Copyright
©2011 Baishideng Publishing Group Co.
World J Gastroenterol. Mar 21, 2011; 17(11): 1383-1399
Published online Mar 21, 2011. doi: 10.3748/wjg.v17.i11.1383
Published online Mar 21, 2011. doi: 10.3748/wjg.v17.i11.1383
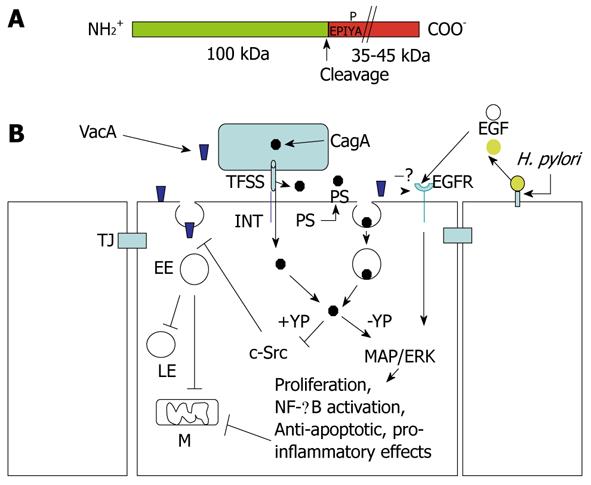
Figure 6 Structure of CagA and signaling cross-talk between VacA and CagA.
A: CagA encompasses two fragments: the N-terminal 100-kDa fragment may contain the cell binding domain [to phosphatidylserine (PS)?]. Cleavage of CagA may take place just at the beginning of the first EPIYA motif, which can be tyrosine-phosphorylated by the c-Src tyrosine kinase. The C-terminal portion of CagA, which contains all the signaling activity of the molecule, may have a different molecular mass (up to 45 kDa) due to the repetition of the EPIYA-containing domain; B: CagA produced within the bacterium is transferred in the external medium by the type IV secretion system (TFSS) machinery. Two possibilities for CagA transfer into the target epithelial cell: (a) by binding to an integrin (INT), the TFSS punches the cell membrane and injects CagA; or (b) the TFSS induces the flipping of PS on the outer cell surface. By its 100-kDa N-terminal fragment, CagA binds PS and, upon endocytosis, which is transferred into the gastric cells. In the cytosol, the CagA signaling domain can be tyrosine-phosphorylated (+YP) and inhibits the c-Src kinase activity that is required to allow the transfer of VacA from glycosylphosphatidylinositol-anchored protein-enriched early endosomal compartments (GEECs) to early endosomes (EEs). This blocks vacuolation in late endosomes (LEs) and mitochondria (M)-dependent apoptosis induced by VacA. In an unphosphorylated (-YP) state, CagA activates mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) and nuclear factor (NF)-κB anti-apototic and pro-inflammatory pathways, which also counteract VacA-induced apoptosis. VacA interferes with epidermal growth factor (EGF) receptor (EGFR) activation and endocytosis, thus impairing the signaling pathway that is triggered by this receptor. Free EGFR ligands (EGF) are liberated from the cell-surface-bound molecules via cleavage triggered by Helicobacter pylori (H. pylori). TJ: Tight junction.
- Citation: Ricci V, Romano M, Boquet P. Molecular cross-talk between Helicobacter pylori and human gastric mucosa. World J Gastroenterol 2011; 17(11): 1383-1399
- URL: https://www.wjgnet.com/1007-9327/full/v17/i11/1383.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i11.1383