. Carcinogenesis. 2005;26:1008-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 86] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 19. | Sugimachi K, Ohno S, Matsuda H, Mori M, Matsuoka H, Kuwano H. Clinicopathologic study of early stage esophageal carcinoma. Br J Surg. 1989;76:759-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Fang W, Kato H, Chen W, Tachimori Y, Igaki H, Sato H. Comparison of surgical management of thoracic esophageal carcinoma between two referral centers in Japan and China. Jpn J Clin Oncol. 2001;31:203-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Gono K, Yamazaki K, Doguchi N, Sano Y, Nonami T, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y. Endoscopic observation of tissue by narrow band illumination. Opt Rev. 2003;10:211-215. [DOI] [Cited in This Article: ] |
| 22. | Muto M, Saito Y, Ohmori T, Kaise M, Kaise M, Inoue H, Ishikawa H, Sugiura H, Ochiai A, Shimoda T. Multicenter prospective randomized controlled study on the detection and diagnosis of superficial squamous cell carcinoma by back-to-back endoscopic examination of narrowband imaging and white light observation. Gastrointest Endosc. 2007;65:AB110. [DOI] [Cited in This Article: ] |
| 23. | Muto M, Horimatsu T, Ezoe Y, Hori K, Yukawa Y, Morita S, Miyamoto S, Chiba T. Narrow-band imaging of the gastrointestinal tract. J Gastroenterol. 2009;44:13-25. [PubMed] [Cited in This Article: ] |
| 24. | Chiu PW, Cheung FK, Tsang RK. Narrow band imaging (NBI) against conventional Lugol staining for detection of superficial oesophageal neoplasia in high risk patients: a prospective comparative study. Gastrointest Endosc. 2007;65:AB159. [Cited in This Article: ] |
| 25. | Ponchon T, Lapalus MG, Saurin JC, Robles-Medranda C, Chemaly M, Parmentier B, Guillaud O. Could narrow band imaging (NBI) replace Lugol staining for the detection of oesophageal squamous cell carcinoma? Gastrointest Endosc. 2007;65:AB343 (abstract). [Cited in This Article: ] |
| 26. | Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, Tanioka D, Tsuzuki T, Uemura M, Ohara N. Narrow-Band Imaging Provides Reliable Screening for Esophageal Malignancy in Patients With Head and Neck Cancers. Am J Gastroenterol. 2009;104:2942-2948. [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Lee YC, Wang CP, Chen CC, Chiu HM, Ko JY, Lou PJ, Yang TL, Huang HY, Wu MS, Lin JT. Transnasal endoscopy with narrow-band imaging and Lugol staining to screen patients with head and neck cancer whose condition limits oral intubation with standard endoscope (with video). Gastrointest Endosc. 2009;69:408-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |