Published online Sep 28, 2011. doi: 10.3748/wjg.v17.i36.4104
Revised: March 4, 2011
Accepted: March 11, 2011
Published online: September 28, 2011
AIM: To analyse the possible association of various Helicobacter species and certain common gut bacteria in patients with Meckel’s diverticulum and appendicitis.
METHODS: A nested-polymerase chain reaction (PCR), specific to 16S rRNA of the Helicobacter genus, was performed on paraffin embedded samples, 50 with acute appendicitis, 50 normal appendixes, and 33 Meckel’s diverticulum with gastric heterotopia and/or ulcer. Helicobacter genus positive samples were sequenced for species identification. All samples were also analysed for certain gut bacteria by PCR.
RESULTS: Helicobacter pullorum DNA was found in one out of 33 cases and Enterobacteria in two cases of Meckel’s diverticulum. Helicobacter pylori (H. pylori) was found in three, Enterobacter in 18, and Bacteroides in 19 out of 100 appendix samples by PCR. Enterococcus was not found in any MD or appendix samples. All H. pylori positive cases were from normal appendixes.
CONCLUSION: Helicobacter is not an etiological agent in the pathogenesis of symptomatic Meckel’s diverticulum or in acute appendicitis.
-
Citation: Karagin PH, Stenram U, Wadström T, Ljungh Å.
Helicobacter species and gut bacterial DNA in Meckel’s diverticulum and the appendix. World J Gastroenterol 2011; 17(36): 4104-4108 - URL: https://www.wjgnet.com/1007-9327/full/v17/i36/4104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i36.4104
Although the stomach is the most frequent site of Helicobacter pylori (H. pylori) infection, H. pylori and enterohepatic Helicobacter spp. (EHS) have also been associated with extragastric diseases[1].
Meckel’s diverticulum (MD) is the most common developmental anomaly of the gastrointestinal tract and is present in 1%-2% of the general population. It often contains ectopic tissue, notably gastric and pancreatic tissue[2]. Gastric mucosa is found in 10%-25% of MD and may be associated with inflammation, ulceration, gastrointestinal bleeding, and perforation[3-5]. H. pylori has been demonstrated in the ectopic gastric epithelium within MD[6]. Campylobacter-like organisms in MD were first reported in 1989[7-9]. However, conflicting results were reported concerning colonisation by H. pylori of such ectopic mucosa[10,11]. There has been no study that investigated EHS in MD.
Acute appendicitis is the most common abdominal surgical emergency and can be seen in all ages, especially in those younger than 30 years[12]. However, the aetiology of acute appendicitis is uncertain, and diagnosis is often difficult. There have been some investigations of H. pylori in appendix tissue[13-15], but none that investigated non-pylori Helicobacters.
We hypothesized that non-pylori Helicobacters, such as enterohepatic Helicobacters, might be associated with these diseases. Most studies have investigated only H. pylori in MD and the appendix and mostly used non-molecular biological techniques; therefore, we aimed to analyse gastric, EHS and certain common gut bacteria in appendicitis and MD patients by genus specific polymerase chain reaction (PCR) and sequencing.
We re-examined all MD patients from 1990-2009 taken from the files of the Department of Pathology, Lund University Hospital. Thirty-three MD patients (two cases of ulcer without heterotopia, 31 cases with gastric heterotopia, of which seven also had an ulcer) (mean age: 11 years; range: 4 wk-73 years; 26 male, 7 female) were included in our study. Abdominal pain was the reason for operation in 16 cases, two of whom had acute appendicitis and one enlarged lymph nodes, nine were operated upon because of gastrointestinal bleeding and six for other abdominal diseases. No indication was given in two of the cases. Histological sections from stored paraffin blocks were stained with Alcian blue-periodic acid Schiff (AB-PAS) pH 2.5, Whartin-Starry silver stain and immunostained with an anti-H. pylori antibody (DAKO, Glostrup, Denmark, diluted 1:300).
We also re-examined mucosa from 50 cases of acute appendicitis (26 male, 24 female, median age: 30 years; range: 9-87 years) and 50 cases of normal appendix (16 male, 34 female, median age: 34 years; range: 10 d-80 years) from 2008-2009. Of the latter patients 26 were operated for a suspected appendicitis (8 male, 18 female, median age: 21 years; range: 8-77 years), 12 for intestinal diseases (8 male, 4 female, median age: 59 years; range: 10 d-80 years), and 12 for female genital disorders (median age: 38 years; range: 11-75 years). Histological sections from the Helicobacter positive cases were stained as mentioned above.
