Published online Sep 7, 2011. doi: 10.3748/wjg.v17.i33.3836
Revised: March 5, 2011
Accepted: March 12, 2011
Published online: September 7, 2011
AIM: To examine the influence of ghrelin on the regenerative potential of gastrointestinal (GI) epithelium.
METHODS: Damage to GI epithelium was induced in mice by two intravenous injections of doxorubicin (10 and 6 mg/kg). Some of the doxorubicin-treated mice received a continuous subcutaneous infusion of ghrelin (1.25 μg/h) for 10 d via implanted mini-osmotic pumps. To label dividing stem cells in the S-phase of the cell cycle, all mice received a single intraperitoneal injection of 5’-bromo-2’-deoxyuridine (BrdU) one hour before sacrifice. The stomach along with the duodenum were then removed and processed for histological examination and immunohistochemistry using anti-BrdU antibody.
RESULTS: The results showed dramatic damage to the GI epithelium 3 d after administration of chemotherapy which began to recover by day 10. In ghrelin-treated mice, attenuation of GI mucosal damage was evident in the tissues examined post-chemotherapy. Immunohistochemical analysis showed an increase in the number of BrdU-labeled cells and an alteration in their distribution along the epithelial lining in response to damage by doxorubicin. In mice treated with both doxorubicin and ghrelin, the number of BrdU-labeled cells was reduced when compared with mice treated with doxorubicin alone.
CONCLUSION: The present study suggests that ghrelin enhances the regenerative potential of the GI epithelium in doxorubicin-treated mice, at least in part, by modulating cell proliferation.
- Citation: Fahim MA, Kataya H, El-Kharrag R, Amer DA, al-Ramadi B, Karam SM. Ghrelin attenuates gastrointestinal epithelial damage induced by doxorubicin. World J Gastroenterol 2011; 17(33): 3836-3841
- URL: https://www.wjgnet.com/1007-9327/full/v17/i33/3836.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i33.3836
Ghrelin is a novel 28-amino acid peptide which was simultaneously discovered by two independent research groups more than 10 years ago[1,2]. It was initially found to be a secretory product of a subset of enteroendocrine cells predominantly present in the stomach with a decreasing gradient toward the small intestine and the colon[1,2]. It was also found to be produced in small amounts by several other organs such as the brain, pancreas, pituitary, kidney, lung and placenta[3].
Functional studies revealed that ghrelin is involved in a wide variety of biological activities including growth hormone release[4], stimulation of food intake and body weight gain[5,6], gastrointestinal (GI) motility[7], and modulation of cardiovascular function[8]. Furthermore, it has been shown that ghrelin may also control proliferation and differentiation programs of neuronal and mesenchymal stem cells[9] and modulate cell proliferation of some tissue progenitors[10,11] and cell lines[12-14].
In the stomach, while ghrelin has been shown to modulate the secretory activity of parietal cells[15,16], it is not known whether this hormone is involved in the regulation of epithelial cell proliferation. It was shown earlier that parietal cells are the source of some instructive signals and their ablation in mice modulates the proliferation and differentiation program of gastric epithelial stem/progenitor cells[17] and with age cause gastric carcinoma[18]. Therefore, modulation of the secretory activity of these cells might also affect the proliferation/differentiation program of the epithelial progenitors. Surprisingly, when ghrelin knockout mice were examined, no abnormalities were reported in the GI mucosa[19].Recently, there has been increasing evidence to suggest a role for ghrelin in protection against gastric mucosal damage[20,21]. The mechanism of mucosal protection was mainly attributed to the release of nitric oxide[22].
The aims of this study were to determine whether ghrelin can be used to protect against GI mucosal damage induced by doxorubicin, and to test whether ghrelin protection, if any, is associated with modulation of cell proliferation in the progenitor cell zone of the GI epithelium.
In this study, female BALB/c mice (2-3 mo old) were evaluated after being housed in sterile microisolator cages with sterile bedding, food, and water ad libitum. The animals were kept under a 12-h light/dark cycle and at room temperature (22-24 °C). The protocols described in this study were approved by the Animal Research Ethics Committee of the Faculty of Medicine, UAE University.
To establish the experimental protocol of this study, we initially injected age-matched mice (n = 40) with a single dose of 5-fluorouracil (100 mg/kg body weight) or doxorubicin (10 mg/kg). Mice were then sacrificed at different time periods varying from 1 to 16 d. Gastroduodenal tissues were collected to identify any changes in mucosal integrity.
Mice (n = 9) were divided into three equal groups. Mice in the first group received ghrelin (Sigma, St. Louis, MO, United States) through Alzet micro-osmotic pumps (Durect Co, Cupertino, CA, United States) implanted subcutaneously which released ghrelin at a rate of 1.25 mg/h for 14 d. The pumps were prepared for implantation according to manufacturer’s instructions and our previously published procedure[23]. On the 8th and 9th day of ghrelin perfusion, mice received two intravenous injections of doxorubicin (10 and 6 mg/kg, respectively). In the second group, mice were subcutaneously infused with saline instead of ghrelin and then received two intravenous injections of doxorubicin on two consecutive days as in the first group. The third group of mice served as controls and, instead of ghrelin and doxorubicin, received only saline by infusion pump and by intravenous injections, respectively. To label dividing cells in the S-phase of the cell cycle, mice in all 3 groups received a single intraperitoneal injection of 5’-bromo-2’-deoxyuridine (BrdU, 120 mg/kg) one hour before sacrifice. At day 4 post-doxorubicin (or saline) second injection, the stomach along with the duodenum were removed under ether anesthesia and processed for morphological and immunohistochemical analysis.
To examine the histopathological changes that occurred in the wall of the GI tract, the stomach and proximal part of the duodenum were dissected from all mice under anesthesia and immediately processed for conventional histological examination[23]. The tissues were fixed immediately in Bouin solution, dehydrated in ethanol, and infiltrated/embedded in paraffin. Five-micron-thick sections were mounted on slides and stained with periodic acid schiff and hematoxylin.
To examine cell proliferation and estimate the number of cells in the S-phase of the cell cycle, paraffin tissue sections from all mice were processed to determine the localization of cells which incorporated BrdU[23]. Briefly, tissue sections were first deparaffinized, hydrated, and incubated with 3% hydrogen peroxide to block endogenous peroxidase. The anti-BrdU antibody used was goat polyclonal[24]. Biotinylated anti-goat immunoglobulin G was used as a secondary antibody which was identified by avidin-peroxidase and then di-aminobenzidine as a coloring agent. Immunoprobed tissue sections were scanned using the Olympus microscope to quantify BrdU-labeled cells. In each mouse, the numbers of BrdU-labeled cells per gastric gland or crypt-villus unit were averaged.
Data are presented as mean ± SD. Differences between groups were evaluated using the Student t test. P < 0.05 was taken as statistically significant.
The damaging effect of the chemotherapeutic agents on GI mucosa was followed by microscopic examination of tissue sections. The dose regimen used for 5-fluorouracil showed inconsistent damaging effects in the mucosal tissues in the form of occasional vacuolation of the lining GI epithelial cells. Doxorubicin treatment induced a slightly more pronounced and consistent effect. Therefore, it was decided to use two intravenous injections of doxorubicin for the experimental protocol.
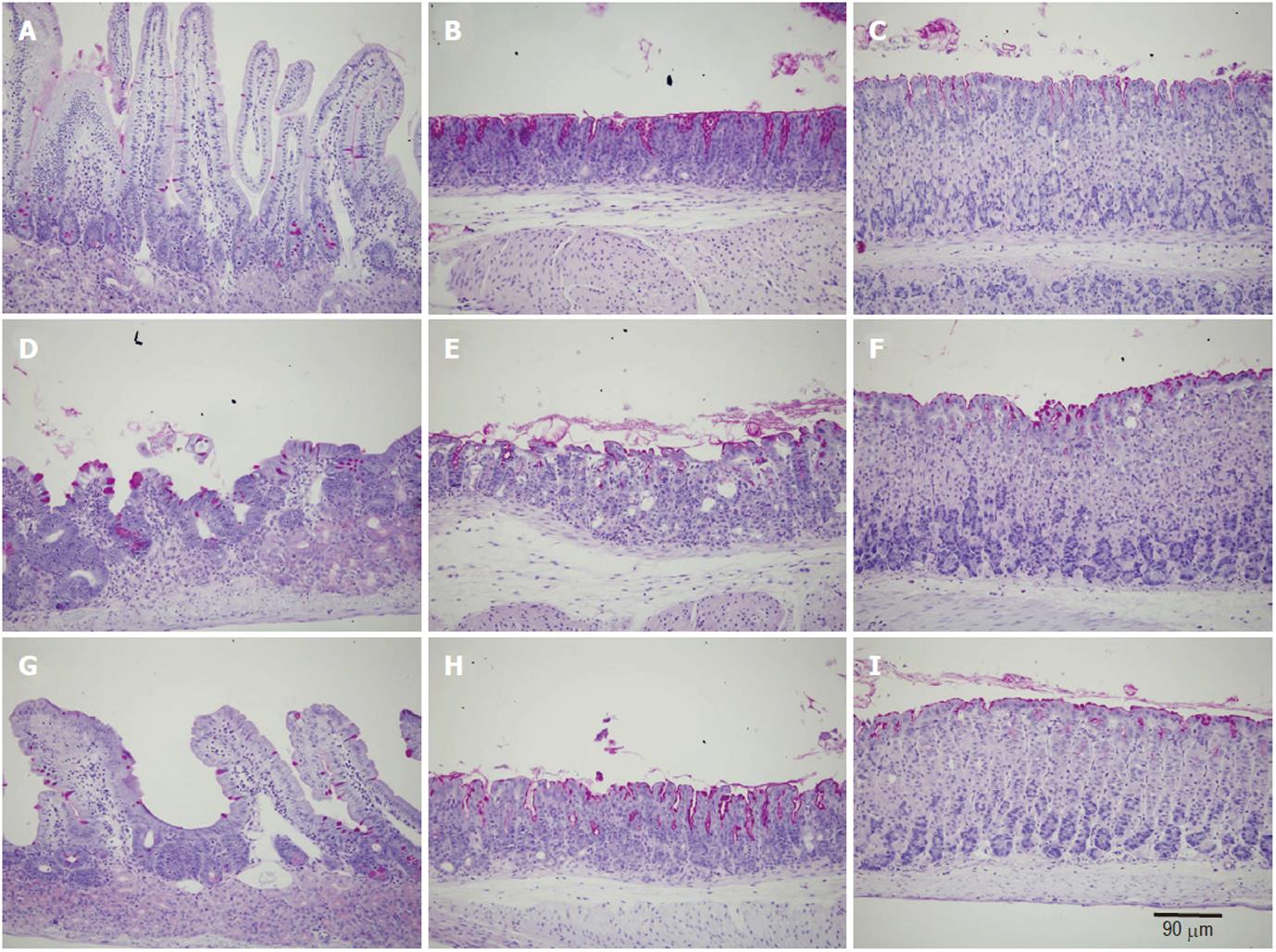
Microscopic examination of gastroduodenal mucosa of control mice revealed the expected histological features of intact long oxyntic glands of the corpus region, short mucous glands of the pyloric antrum, and the very long crypt-villus units of the duodenum (Figure 1) as previously described[25]. In the second group of doxorubicin-treated mice, while little changes were observed in the oxyntic glands in the form of a few scattered cells with vacuolated cytoplasm, the antral glands showed more aggressive changes which induced dilatations of the glandular lumen. Massive mucosal changes in the duodenum were observed (Figure 1D-F). The villi appeared blunt or much shorter and broader than those of control mice. In addition, the integrity of the villus epithelium was not intact. Signs of vacuolation and cell damage were evident throughout. In the ghrelin-plus-doxorubicin-treated mice, the glands in the corpus and antral regions appeared similar to those of control mice. Even the duodenal villi appeared intact, long and populated mainly by absorptive and goblet cells as in control mice (Figure 1G-I).
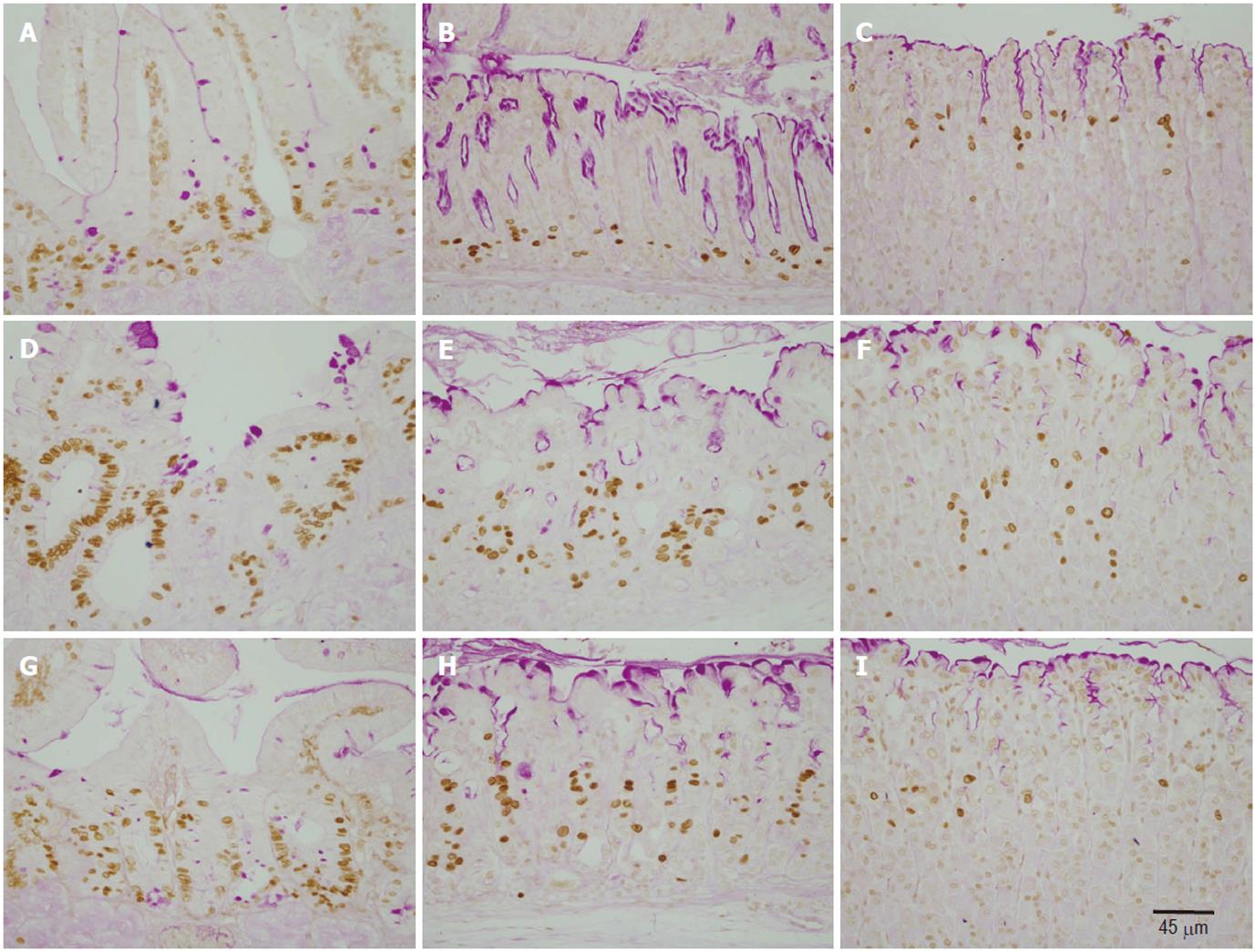
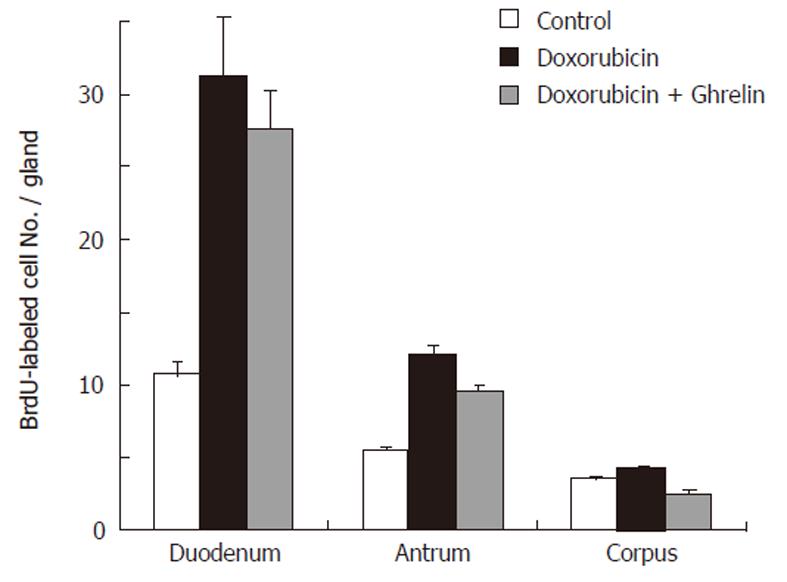
To correlate the morphological changes with cell proliferation, we used the BrdU labeling method. BrdU was made available to cells in the S-phase of the cell cycle one hour before sacrifice. Gastroduodenal tissue sections were then immunolabeled using anti-BrdU antibody (Figure 2). In control mice, BrdU-labeled cells in the corpus and antral mucosae were located at the pit-gland junction close to the luminal surface (corpus) or the gland bottom (antrum). Counts revealed the presence of 3.5 ± 0.18 and 5.5 ± 0.18 cells per gland in the corpus and antrum, respectively (Figure 3). In the duodenum, dividing BrdU-labeled cells were in the lower portion of the crypts and averaged 10.7 ± 0.85 (Figure 3).
In doxorubicin-treated mice, the labeling pattern of BrdU-immunoreactive cells was altered in the corpus, antrum and duodenum. There was a general increase in the number of BrdU-labeled cells in the gastroduodenal mucosa. In addition, the distribution of BrdU-labeled cells was expanded and tended to be more scattered rather than localized to the gastric isthmus (Figure 2E, F). Counts revealed an increase in the number of BrdU-labeled cells up to 31.2 ± 4.07, 12.1 ± 0.59, 4.3 ± 0.18 in the duodenum, antrum and corpus, respectively. Each of these values was significantly higher than its corresponding value in control mice (P > 0.01).
Ghrelin-treated mice which also received doxorubicin showed an usual pattern in the distribution of dividing cells, however, the number of BrdU-labeled cells in the antrum and duodenum remained at a higher level than the control (Figures 2G, H, 3). These findings were confirmed when all mice were examined and BrdU-labeled cells were quantified. When compared with control, the data showed a significant increase in the number of BrdU-labeled cells in the pyloric antrum and duodenum (9.6 ± 0.38 and 27.6 ± 2.75, respectively). However, when these values were compared with those obtained from the second group of mice (treated only with doxorubicin), there was a decrease in S-phase labeled cells, but this was only significant in the antrum (P > 0.02).
Despite the tremendous effort in modern drug discovery and development, dyspepsia and GI mucosal damage remain frequent complications affecting life quality in cancer patients receiving chemotherapy. The available data demonstrate that ghrelin could be a potential protective agent against these complications. In rats treated with cisplatin, it was demonstrated that ghrelin can be used to prevent delayed gastric emptying, early satiety, anorexia, nausea and vomiting, all characteristic of cancer-associated dyspepsia syndrome[26]. In the present study we induced GI mucosal damage using doxorubicin and demonstrated that ghrelin administration can also prevent the damaging effects of this chemotherapeutic agent.
Ghrelin appears to be a potent GI mucosal protective agent. Its effect on maintaining GI mucosal integrity was achieved using different experimental animal models. A recent series of studies showed that administration of ghrelin to rodents attenuated gastric mucosal lesions induced by ethanol[20], stress[21], ischemia-reperfusion[27,28] and even HCl administration[29]. The ghrelin effects on ethanol and stress models were mediated by release of nitric oxide as they were prevented by blocking nitric oxide synthase activity[22]. The effects of ghrelin on the HCl-model was mediated not only by growth hormone secretagogue receptor, but also histamine H3 receptor, suggesting the involvement of histamine release in ghrelin-induced protection[29].
It has been suggested that ghrelin is involved in several cell biological processes via different modes of actions: endocrine, paracrine and autocrine. We speculate that ghrelin exerted its GI mucosal protection via a paracrine effect. Examination of the GI mucosae of mice treated with two intravenous injections of doxorubicin revealed degenerative changes in their epithelial cell lining. However, mice which received ghrelin by continuous subcutaneous infusion showed minimal effects and appeared more or less similar to control mice when treated with the same dosage regimen of doxorubicin. The available data from the present study may suggest a mechanism which involves modulation of the cell cycle and induction of cell differentiation to substitute for the damaging effect of doxorubicin.
Several lines of evidence suggest that ghrelin modulates the proliferation of various cell types. It has been demonstrated that ghrelin stimulates proliferation of osteoprogenitor cells in bone tissue[30] and neuronal progenitor cells in the spinal cord[31]. Since cell proliferation and epithelial renewal are regarded as one of the protective mechanisms against GI mucosal damage, the question arises whether ghrelin also modulates proliferation of GI epithelial cells and hence could protect their integrity against noxious agents (such as chemotherapy). To answer this question we injected all mice used in our experiments with BrdU to label dividing cells during the S-phase of the cell cycle using immunohistochemistry.As expected, control mice showed BrdU-labeled cells in the isthmus regions of gastric glands and at the bottom of intestinal crypts. Doxorubicin-treated mice showed many more BrdU-labeled cells in both the gastric glands and intestinal crypts, probably to compensate for the damaged cells. However, in the presence of excess ghrelin, the damaging effects of doxorubicin were minimal and the number of BrdU-labeled cells was reduced, perhaps due to enhancement of cell differentiation. The other possibility is that ghrelin enhances cell differentiation and the increased proliferating cells observed in doxorubicin-treated mice were instructed by ghrelin to differentiate and migrate to restore the normal organization of the epithelium.
The protective effect of ghrelin against tissue damage is not restricted to the GI mucosa. Recent studies suggested that ghrelin promotes neuroprotective effects via stimulation of the regenerative potential of hippocampal neuroprogenitor cells to form new neurons. Incidentally, the expression of GHS-R was also demonstrated in the hippocampus[10,11].
In conclusion, this study demonstrates the protective effect of ghrelin against GI mucosal damage induced by doxorubicin and provides an addition justification for its potential use during chemotherapy in cancer patients to improve their quality of life.
Normal gastrointestinal (GI) mucosa is characterized by its regenerative potential following damage. However, cancer patients receiving chemotherapy develop severe GI complications due to mucosal damage. Ghrelin has been suggested to play a protective role against these mucosal damaging effects.
It is important to define the factors involved in GI mucosal protection and regulation of stem cell proliferation and differentiation. Here the authors provide evidence in support of the role of ghrelin in GI mucosal protection.
This study provides evidence that ghrelin protects against GI mucosal damage caused by doxorubicin.
The findings of this article could help in designing new modalities for GI mucosal protection and regeneration in cancer patients undergoing chemotherapy.
This is an interesting and elegant experimental paper examining the effect of ghrelin on the intestinal tract of doxorubicin-treated mice. The investigators showed that ghrelin protects against doxorubicin-induced epithelial damage. In addition, they showed it modulates epithelial proliferation.
This paper is dedicated to our colleague Dr. H Kataya who initiated this collaborative study and suddenly passed away before its completion. The authors acknowledge the Research Affairs section of the United Arab Emirates University which funded this interdisciplinary project.
Peer reviewers: Dr. Jianyuan Chai, PhD, MS, BS, Assistant Professor, Research (09-151), VA Long Beach Healthcare System, 5901 E. 7th St, Long Beach, CA 90822, United States; Julio Mayol, MD, PhD, Department of Digestive Surgery, Hospital Clinico San Carlos, MARTIN-LAGOS S/n, Madrid, 28040, Spain
S- Editor Tian L L- Editor Webster JR E- Editor Li JY
| 1. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5961] [Cited by in F6Publishing: 5702] [Article Influence: 228.1] [Reference Citation Analysis (0)] |
| 2. | Tomasetto C, Karam SM, Ribieras S, Masson R, Lefèbvre O, Staub A, Alexander G, Chenard MP, Rio MC. Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology. 2000;119:395-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 305] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908-4911. [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2466] [Cited by in F6Publishing: 2399] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 6. | Camina JP, Carreira MC, Micic D, Pombo M, Kelestimur F, Dieguez C, Casanueva FF. Regulation of ghrelin secretion and action. Endocrine. 2003;22:5-12. [DOI] [Cited in This Article: ] |
| 7. | Poitras P, Tomasetto C. The potential of ghrelin as a prokinetic. Regul Pept. 2009;155:24-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Nagaya N, Kangawa K. Ghrelin, a novel growth hormone-releasing peptide, in the treatment of chronic heart failure. Regul Pept. 2003;114:71-77. [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Xu G, Li Y, An W, Zhang W. Ghrelin and cell differentiation. Acta Biochim Biophys Sin (Shanghai). 2008;40:841-847. [Cited in This Article: ] |
| 10. | Johansson I, Destefanis S, Aberg ND, Aberg MA, Blomgren K, Zhu C, Ghè C, Granata R, Ghigo E, Muccioli G. Proliferative and protective effects of growth hormone secretagogues on adult rat hippocampal progenitor cells. Endocrinology. 2008;149:2191-2199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Moon M, Kim S, Hwang L, Park S. Ghrelin regulates hippocampal neurogenesis in adult mice. Endocr J. 2009;56:525-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Duxbury MS, Waseem T, Ito H, Robinson MK, Zinner MJ, Ashley SW, Whang EE. Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem Biophys Res Commun. 2003;309:464-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Kim MS, Yoon CY, Jang PG, Park YJ, Shin CS, Park HS, Ryu JW, Pak YK, Park JY, Lee KU. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2291-2301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Nanzer AM, Khalaf S, Mozid AM, Fowkes RC, Patel MV, Burrin JM, Grossman AB, Korbonits M. Ghrelin exerts a proliferative effect on a rat pituitary somatotroph cell line via the mitogen-activated protein kinase pathway. Eur J Endocrinol. 2004;151:233-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Levin F, Edholm T, Ehrström M, Wallin B, Schmidt PT, Kirchgessner AM, Hilsted LM, Hellström PM, Näslund E. Effect of peripherally administered ghrelin on gastric emptying and acid secretion in the rat. Regul Pept. 2005;131:59-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Sibilia V, Lattuada N, Rapetti D, Pagani F, Vincenza D, Bulgarelli I, Locatelli V, Guidobono F, Netti C. Ghrelin inhibits inflammatory pain in rats: involvement of the opioid system. Neuropharmacology. 2006;51:497-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Karam SM, Li Q, Gordon JI. Gastric epithelial morphogenesis in normal and transgenic mice. Am J Physiol. 1997;272:G1209-1220. [Cited in This Article: ] |
| 18. | Syder AJ, Karam SM, Mills JC, Ippolito JE, Ansari HR, Farook V, Gordon JI. A transgenic mouse model of metastatic carcinoma involving transdifferentiation of a gastric epithelial lineage progenitor to a neuroendocrine phenotype. Proc Natl Acad Sci USA. 2004;101:4471-4476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA. 2004;101:8227-8232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 333] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Sibilia V, Rindi G, Pagani F, Rapetti D, Locatelli V, Torsello A, Campanini N, Deghenghi R, Netti C. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Brzozowski T, Konturek PC, Konturek SJ, Kwiecień S, Drozdowicz D, Bielanski W, Pajdo R, Ptak A, Nikiforuk A, Pawlik WW. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul Pept. 2004;120:39-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Sibilia V, Pagani F, Rindi G, Lattuada N, Rapetti D, De Luca V, Campanini N, Bulgarelli I, Locatelli V, Guidobono F. Central ghrelin gastroprotection involves nitric oxide/prostaglandin cross-talk. Br J Pharmacol. 2008;154:688-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Karam SM, John R, Alpers DH, Ponery AS. Retinoic acid stimulates the dynamics of mouse gastric epithelial progenitors. Stem Cells. 2005;23:433-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Cohn SM, Lieberman MW. The use of antibodies to 5-bromo-2'-deoxyuridine for the isolation of DNA sequences containing excision-repair sites. J Biol Chem. 1984;259:12456-12462. [PubMed] [Cited in This Article: ] |
| 25. | Karam SM. Lineage commitment and maturation of epithelial cells in the gut. Front Biosci. 1999;4:D286-D298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 167] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Liu YL, Malik NM, Sanger GJ, Andrews PL. Ghrelin alleviates cancer chemotherapy-associated dyspepsia in rodents. Cancer Chemother Pharmacol. 2006;58:326-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Brzozowski T, Konturek PC, Sliwowski Z, Pajdo R, Drozdowicz D, Kwiecien S, Burnat G, Konturek SJ, Pawlik WW. Prostaglandin/cyclooxygenase pathway in ghrelin-induced gastroprotection against ischemia-reperfusion injury. J Pharmacol Exp Ther. 2006;319:477-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Wu R, Dong W, Ji Y, Zhou M, Marini CP, Ravikumar TS, Wang P. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS One. 2008;3:e2026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Adami M, Pozzoli C, Leurs R, Stark H, Coruzzi G. Histamine H(3) receptors are involved in the protective effect of ghrelin against HCl-induced gastric damage in rats. Pharmacology. 2010;86:259-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Maccarinelli G, Sibilia V, Torsello A, Raimondo F, Pitto M, Giustina A, Netti C, Cocchi D. Ghrelin regulates proliferation and differentiation of osteoblastic cells. J Endocrinol. 2005;184:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 31. | Sato M, Nakahara K, Goto S, Kaiya H, Miyazato M, Date Y, Nakazato M, Kangawa K, Murakami N. Effects of ghrelin and des-acyl ghrelin on neurogenesis of the rat fetal spinal cord. Biochem Biophys Res Commun. 2006;350:598-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |