Published online Oct 7, 2011. doi: 10.3748/wjg.v17.i37.4166
Revised: June 20, 2011
Accepted: June 27, 2011
Published online: October 7, 2011
The thiopurine drugs, 6-mercaptopurine (6-MP) and azathioprine, are efficacious in the arsenal of inflammatory bowel disease (IBD) therapy. Previous reports indicate that 6-thioguanine nucleotide (6-TGN) levels correlate with therapeutic efficacy, whereas high 6-methylmercaptopurine (6-MMP) levels are associated with hepatotoxicity and myelotoxicity. Due to their complex metabolism, there is wide individual variation in patient response therein, both in achieving therapeutic drug levels as well as in developing adverse reactions. Several strategies to optimize 6-TGN while minimizing 6-MMP levels have been adopted to administer the thiopurine class of drugs to patients who otherwise would not tolerate these drugs due to side-effects. In this report, we will review different approaches to administer the thiopurine medications, including the administration of 6-mercaptopurine in those unsuccessfully treated with azathioprine; co-administration of thiopurine with allopurinol; co-administration of thiopurine with anti-tumor necrosis factor α; 6-TGN administration; desensitization trials; and split dosing of 6-MP.
- Citation: Bradford K, Shih DQ. Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease. World J Gastroenterol 2011; 17(37): 4166-4173
- URL: https://www.wjgnet.com/1007-9327/full/v17/i37/4166.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i37.4166
Inflammatory bowel disease (IBD) encompassing Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic inflammatory disorder caused by dysregulated immune responses in a genetically predisposed individual. Given the role that the immune system plays in IBD, the hallmark of therapy is immune modulation. The thiopurine drugs, 6-mercaptopurine (6-MP) and its prodrug azathioprine (AZA), remain the mainstay of immunomodulator therapy for IBD and are indicated in steroid-dependent and -refractory patients, as prophylaxis in CD[1-3]. Chebli found that AZA maintained steroid-free clinical remission for three years in UC patients, previously steroid-dependent[2]. Of note, however, AZA has not been shown to be effective in treating active UC flare[4]. Rather, thiopurines have also been noted to induce and maintain remission in UC and CD patients, more effectively than 5-aminosalicylic acid[1,5-10]. However efficacious, their use is often limited, as an estimated 30% to 50% of patients discontinue these drugs due to either side-effects or lack of clinical efficacy[11-13]. The lack of response to these immunomodulators has been attributed to differences in individual variations in drug metabolism[14,15]. The 6-thioguanine nucleotide (6-TGN) metabolite of 6-MP and AZA appears to be the predominant active metabolite responsible for therapeutic efficacy, whereas 6-methylmercaptopurine (6-MMP) levels correlate with the risk of hepatotoxicity and possibly myelotoxicity[15,16]. Theoretically, if the thiopurine metabolite profile can be shifted to 6-TGN, a greater percentage of IBD patients would benefit from immunomodulator therapy. A meta-analysis has confirmed that higher 6-TGN levels are associated with remission among IBD patients[17]. In this review, we will discuss the thiopurine metabolic pathway, monitor the drug metabolite levels, and evaluate the different approaches that have been developed to enhance clinical efficacy and minimize the side-effects of AZA and 6-MP.
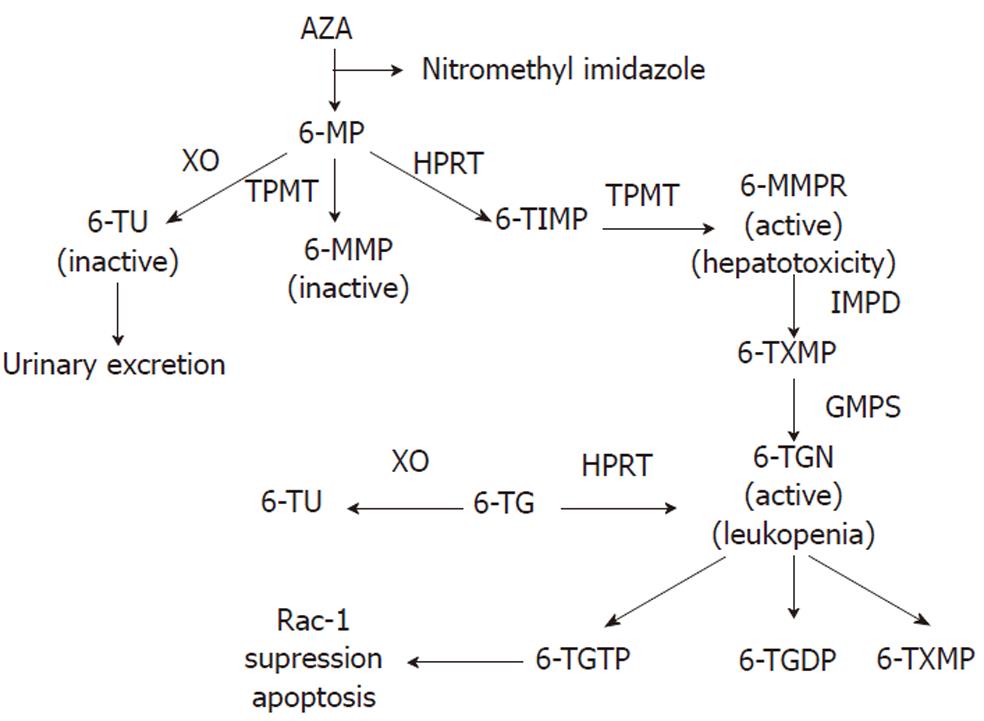
To achieve the active cytotoxic form, AZA is metabolized via a series of biochemical pathways summarized in Figure 1. Initially, approximately 90% is non-enzymatically cleaved to 6-MP in the liver[18,19]. There are three competitive metabolic pathways in 6-MP metabolism. It can be inactivated to 6-thiouric acid (6-TU) via xanthine oxidase (XO), activated to 6-MMP via thiopurine methyltransferase (TMPT), or to the therapeutic 6-TGN via enzymes hypoxanthine phosphoribosyl transferase (HPRT), inosine monophosphate dehydrogenase (IMPDH), and guanosine monophosphate synthetase (GMPS)[11,20,21]. A complete understanding of its mode of action is unknown[19]; however, based on its structural similarity to the purine guanine, 6-TGN is a purine antagonist that inserts within the DNA of leukocytes[22]. Intracellular build up of 6-TGN is thought to be the cytoactive form that inhibits DNA synthesis and downstream T cell proliferation for its immunosuppressive activity[20,23,24]. Using a genome-wide expression profiling approach, 6-TGN was found to inhibit several immune and inflammation-related genes including tumor necrosis factor-related apoptosis-inducing ligand, tumor necrosis factor receptor superfamily member 7, and α4-integrin in activated but not resting T lymphocytes[25]. Thus, 6-TGN may additionally exert its immunosuppressive effect by down-regulating the expression of pro-inflammatory and gut-homing factors. Another report found that the immunosuppressive role of thiopurine medications may in part be due to its metabolite 6-thioguanine triphosphate (6-TGTP) suppression of the Rac1 protein, which participates in T cell maturation and proliferation, thus inducing T lymphocyte apoptosis[19,26].
Because AZA is 55% of 6-MP by molecular weight and 88% of AZA is converted to 6-MP, historically, thiopurines are dosed by the patient’s weight; the maintenance dose of AZA is 2-2.5 mg/kg per day and 6-MP is dosed at half that of AZA, or 1-1.5 mg/kg per day in IBD patients[1,22,7-29]. Individual variation in drug metabolism account for the differences in therapeutic efficacy and development of adverse reactions[30,31]. Fortunately, advances in thiopurine metabolite monitoring can help predict which patients are more at risk of developing side effects, allowing for adjustments in drug dosages[11,15].
TPMT is a key enzyme whose activity determines the level of 6-MMP as well as 6-TGN metabolite levels[15,20,24,30]. TPMT methylates 6-MP to 6-MMP and 6-TIMP to 6-methylmercaptopurine ribonucleotide (MMPR) (Figure 1)[15]. Elevated levels of 6-MMP (> 5700 pmol/ 8 × 108 erythrocytes) are associated with hepatotoxicity, whereas 6-TGN is the metabolite responsible for the therapeutic activity of thiopurines. Monitoring the thiopurine metabolite levels can help to optimize immunomodulator therapy and minimize adverse events. A retrospective study showed that patients who did not respond to AZA or 6-MP either had a high 6-MMP concentration or 6-MMP/6-TGN ratio[12]. Furthermore, a subset of IBD patients preferentially metabolize thiopurines to the hepatotoxic 6-MMPR which explains why some patients develop toxic metabolite accumulation, side effects and ultimately cannot be maintained on thiopurine therapy[30]. Even though 6-TGN is associated with therapeutic immunosuppressive activity, an excess amount of this metabolite poses an increased risk for myelosuppression[32]. The therapeutic efficacy of 6-MP or AZA are correlated with 6-TGN levels between 235-450 pmol/8 × 108 erythrocytes[15,22,33,34]. Because several studies have shown that weight-based dosing is poorly correlated with 6-TGN levels[16,35,36], monitoring thiopurine metabolite levels can help optimize immunomodulatory therapy while minimizing adverse effects[15,30,34].
Differences in patient response to thiopurines may in part be due to patient-specific metabolism and genetic variation[30]. TPMT activity is inversely related to clinical response to AZA[11]. Different genetic polymorphisms code for the level of TPMT activity[37]. 0.3% of the Caucasian population are homozygous for low enzyme activity; 11% are heterozygous and 89% are homozygous for high enzyme activity[38]. Allelic frequency patterns vary among different ethnic groups. In Caucasian populations, intermediate or low TPMT activity is most frequently associated with TPMT*2, TPMT*3A or TPMT*3C alleles[38], while in African-Americans, TPMT*3C is the most prevalent variant allele[39]. Wild-type or heterozygous TPMT deficient patients have high TPMT activity > 14 units/mL RBC, which were associated with higher levels of 6-MMP and lower levels of 6-TGN, and thus a decreased likelihood of achieving complete remission (termed 6-MP resistance) and increased risk for hepatotoxicity[11,13,30]. Dose escalation of thiopurine level may optimize 6-TGN levels, but must be done under caution given that TPMT also catalyzes the formation of the toxic metabolite 6-MMPR[40]. A meta-analysis found that TPMT polymorphisms are related to adverse drug reactions and myelotoxicity, but not hepatotoxicity or pancreatitis[37]. It is thought that the higher level of TPMT activity may cause higher 6-MP catabolism resulting in higher 6-MMP and decreased 6-TGN levels[11]. Low TMPT and thus high 6-TGN is associated with a higher risk for leukopenia[13,15].
Studies have found that checking TMPT activity may be cost-effective as compared to standard therapeutic dose administration[33,41]. Traditionally, AZA or 6-MP was started at a low dose and progressively titrated up because of safety concerns (bone marrow suppression, hepatotoxicity, etc.). Using this strategy, time to initial response is delayed and can take up to 6 mo to reach therapeutic response[6,33,42,43]. Compared to traditional thiopurine dosing, monitoring TMPT can allow faster achievement of initial response (22.4 wk vs 18.9 wk) and lower costs at 1 year ($7142 vs $3861)[33]. Thus, patients found to have normal TPMT could have dose escalation sooner therefore avoiding delay in achieving response[33]. The cost-effectiveness of measuring TPMT activity was independently shown in a separate study[41]. Furthermore, awareness of TMPT activity can help to avoid potential deleterious consequences of thiopurine therapy. For example, in patients with low TPMT activity, a lower initiation dose or avoidance of either 6-MP or AZA is recommended due to risks of leukopenia[16,44]. Albeit, TMPT activity monitoring is not universally available to all practitioners; in these cases, thiopurine may be started at a low-dose (50 mg daily) and titrated up with weekly monitoring of CBC and liver function tests during the first 2 mo, and once every 3 mo thereafter[20].
In addition to bone marrow suppression and hepatotoxicity, early hypersensitivity reactions including fever and gastrointestinal side effects including diarrhea, nausea, and emesis can occur in as many as 10% of patients[45]. These adverse reactions often cause IBD patients to discontinue thiopurine therapy. Several studies have shown that among patients intolerant of AZA, 6-MP may be a safe and effective alternative[29,45,46]. One study showed that 20 of 29 (69%) IBD patients with a history of AZA hypersensitivity tolerated 6-MP[46]. An AZA to 6-MP change appears to be more effective in UC compared to CD patients as by the end of the first year, none of the CD patients were maintained on 6-MP[46]. In addition to hypersensitivity reactions, up to 60% of IBD patients with AZA intolerance due to nausea, emesis, and flu-like illness tolerated switching to 6-MP[45]. In contrast, patients who discontinued AZA due to hepatotoxicity or pancreatitis were less likely to tolerate 6-MP[45]. Another study found that 48% of patients previously intolerant to AZA due to myalgia and arthralgia were able to tolerate 6-MP[16]. Another report showed that 11 of 15 (11 CD, 4 UC) patients (73.3%) who discontinued AZA due to epigastric pain, nausea and vomiting tolerated 6-MP and reached therapeutic goals[47]. A retrospective study showed that 19 out of 140 patients discontinued AZA therapy (4 patients for clinical inefficacy, 13 due to side-effects, 2 due to leucopenia)[48]. Of these 19 patients, 11 (58%) tolerated the switch to 6-MP[48]. Consistent with the above findings, another report showed that 6 of 11 patients who initially could not tolerate AZA, were able to tolerate 6-MP and achieve response[18]. The reasons behind the observation that 6-MP bypasses the adverse reactions caused by AZA are unclear, but may in part be due to the nitro-imidazole structure that is released as AZA is cleaved to 6-MP[49].
Based upon the above studies, we propose that 6-MP should be considered in IBD patients who require continuing immunosuppressive therapy but are intolerant of AZA. We caution that there has been variable success among those who are switched to 6-MP (Table 1), and unfortunately many of the same reactions to AZA develop with 6-MP over time.
| Method | Effectiveness (%) | Side effect |
| AZA to 6-MP | 48-73[45-48] | Nausea, vomiting, hepatotoxicity, neutropenia, pancreatitis |
| 6-MP to AZA | Not effective[48,50,51] | Nausea, vomiting, hepatotoxicity, neutropenia, pancreatitis |
| Desensitization | 25[51] | Hypersensitivity reaction |
| Combination infliximab/thiopurine | 25[72,74] | Lymphoma, infection |
| 6-TG | 46-82[50,58] | NRH, veno-occlusive disease and possible tumor |
| Allopurinol supplementation | 25-75[68,69] | Skin rash, renal impairment, leukopenia |
| Split-dosing | 60[75] | Reduces 6-MP, AZA associated adverse effects |
The converse treatment strategy of administering AZA to patients who did not tolerate initial 6-MP therapy has not proved as effective[48,50,51]. In a trial of AZA after 6-MP adverse reactions, similar side-effect profiles were seen[18,48,50,51]. This is likely due to the fact that AZA is converted in the liver to 6-MP (Figure 1), thereby, yielding similar adverse reactions (Table 1). Based upon the lack of clinical efficacy, we do not recommend using AZA in patients who were previously intolerant of 6-MP.
Some investigators propose desensitization in the subset of patients who experience hypersensitivity reactions to AZA or 6-MP within the first month of treatment. Korelitz et al[51,52] retrospectively reviewed 591 charts of IBD patients treated with 6-MP. Four of 16 patients who had early hypersensitivity reactions were successfully desensitized to 6-MP or AZA and achieved long-term clinical remission. One patient tolerated the direct switch from 6-MP to AZA. Of the remaining 11 patients, 5 needed surgery, 2 were changed to methotrexate (MTX), and 4 had chronic symptoms. In this study, desensitization began at one-quarter tablet per day, with an increase in the dose every 3 d for several weeks until a full dose was reached[51,52]. A similar case report describes a CD patient who developed skin rash after 4 wk of treatment with AZA[53]. Upon drug withdrawal, the rash resolved. A skin test was positive for AZA allergy, suggesting an IgE mediated hypersensitivity; and the patient was desensitized with AZA, with the patient’s CD successfully in remission[53]. Another case report describes a CD patient who developed a macular, erythematous truncal rash and fever after treatment with 6-MP[54]. This patient was able to tolerate 6-MP after desensitization[54]. The process of desensitization for patients with hypersensitivity reactions to AZA or 6-MP may be an empiric strategy for maintenance of immunomodulator therapy. However, we caution that more studies are needed to confirm the efficacy of this strategy.
As a possible alternative to those who cannot be maintained on 6-MP or AZA, treatment using 6-TG has been proposed. This drug has been used in children with acute lymphoblastic leukemia[55]. Compared to AZA, 6-TG is directly converted to 6-TGN by HPRT (Figure 1)[56,57]. Since 6-TG is a poor substrate for TPMT, hepatotoxic 6-MMPR production would be low[50,55]. Therefore, 6-TG bypasses several of the steps in thiopurine metabolism that are responsible for producing toxic metabolite build-up and its association with the aforementioned potential adverse effects[13,50]. One study found that of the 49 CD patients who were either resistant or intolerant to AZA or 6-MP, 46% of patients at 6 mo and 79% of patients at 12 mo were in remission and none of the patients developed pancreatitis or bone marrow toxicity[58]. Although up to 82% of patients tolerated 6-TG[50], this drug has been associated with several possible toxicities, notably nodular regenerative hyperplasia (NRH). One study found that the prevalence of NRH to be 16 out of 26 (62%) biopsies taken from patients treated with 6-TG[59]. Several studies further found that the incidence of NRH associated with 6-TG use varied from 4%-27% among thiopurine naive patients (Table 1)[60-62]. Other 6-TG associated adverse effects include secondary liver tumors, veno-occlusive disease, and other vascular liver pathologies[50,58,63-65]. Formal dose-ranging studies for 6-TG are lacking and only limited data are available on the therapeutic efficacy and dosing regimes[13]. As a general rule, dosage should not exceed 25 mg daily, as higher dosing has been associated with an increased risk of developing NRH[13,66].
6-TG can be considered a rescue drug in IBD patients intolerant of or refractory to AZA or 6-MP. However, given the potential complications including NRH, and the small number of long-term safety monitoring and limited formal dose-range studies, we do not recommend 6-TG therapy at this time.
In some patients, with AZA or 6-MP dose escalation, rather than achieving therapeutic levels of TGN, 6-MMP levels increase and resultant hepatotoxicity ensues[67]. These patients are known as preferential 6-MMP metabolizers[30]. Among IBD patients with high TPMT activity who favor 6-MMP production with reduced 6-TGN levels, adding the XO inhibitor allopurinol can favor the production of 6-TGN over 6-MMP[68,69]. The addition of allopurinol to 6-MP or AZA resulted in improved disease activity as measured by the partial Harvey Bradshaw index in CD and Mayo scores in UC patients; decreased prednisone maintenance dose, and improved liver function laboratory values[68,69]. However, at the 18 mo follow-up, 25% of these patients were escalated to anti-tumor necrosis factor (TNF) α therapy and 2 required surgery.
The exact mechanism of shifting thiopurine metabolites from 6-MMP to 6-TGN by the XO inhibitor is unknown, but may involve reduced production of the inactive thiouric acid (TU) metabolite in favor of the cytoactive metabolites 6-MMP and 6-TGN[67,70,71]. However, if XO inhibition favors MMP and TGN production, then the toxic 6-MMPR would also be expected to increase (Figure 1). Interestingly, allopurinol does not increase the level of 6-MMPR or its associated hepatotoxicity, but mainly shifts the metabolite to 6-TGN.
Potential side effects of allopurinol include skin rash and renal impairment[68]. In addition, the addition of allopurinol to thiopurine therapy may lead to supra-therapeutic levels of 6-TGN, leading to leukopenia[40,68]. Therefore, it is recommended that if allopurinol is used in combination with 6-MP or AZA, the dose of thiopurine medications should be reduced by at least 50% with close laboratory monitoring for leukopenia with weekly CBC monitoring during the first month, followed by every other week for the next month.
Anti-TNF therapies are generally used for patients who are refractory to first-line medications[72,73]. Colombel et al[72] conducted a randomized, double blind study of moderate-to-severe CD patients, comparing infliximab and AZA alone vs in combination and found that the primary endpoint, steroid-free remission at 26 wk, was achieved in a greater number among those treated in combination vs monotherapy. Mucosal healing, a secondary endpoint was also greater among patients who received combination therapy. These patients were immunosuppressant- and biologic therapy-naïve patients. Caution must be used, however, as studies have found an increased risk of non-Hodgkin’s lymphoma in patients treated with anti-TNF with a history of thiopurine use[74].
As discussed above, patients who are preferential 6-MMP metabolizers exhibit high 6-MMP levels with subtherapeutic 6-TGN levels when thiopurines are dosed in the traditional weight-based, once-a-day fashion. 6-MP/AZA dose escalation in this subset of patients in an attempt to push the 6-TGN level into the “therapeutic range”-often results in dose-dependent leukopenia, transaminitis and/or flu-like symptoms (headache, nausea, myalgia, fatigue, general malaise). Overproduction of 6-MMP and side-effects resolve with dose reduction, but the lower dose often fails to adequately suppress IBD disease activity, resulting in suboptimal symptom control.
Anecdotally, we observed that simply splitting the daily dose of thiopurine (e.g., 50 mg BID rather than 100 mg once daily) can reduce the 6-MMP metabolites while maintaining 6-TGN levels. To confirm our observation, we performed a retrospective chart review of patients with baseline 6-MMP levels greater than 7000 pmol/8 × 108 red blood cells (RBC) who underwent split dosing (n = 20). Dividing the daily thiopurine dose led to a significant reduction in 6-MMP levels (11 879 vs 5955 pmol/8 × 108 RBC; P < 0.0001) without adversely affecting clinical disease activity (HBI) or 6-TGN levels (250 vs 227 pmol/8 × 108, P = NS)[75]. Side-effects associated with 6-MMP, such as abnormal liver function test (LFT), leukopenia and flu-like symptoms, improved in seven of eight patients[75]. After a mean follow-up of 42 mo, 12 of 20 patients were able to be maintained on a split dose of 6-MP with control of their IBD activity[75]. To our knowledge, this is the first study to demonstrate the effectiveness of dose splitting on preferential metabolism. This approach has several advantages over other strategies. Dose splitting does not sacrifice potential efficacy associated with dose reduction, and may even allow for further upward titration of thiopurine to efficacy if needed. It avoids the introduction of possible additional medication side effects as can be seen with co-administration of allopurinol and the potential cost burden of designer biologic inventions. This maneuver is relatively simple for both patients and practitioners alike.
Independent studies are needed to confirm that split-dose administration of thiopurine is an effective approach to manage 6-MMP preferential metabolizers. However, in an IBD patient who might otherwise not tolerate immunomodulator therapy and require ongoing steroid exposure and/or escalation of therapy to biologics, splitting the daily dose of 6MP or AZA may be attempted.
Thiopurines have been linked with chromosomal abnormalities and an increased risk of lymphoma among rheumatoid arthritis patients[76]. Whether the same risk exists in IBD patients is controversial. A meta-analysis of six studies showed a four-fold increased risk of developing lymphoma among IBD patients treated with immunomodulators[77]. Similarly, in the CESAME prospective cohort study by Beaugerie, a five-fold increased risk of lymphoproliferative diseases was shown among IBD patients on thiopurines[78]. Whether this risk stems from underlying disease or iatrogenic medication is unclear. Studies have shown that the risk is greater in IBD patients who are male and between 15 to 40 years old[79,80]. One study focused on hepatosplenic T-Cell lymphoma and noted cases among men younger than 35 years who had been treated with either anti-TNF and thipopurines or thiopurine monotherapy[79]. In contrast, a study by Vos et al[81] found no increased risk of lymphoma among a nationwide study of 17 834 IBD patients. Interestingly, however, they did find an association between Epstein-Barr Virus-positive lymphoma and thiopurine use. EBV-positive lymphoma implies the effect of immunosuppression as a factor[77,80,81]. Nevertheless, the consensus has been that the benefits of thiopurines outweigh the risk[77,82]. Lewis et al[82] conducted a decision analysis and found that a 9.8-fold increase in lymphoma is needed to favor an alternative therapy over AZA. Further studies are needed, as it has yet to be determined how this risk changes with discontinuation of thiopurines[77].
Given the complex metabolism of thiopurines and the individual variability among patients in response to this medication, various dosing strategies have been adopted. Several approaches have been promising among patients who develop toxicities to the initial strategy. However, no single strategy has proven completely effective in all patients. Further studies will inevitably provide more information to assist in optimizing administration of these vital IBD medications. This is significant given that there are a limited number of medications available in the IBD arsenal. In all dosing strategies, however, close monitoring of metabolites as well as determination of pharmacogenetics are pivotal for patient safety and medication efficacy.
We thank Cindy Ting, PharmD for critical reading of this manuscript.
Peer reviewers: Ferenc Sipos, MD, PhD, Cell Analysis Labo-ratory, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi u 46, Budapest 1088, Hungary; Wojciech Blonski, MD, PhD, University of Pennsylvania, GI Research-Ground Centrex, 3400 Spruce St, Philadelphia, PA 19104, United States
S- Editor Tian L L- Editor Webster JR E- Editor Zhang DN
| 1. | Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1-V16. [PubMed] [Cited in This Article: ] |
| 2. | Chebli LA, Chaves LD, Pimentel FF, Guerra DM, Barros RM, Gaburri PD, Zanini A, Chebli JM. Azathioprine maintains long-term steroid-free remission through 3 years in patients with steroid-dependent ulcerative colitis. Inflamm Bowel Dis. 2010;16:613-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Kirk AP, Lennard-Jones JE. Controlled trial of azathioprine in chronic ulcerative colitis. Br Med J (Clin Res Ed). 1982;284:1291-1292. [PubMed] [Cited in This Article: ] |
| 4. | Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. Br Med J. 1974;4:627-630. [PubMed] [Cited in This Article: ] |
| 5. | Adler DJ, Korelitz BI. The therapeutic efficacy of 6-mercaptopurine in refractory ulcerative colitis. Am J Gastroenterol. 1990;85:717-722. [PubMed] [Cited in This Article: ] |
| 6. | Pearson DC, May GR, Fick G, Sutherland LR. Azathioprine for maintaining remission of Crohn's disease. Cochrane Database Syst Rev. 2000;(2):CD000067. [PubMed] [Cited in This Article: ] |
| 7. | Sandborn WJ. Azathioprine: state of the art in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1998;225:92-99. [PubMed] [Cited in This Article: ] |
| 8. | Timmer A, McDonald JW, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(1):CD000478. [PubMed] [Cited in This Article: ] |
| 9. | Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55:47-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 10. | Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2010;(6):CD000545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Ansari A, Hassan C, Duley J, Marinaki A, Shobowale-Bakre EM, Seed P, Meenan J, Yim A, Sanderson J. Thiopurine methyltransferase activity and the use of azathioprine in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:1743-1750. [PubMed] [Cited in This Article: ] |
| 12. | Jharap B, Seinen ML, de Boer NK, van Ginkel JR, Linskens RK, Kneppelhout JC, Mulder CJ, van Bodegraven AA. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16:1541-1549. [PubMed] [Cited in This Article: ] |
| 13. | Seinen ML, van Asseldonk DP, Mulder CJ, de Boer NK. Dosing 6-thioguanine in inflammatory bowel disease: expert-based guidelines for daily practice. J Gastrointestin Liver Dis. 2010;19:291-294. [PubMed] [Cited in This Article: ] |
| 14. | Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn's disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981-987. [PubMed] [Cited in This Article: ] |
| 15. | Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705-713. [PubMed] [Cited in This Article: ] |
| 16. | Hindorf U, Lindqvist M, Peterson C, Söderkvist P, Ström M, Hjortswang H, Pousette A, Almer S. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55:1423-1431. [PubMed] [Cited in This Article: ] |
| 17. | Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130:1047-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 279] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Bowen DG, Selby WS. Use of 6-mercaptopurine in patients with inflammatory bowel disease previously intolerant of azathioprine. Dig Dis Sci. 2000;45:1810-1813. [PubMed] [Cited in This Article: ] |
| 19. | Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133-1145. [PubMed] [Cited in This Article: ] |
| 20. | Derijks LJ, Gilissen LP, Hooymans PM, Hommes DW. Review article: thiopurines in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:715-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Welch J, Lennard L, Morton GC, Lilleyman JS. Pharmacokinetics of mercaptopurine: plasma drug and red cell metabolite concentrations after an oral dose. Ther Drug Monit. 1997;19:382-385. [PubMed] [Cited in This Article: ] |
| 22. | Cuffari C, Hunt S, Bayless T. Utilisation of erythrocyte 6-thioguanine metabolite levels to optimise azathioprine therapy in patients with inflammatory bowel disease. Gut. 2001;48:642-646. [PubMed] [Cited in This Article: ] |
| 23. | Deshpande AR, Abreu MT. Optimizing therapy with 6-mercaptopurine and azathioprine: to measure or not to measure? Therap Adv Gastroenterol. 2010;3:275-279. [PubMed] [Cited in This Article: ] |
| 24. | Lennard L, Singleton HJ. High-performance liquid chromatographic assay of human red blood cell thiopurine methyltransferase activity. J Chromatogr B Biomed Appl. 1994;661:25-33. [PubMed] [Cited in This Article: ] |
| 25. | Thomas CW, Myhre GM, Tschumper R, Sreekumar R, Jelinek D, McKean DJ, Lipsky JJ, Sandborn WJ, Egan LJ. Selective inhibition of inflammatory gene expression in activated T lymphocytes: a mechanism of immune suppression by thiopurines. J Pharmacol Exp Ther. 2005;312:537-545. [PubMed] [Cited in This Article: ] |
| 26. | Li B, Yu H, Zheng W, Voll R, Na S, Roberts AW, Williams DA, Davis RJ, Ghosh S, Flavell RA. Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science. 2000;288:2219-2222. [PubMed] [Cited in This Article: ] |
| 27. | Elion GB. The George Hitchings and Gertrude Elion Lecture. The pharmacology of azathioprine. Ann N Y Acad Sci. 1993;685:400-407. [PubMed] [Cited in This Article: ] |
| 28. | Sandborn WJ. A review of immune modifier therapy for inflammatory bowel disease: azathioprine, 6-mercaptopurine, cyclosporine, and methotrexate. Am J Gastroenterol. 1996;91:423-433. [PubMed] [Cited in This Article: ] |
| 29. | Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Dubinsky MC, Yang H, Hassard PV, Seidman EG, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology. 2002;122:904-15. [PubMed] [Cited in This Article: ] |
| 31. | Neurath MF, Kiesslich R, Teichgräber U, Fischer C, Hofmann U, Eichelbaum M, Galle PR, Schwab M. 6-thioguanosine diphosphate and triphosphate levels in red blood cells and response to azathioprine therapy in Crohn's disease. Clin Gastroenterol Hepatol. 2005;3:1007-1014. [PubMed] [Cited in This Article: ] |
| 32. | Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2:731-743. [PubMed] [Cited in This Article: ] |
| 33. | Dubinsky MC, Reyes E, Ofman J, Chiou CF, Wade S, Sandborn WJ. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn's disease treated with azathioprine or 6-mercaptopurine. Am J Gastroenterol. 2005;100:2239-2247. [PubMed] [Cited in This Article: ] |
| 34. | Roblin X, Serre-Debeauvais F, Phelip JM, Faucheron JL, Hardy G, Chartier A, Helluwaert F, Bessard G, Bonaz B. 6-tioguanine monitoring in steroid-dependent patients with inflammatory bowel diseases receiving azathioprine. Aliment Pharmacol Ther. 2005;21:829-839. [PubMed] [Cited in This Article: ] |
| 35. | Achkar JP, Stevens T, Easley K, Brzezinski A, Seidner D, Lashner B. Indicators of clinical response to treatment with six-mercaptopurine or azathioprine in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:339-345. [PubMed] [Cited in This Article: ] |
| 36. | Morales A, Salguti S, Miao CL, Lewis JD. Relationship between 6-mercaptopurine dose and 6-thioguanine nucleotide levels in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:380-385. [PubMed] [Cited in This Article: ] |
| 37. | Dong XW, Zheng Q, Zhu MM, Tong JL, Ran ZH. Thiopurine S-methyltransferase polymorphisms and thiopurine toxicity in treatment of inflammatory bowel disease. World J Gastroenterol. 2010;16:3187-3195. [PubMed] [Cited in This Article: ] |
| 38. | Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651-662. [PubMed] [Cited in This Article: ] |
| 39. | Hon YY, Fessing MY, Pui CH, Relling MV, Krynetski EY, Evans WE. Polymorphism of the thiopurine S-methyltransferase gene in African-Americans. Hum Mol Genet. 1999;8:371-376. [PubMed] [Cited in This Article: ] |
| 40. | Snow JL, Gibson LE. A pharmacogenetic basis for the safe and effective use of azathioprine and other thiopurine drugs in dermatologic patients. J Am Acad Dermatol. 1995;32:114-116. [PubMed] [Cited in This Article: ] |
| 41. | Winter J, Walker A, Shapiro D, Gaffney D, Spooner RJ, Mills PR. Cost-effectiveness of thiopurine methyltransferase genotype screening in patients about to commence azathioprine therapy for treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:593-599. [PubMed] [Cited in This Article: ] |
| 42. | Sandborn W, Sutherland L, Pearson D, May G, Modigliani R, Prantera C. Azathioprine or 6-mercaptopurine for inducing remission of Crohn's disease. Cochrane Database Syst Rev. 2000;CD000545. [PubMed] [Cited in This Article: ] |
| 43. | Robinson M. Optimizing therapy for inflammatory bowel disease. Am J Gastroenterol. 1997;92:12S-17S. [PubMed] [Cited in This Article: ] |
| 44. | Winter JW, Gaffney D, Shapiro D, Spooner RJ, Marinaki AM, Sanderson JD, Mills PR. Assessment of thiopurine methyltransferase enzyme activity is superior to genotype in predicting myelosuppression following azathioprine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:1069-1077. [PubMed] [Cited in This Article: ] |
| 45. | Lees CW, Maan AK, Hansoti B, Satsangi J, Arnott ID. Tolerability and safety of mercaptopurine in azathioprine-intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;27:220-227. [PubMed] [Cited in This Article: ] |
| 46. | Nagy F, Molnar T, Szepes Z, Farkas K, Nyari T, Lonovics J. Efficacy of 6-mercaptopurine treatment after azathioprine hypersensitivity in inflammatory bowel disease. World J Gastroenterol. 2008;14:4342-4346. [PubMed] [Cited in This Article: ] |
| 47. | Domènech E, Nos P, Papo M, López-San Román A, Garcia-Planella E, Gassull MA. 6-mercaptopurine in patients with inflammatory bowel disease and previous digestive intolerance of azathioprine. Scand J Gastroenterol. 2005;40:52-55. [PubMed] [Cited in This Article: ] |
| 48. | Boulton-Jones JR, Pritchard K, Mahmoud AA. The use of 6-mercaptopurine in patients with inflammatory bowel disease after failure of azathioprine therapy. Aliment Pharmacol Ther. 2000;14:1561-1565. [PubMed] [Cited in This Article: ] |
| 49. | McGovern DP, Travis SP, Duley J, Shobowale-Bakre el M, Dalton HR. Azathioprine intolerance in patients with IBD may be imidazole-related and is independent of TPMT activity. Gastroenterology. 2002;122:838-839. [PubMed] [Cited in This Article: ] |
| 50. | Dubinsky MC, Feldman EJ, Abreu MT, Targan SR, Vasiliauskas EA. Thioguanine: a potential alternate thiopurine for IBD patients allergic to 6-mercaptopurine or azathioprine. Am J Gastroenterol. 2003;98:1058-1063. [PubMed] [Cited in This Article: ] |
| 51. | Korelitz BI, Zlatanic J, Goel F, Fuller S. Allergic reactions to 6-mercaptopurine during treatment of inflammatory bowel disease. J Clin Gastroenterol. 1999;28:341-344. [PubMed] [Cited in This Article: ] |
| 52. | Korelitz BI, Reddy B, Bratcher J. Desensitization of patients with allergic reactions to immunosuppressives in the treatment of inflammatory bowel disease. Expert Opin Drug Saf. 2010;9:379-382. [PubMed] [Cited in This Article: ] |
| 53. | Lavaud F, Abdelli N, Thiefin G. Successful desensitization for azathioprine skin rash in a patient with severe Crohn's disease. Dig Dis Sci. 1997;42:823. [PubMed] [Cited in This Article: ] |
| 54. | Mutinga M, Castells M, Horan R, Farraye FA. Successful desensitization to 6-mercaptopurine in a patient with Crohn's disease. Am J Gastroenterol. 2000;95:1383-1384. [PubMed] [Cited in This Article: ] |
| 55. | Lennard L, Davies HA, Lilleyman JS. Is 6-thioguanine more appropriate than 6-mercaptopurine for children with acute lymphoblastic leukaemia? Br J Cancer. 1993;68:186-190. [PubMed] [Cited in This Article: ] |
| 56. | Lennard L. TPMT in the treatment of Crohn's disease with azathioprine. Gut. 2002;51:143-146. [PubMed] [Cited in This Article: ] |
| 57. | Erb N, Harms DO, Janka-Schaub G. Pharmacokinetics and metabolism of thiopurines in children with acute lymphoblastic leukemia receiving 6-thioguanine versus 6-mercaptopurine. Cancer Chemother Pharmacol. 1998;42:266-272. [PubMed] [Cited in This Article: ] |
| 58. | Bonaz B, Boitard J, Marteau P, Lémann M, Coffin B, Flourié B, Belaiche J, Cadiot G, Metman EH, Cortot A. Tioguanine in patients with Crohn's disease intolerant or resistant to azathioprine/mercaptopurine. Aliment Pharmacol Ther. 2003;18:401-408. [PubMed] [Cited in This Article: ] |
| 59. | Dubinsky MC, Hassard PV, Seidman EG, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. An open-label pilot study using thioguanine as a therapeutic alternative in Crohn's disease patients resistant to 6-mercaptopurine therapy. Inflamm Bowel Dis. 2001;7:181-189. [PubMed] [Cited in This Article: ] |
| 60. | Seiderer J, Zech CJ, Reinisch W, Lukas M, Diebold J, Wrba F, Teml A, Chalupna P, Stritesky J, Schoenberg SO. A multicenter assessment of liver toxicity by MRI and biopsy in IBD patients on 6-thioguanine. J Hepatol. 2005;43:303-309. [PubMed] [Cited in This Article: ] |
| 61. | Teml A, Schwab M, Hommes DW, Almer S, Lukas M, Feichtenschlager T, Florin T, Seiderer J, Petritsch W, Bokemeyer B. A systematic survey evaluating 6-thioguanine-related hepatotoxicity in patients with inflammatory bowel disease. Wien Klin Wochenschr. 2007;119:519-526. [PubMed] [Cited in This Article: ] |
| 62. | van Asseldonk DP, Jharap B, De Boer NK, Zondervan PE, Bloemena E, den Hartog G, Westerveld BD, Kolkman JJ, Engels LG, van Bodegraven AA. Liver histology of IBD patients who are treated with 6-Thioguanine due to failure of conventional thiopurines reveals very few cases of nodular regenerative hyperplasia. Gastroenterology. 2010;138:S62. [Cited in This Article: ] |
| 63. | Bo J, Schrøder H, Kristinsson J, Madsen B, Szumlanski C, Weinshilboum R, Andersen JB, Schmiegelow K. Possible carcinogenic effect of 6-mercaptopurine on bone marrow stem cells: relation to thiopurine metabolism. Cancer. 1999;86:1080-1086. [PubMed] [Cited in This Article: ] |
| 64. | Stork LC, Matloub Y, Broxson E, La M, Yanofsky R, Sather H, Hutchinson R, Heerema NA, Sorrell AD, Masterson M. Oral 6-mercaptopurine versus oral 6-thioguanine and veno-occlusive disease in children with standard-risk acute lymphoblastic leukemia: report of the Children's Oncology Group CCG-1952 clinical trial. Blood. 2010;115:2740-2748. [PubMed] [Cited in This Article: ] |
| 65. | Broxson EH, Dole M, Wong R, Laya BF, Stork L. Portal hypertension develops in a subset of children with standard risk acute lymphoblastic leukemia treated with oral 6-thioguanine during maintenance therapy. Pediatr Blood Cancer. 2005;44:226-231. [PubMed] [Cited in This Article: ] |
| 66. | de Boer NK, Derijks LJ, Gilissen LP, Hommes DW, Engels LG, de-Boer SY, den Hartog G, Hooymans PM, Mäkelburg AB, Westerveld BD. On tolerability and safety of a maintenance treatment with 6-thioguanine in azathioprine or 6-mercaptopurine intolerant IBD patients. World J Gastroenterol. 2005;11:5540-5544. [PubMed] [Cited in This Article: ] |
| 67. | Witte TN, Ginsberg AL. Use of allopurinol with low-dose 6-mercaptopurine in inflammatory bowel disease to achieve optimal active metabolite levels: a review of four cases and the literature. Can J Gastroenterol. 2008;22:181-185. [PubMed] [Cited in This Article: ] |
| 68. | Sparrow MP, Hande SA, Friedman S, Cao D, Hanauer SB. Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin Gastroenterol Hepatol. 2007;5:209-214. [PubMed] [Cited in This Article: ] |
| 69. | Sparrow MP, Hande SA, Friedman S, Lim WC, Reddy SI, Cao D, Hanauer SB. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther. 2005;22:441-446. [PubMed] [Cited in This Article: ] |
| 70. | Oláh T, Régely K, Mándi Y. The inhibitory effects of allopurinol on the production and cytotoxicity of tumor necrosis factor. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:96-99. [PubMed] [Cited in This Article: ] |
| 71. | Sasaki H, Tsuru K, Nakamura J, Konishi R, Shibasaki J. Effect of allopurinol on the intestinal absorption of 6-mercaptopurine in rats. J Pharmacobiodyn. 1987;10:697-702. [PubMed] [Cited in This Article: ] |
| 72. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2221] [Cited by in F6Publishing: 2214] [Article Influence: 158.1] [Reference Citation Analysis (0)] |
| 73. | Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn's disease in adults. Am J Gastroenterol. 2009;104:465-483; quiz 464, 484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 619] [Cited by in F6Publishing: 618] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 74. | Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 75. | Shih DQ, Nguyen M, Ibanez P, Kwan LY, Targan SR, vasiliauskas EA. Split-dose administration of 6MP/Azathiopurine: a novel and effective strategy for IBD patients with preferential 6MMP metabolism. Gastroenterology. 2009;136:A677-A678. [DOI] [Cited in This Article: ] |
| 76. | Knipp S, Hildebrandt B, Richter J, Haas R, Germing U, Gattermann N. Secondary myelodysplastic syndromes following treatment with azathioprine are associated with aberrations of chromosome 7. Haematologica. 2005;90:691-693. [PubMed] [Cited in This Article: ] |
| 77. | Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121-1125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 583] [Cited by in F6Publishing: 564] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 78. | Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617-1625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 774] [Cited by in F6Publishing: 748] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 79. | Kotlyar DS, Osterman MT, Diamond RH, Porter D, Blonski WC, Wasik M, Sampat S, Mendizabal M, Lin MV, Lichtenstein GR. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:36-41.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 323] [Cited by in F6Publishing: 297] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 80. | Shale M, Kanfer E, Panaccione R, Ghosh S. Hepatosplenic T cell lymphoma in inflammatory bowel disease. Gut. 2008;57:1639-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 81. | Vos AC, Bakkal N, Minnee RC, Casparie MK, de Jong DJ, Dijkstra G, Stokkers P, van Bodegraven AA, Pierik M, van der Woude CJ. Risk of malignant lymphoma in patients with inflammatory bowel diseases: A Dutch nationwide study. Inflamm Bowel Dis. 2010;Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 82. | Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn's disease: benefits outweigh the risk of lymphoma. Gastroenterology. 2000;118:1018-1024. [PubMed] [Cited in This Article: ] |