From the cases of MD, heterotopic mucosa of the gastric as well as the antral type were obtained from the paraffin blocks for PCR-assay with the tip of a scalpel. Ulcers were examined separately. In the case positive for Helicobacter DNA, the intestinal type mucosa surrounding the heterotopia was also studied. Corresponding areas from appendix samples of mucosa, or of necrotic appendicitis were sampled for PCR. It was not possible to avoid including material from the appendical lumen in these samples. In the cases positive for Helicobacter DNA, other tissues removed at the same operation were also examined by PCR.
DNA was extracted from the paraffin-embedded tissue samples by de-embedding, as previously described[1]. DNA was extracted by a QIAamp DNA Mini Kit Tissue protocol (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
DNA extracts were amplified in a GeneAmp 2700 Thermocycler (Applied Biosystems, Foster City, CA, United States) using a semi-nested PCR assay specific for Helicobacter spp. 16S rDNA, as previously described[1]. H. pylori (CCUG 17874) was used as a positive control in all PCR reactions. The 416-bp PCR products were visualized by 1.3% agarose gel electrophoresis.
PCR specific for Enterobacteriaceae, the Bacterioides-Prevotella group, and Enterococcus were performed. The reaction mixture and amplification conditions, except for annealing temperatures, for non-Helicobacter PCR assays were the same as in the first step of the semi-nested Helicobacter PCR. The annealing temperatures and primers used for detection of Enterobacteriaceae, Bacterioides-prevotella group, and Enterococcus were as described by Karagin et al[1] 2010. As positive controls, Escherchia coli (CCUG 17620), Bacteroides fragilis (CCUG 4856), and Enterococcus faecalis (CCUG 9997) were used in all PCR reactions. The 112-bp PCR product of Enterococcus, the 418-bp product of Bacteroides, and the 195-bp product of Enterobacteriaceae were visualized by 1.3% agarose gel electrophoresis.
Helicobacter specific PCR products were purified from agarose gels using the Montage DNA Gel Extraction Kit (Millipore, Bedford, MA, United States), according to the manufacturer’s instructions. DNA sequence reactions were performed using the ABI PRISMTM dRhodamine Terminator Cycle Sequencing Ready Reaction Kit version 3.0 (Applied Biosystems) as described by Tolia et al[16]. Products of the sequencing reaction were aligned and the closest homologous DNA was identified by BLASTn-analysis.
The study was approved by the Research Ethics Committee at Lund University, permit number 588/2006.
The most dominant heterotopia seen in MD was of the corpus type with, in most cases, small areas of antral heterotopia. It was therefore easy to include both types of heterotopia, if present, in the same sample. No Helicobacter was found by histological and immunohistological examinations, neither in heterotopia nor in ulcus. In 3/33 of the MD cases, a mild chronic inflammation in the heterotopic area with slightly increased amounts of lymphocytes was seen. The ulcer was infiltrated with polymorphonuclear cells but there was no general, active gastritis. The normal intestinal mucosa in the MD outside the heterotopia did not have an increased amount of lympathic tissue, in except the Helicobacter pullorum (H. pullorum) positive case. The nine ulcers and their surrounding mucosa were negative for Helicobacter DNA.
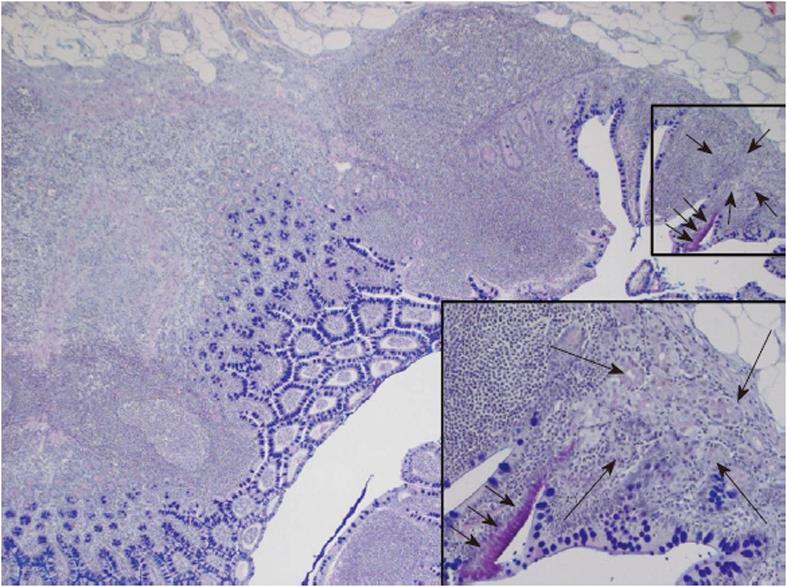
One of the heterotopic mucosa specimens was positive for Helicobacter DNA, namely that from a 44-year-old male. He was operated on for acute appendicitis. The appendix was not sent for histological analysis. The MD was also removed. Histology displayed a few gastric glands of the corpus type and a small strip of surface epithelium of the gastric type. There were a moderate number of lymphocytes and plasma cells in the heterotopic area. The surrounding mucosa of the intestinal type displayed an unusually well developed lymphatic tissue with germinal centres, a predominance of lymphocytes, and very few polymorphonuclear cells (Figure 1). There was no Helicobacter DNA detected by PCR in this sample.
Three normal appendixes were positive for Helicobacter DNA: from one an 18-year-old female with suspicion of appendicitis, one from a 63-year-old male with colon adenoma, one from a 55-year-old male with colon diverticulitis. Adenoma, diverticulitis, and normal colon tissue removed from the two latter patients were negative for Helicobacter. No tissue other than the appendix was removed from the first patient. All cases revealed H. pylori on sequence analysis. There was no gastric metaplasia in any of the appendixes, and no immunopositive H. pylori structures in the mucosa of the samples that were PCR-positive for Helicobacter.
Using the Helicobacter specific PCR assay and agarose gel electrophoresis, Helicobacter spp. was detected in 1/33 (3%) of specimens from patients with MD by genus specific nested-PCR. The sequenced PCR amplicon showed 98% similarity to H. pullorum. There were 3/50 (6%) samples that were positive for Helicobacter spp., among normal appendixes. All of them showed 98%-99% sequence similarity to H. pylori. However Helicobacter spp. was not found in any samples of acute appendicitis.
Using the Enterobacteria specific PCR assay and agarose gel electrophoresis, Enterobacteria spp. was detected in 10/50 (20%) acute appendicitis cases and 8/50 (16%) normal appendixes. There were 7/50 (14%) and 12/50 (24%) samples that were positive for Bacteroides spp., among the acute appendicitis and normal appendix sample, respectively. However, all MD and appendix samples were negative for Enterococcus spp. MD samples were also negative for Bacteroides; however, 2/33 (6%) were positive for Enterobacteria spp.
In this study, we screened for the presence of DNA of Helicobacter spp. and certain common intestinal bacteria by PCR in MD with gastric heterotopia and in appendix samples. We detected H. pullorum DNA in one out of 33 MD cases (3%) and Enterobacteria in two (6%). No Enterococus or Bacteroides were found in the MD cases.
The H. pullorum case was positive only in the heterotopic area, not in the surrounding diverticulum mucosa of the intestinal type. No luminal contents were seen in these samples. This argues for the interpretation that the H. pullorum DNA originated from the heterotopic mucosa and not from the lumen. This assumption is further strengthened by the very low prevalence of other bacterial DNA in the MD samples. H. pullorum has, however, been described in stools from cases with gastroenteritis[17], but our patient did not have such symptoms.
No Helicobacter was seen by immunohistochemistry. However, PCR is a more sensitive method and does not require intact bacteria. Our PCR technique is considered to be highly reliable for genus identification of Helicobacter spp.[18,19]. Some authors have found H. pylori by immunohistochemistry in MD with active gastritis, implying the presence of polymorphonuclear cells; the prevalence varied between 2 and 28%[6-8,20-23]. We had no cases with such inflammation and found no H. pylori DNA.
Interestingly, there was an increased amount of lymphatic tissue in the intestinal type mucosa of the H. pullorum positive case. However, no conclusions can be drawn from just one case.
EHS are known to cause inflammatory bowel diseases[24,25]. We have previously found H. pullorum DNA in cholecystitis samples with gastric metaplasia[1]. Perhaps H. pullorum has some preference for the gastric epithelium.
We found H. pylori DNA in three out of 50 normal appendixes (6%) and none in the 50 cases of acute appendicitis. Other bacterial DNA was found in up to 24% of samples. We could not avoid including some luminal material in the appendix samples and therefore H. pylori DNA in the appendixes might be a contamination. Pavlidis et al[14] found H. pylori by PCR in two out of 46 samples (4%) of acute appendicitis. However, most authors have failed to demonstrate the presence of H. pylori in the appendix[13,15,26]. H. pylori commonly colonises the gastrointestinal tract. However, our results suggest that Helicobacter is without importance in the etiology of acute appendicitis.
In conclusion, H. pullorum has, for the first time, been detected by PCR in MD patients with gastric heterotopias. However, there is no association between H. pullorum and MD pathogenesis. Moreover, H. pylori has no role in the aetiology of acute appendicitis. Its presence might have been that of a passenger.
Hans-Olof Nilsson’s expert knowledge on PCR and specimen handling and PCR inhibitor removal and Ingrid Nilsson’s valuable expertise on Helicobacters are highly appreciated. We also thank Roland Andersson, MD PhD, Department of Surgery, for contributing the case story of the H. pullorum positive patient.
Helicobacter-like bacteria in Meckel´s diverticulum (MD) have been reported by histological methods. However, no study has reported Helicobacter DNA in such specimens by polymerase chain reaction(PCR) and there is some doubt as to the presence of Helicobacter in patients with appendicitis.
Most studies have analyzed only Helicobacter pylori (H. pylori) in Meckel’s diverticulum and appendix samples. However, enterohepatic Helicobacter species might also be important in the etiology of such diseases. The authors demonstrated the presence of Helicobacter pullorum in Meckel’s diverticulum for the first time and concluded that Helicobacter might be a passenger in such patients.
By understanding the role of Helicobacters in the pathology of Meckel’s diverticulum and appendicitis, this study could represent a future strategy for further pathological studies.
Enterohepatic Helicobacter spp. (EHS) are the species of the genus Helicobacter that colonize the hepatobiliary tract and can cause extragastric diseases in humans or in animals.
This work has been had the objective of seeking any association between Helicobacter species other than H. pylori with Meckel’s diverticulum by very sensitive method, i.e., Nested PCR. Most previous studies were based on conventional methods, such as culture isolation, whereas in this study, the authors have used molecular techniques. Although they did not find any association between MD and Helicobacter species, it does not undermine the importance of the study.
Peer reviewers: Tamara Vorobjova, Senior Researcher in Immunology, Department of Immunology, Institute of General and Molecular Pathology, University of Tartu, Ravila, 19, Tartu 51014, Estonia; Gopal Nath, MD, PhD, Professor, Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005, India
S- Editor Tian L L- Editor Stewart GJ E- Editor Zhang DN
| 1. | Karagin PH, Stenram U, Wadström T, Ljungh A. Helicobacter species and common gut bacterial DNA in gallbladder with cholecystitis. World J Gastroenterol. 2010;16:4817-4822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 26] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Mackey WC, Dineen P. A fifty year experience with Meckel's diverticulum. Surg Gynecol Obstet. 1983;156:56-64. [PubMed] [Cited in This Article: ] |
| 3. | Diamond T, Russell CF. Meckel's diverticulum in the adult. Br J Surg. 1985;72:480-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Leijonmarck CE, Bonman-Sandelin K, Frisell J, Räf L. Meckel's diverticulum in the adult. Br J Surg. 1986;73:146-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 105] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Vane DW, West KW, Grosfeld JL. Vitelline duct anomalies. Experience with 217 childhood cases. Arch Surg. 1987;122:542-547. [PubMed] [Cited in This Article: ] |
| 6. | Bemelman WA, Bosma A, Wiersma PH, Rauws EA, Brummelkamp WH. Role of Helicobacter pylori in the pathogenesis of complications of Meckel's diverticula. Eur J Surg. 1993;159:171-175. [PubMed] [Cited in This Article: ] |
| 7. | de Cothi GA, Newbold KM, O'Connor HJ. Campylobacter-like organisms and heterotopic gastric mucosa in Meckel's diverticula. J Clin Pathol. 1989;42:132-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 52] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Morris A, Nicholson G, Zwi J, Vanderwee M. Campylobacter pylori infection in Meckel's diverticula containing gastric mucosa. Gut. 1989;30:1233-1235. [PubMed] [Cited in This Article: ] |
| 9. | Stolte M, Lauer E. [Campylobacter pylori in heterotopic gastric mucosa in Meckel's diverticulum]. Leber Magen Darm. 1989;19:209-210. [PubMed] [Cited in This Article: ] |
| 10. | Ergün O, Celik A, Akarca US, Sen T, Alkanat M, Erdener A. Does colonization of Helicobacter pylori in the heterotopic gastric mucosa play a role in bleeding of Meckel's diverticulum? J Pediatr Surg. 2002;37:1540-1542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Tuzun A, Polat Z, Kilciler G, Turan I, Kilic A, Ozcan A, Uygun A. Evaluation for Helicobacter pylori in Meckel's diverticulum by using real-time PCR. Dig Dis Sci. 2010;55:1969-1974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Simpson J, Samaraweera AP, Sara RK, Lobo DN. Acute appendicitis--a benign disease? Ann R Coll Surg Engl. 2008;90:313-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Fanning NF, Horgan PG, Tanner WA, Keane FB. Helicobacter pylori does not play a role in the aetiology of acute appendicitis. Ir J Med Sci. 1998;167:39-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Pavlidis TE, Atmatzidis KS, Papaziogas BT, Souparis A, Koutelidakis IM, Papaziogas TB. Helicobacter pylori infection in patients undergoing appendectomy. Swiss Surg. 2002;8:110-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Kell MR, Winter DC, Ryan D, Lynch M, Brew B, Rajpal P, Kirwan WO, Redmond HP. Nitric oxide synthetase and Helicobacter pylori in patients undergoing appendicectomy. Br J Surg. 1999;86:1538-1542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Tolia V, Nilsson HO, Boyer K, Wuerth A, Al-Soud WA, Rabah R, Wadström T. Detection of Helicobacter ganmani-like 16S rDNA in pediatric liver tissue. Helicobacter. 2004;9:460-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Ceelen L, Decostere A, Verschraegen G, Ducatelle R, Haesebrouck F. Prevalence of Helicobacter pullorum among patients with gastrointestinal disease and clinically healthy persons. J Clin Microbiol. 2005;43:2984-2986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Moyaert H, Pasmans F, Ducatelle R, Haesebrouck F, Baele M. Evaluation of 16S rRNA gene-based PCR assays for genus-level identification of Helicobacter species. J Clin Microbiol. 2008;46:1867-1869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Al-Soud WA, Ouis IS, Li DQ, Ljungh S, Wadström T. Characterization of the PCR inhibitory effect of bile to optimize real-time PCR detection of Helicobacter species. FEMS Immunol Med Microbiol. 2005;44:177-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Hill P, Rode J. Helicobacter pylori in ectopic gastric mucosa in Meckel's diverticulum. Pathology. 1998;30:7-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Finn LS, Christie DL. Helicobacter pylori and Meckel's diverticula. J Pediatr Gastroenterol Nutr. 2001;32:150-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Ackerman Z, Peston D, Cohen P. Role of Helicobacter pylori infection in complications from Meckel's diverticulum. Dig Dis Sci. 2003;48:1068-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 72] [Reference Citation Analysis (1)] |
| 23. | Oğuzkurt P, Talim B, Tanyel FC, Cağlar M, Senocak ME, Büyükpamukçu N. The role of heterotopic gastric mucosa with or without colonization of Helicobacter pylori upon the diverse symptomatology of Meckel's diverticulum in children. Turk J Pediatr. 2001;43:312-316. [PubMed] [Cited in This Article: ] |
| 24. | Laharie D, Asencio C, Asselineau J, Bulois P, Bourreille A, Moreau J, Bonjean P, Lamarque D, Pariente A, Soulé JC. Association between entero-hepatic Helicobacter species and Crohn's disease: a prospective cross-sectional study. Aliment Pharmacol Ther. 2009;30:283-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Wadstöm T, Hanninen M-L. Other helicobacters in the digestive tract. Curr Opin in Gastroenterol. 1999;15:S53-S56. [Cited in This Article: ] |
| 26. | Paredes Esteban RM, Muñoz Villanueva JR, Velasco Sánchez B, González Mariscal M, Rodríguez Vargas J, Martínez Sánchez M, García Ruiz M. [Role of the Helicobacter pylori in the aetiology of acute appendicitis. Preliminary studies]. Cir Pediatr. 2007;20:156-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |