Published online Jan 7, 2011. doi: 10.3748/wjg.v17.i1.105
Revised: August 16, 2010
Accepted: August 23, 2010
Published online: January 7, 2011
AIM: To evaluate the effects of ginger on gastric motility and emptying, abdominal symptoms, and hormones that influence motility in dyspepsia.
METHODS: Eleven patients with functional dyspepsia were studied twice in a randomized double-blind manner. After an 8-h fast, the patients ingested three capsules that contained ginger (total 1.2 g) or placebo, followed after 1 h by 500 mL low-nutrient soup. Antral area, fundus area and diameter, and the frequency of antral contractions were measured using ultrasound at frequent intervals, and the gastric half-emptying time was calculated from the change in antral area. Gastrointestinal sensations and appetite were scored using visual analog questionnaires, and blood was taken for measurement of plasma glucagon-like peptide-1 (GLP-1), motilin and ghrelin concentrations, at intervals throughout the study.
RESULTS: Gastric emptying was more rapid after ginger than placebo [median (range) half-emptying time 12.3 (8.5-17.0) min after ginger, 16.1 (8.3-22.6) min after placebo, P≤ 0.05]. There was a trend for more antral contractions (P = 0.06), but fundus dimensions and gastrointestinal symptoms did not differ, nor did serum concentrations of GLP-1, motilin and ghrelin.
CONCLUSION: Ginger stimulated gastric emptying and antral contractions in patients with functional dyspepsia, but had no impact on gastrointestinal symptoms or gut peptides.
- Citation: Hu ML, Rayner CK, Wu KL, Chuah SK, Tai WC, Chou YP, Chiu YC, Chiu KW, Hu TH. Effect of ginger on gastric motility and symptoms of functional dyspepsia. World J Gastroenterol 2011; 17(1): 105-110
- URL: https://www.wjgnet.com/1007-9327/full/v17/i1/105.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i1.105
Functional dyspepsia is a clinical syndrome that is characterized by chronic or recurrent upper abdominal pain or discomfort in the absence of underlying organic disease that can explain the symptoms[1]. Pharmacological therapy for patients with functional dyspepsia remains unsatisfactory[2]. The results of controlled trials have generally been disappointing, and only small benefits relative to placebo have been found with histamine H2-receptor antagonists[3], proton-pump inhibitors[4], and Helicobacter pylori eradication[5]. In addition to poor efficacy, pharmacological agents (e.g. cisapride) are associated with a risk of adverse effects.
Herbal medicine might be an attractive alternative based on the perception of its natural approach and low risk of adverse effects. However, the lack of standardization of herbal ingredients has limited the number of rigorous clinical studies available.
Ginger (Zingiber officinale) has been used to treat a number of medical conditions, including those affecting the digestive tract[6,7], such as dyspepsia, flatulence, nausea and abdominal pain. However, the mechanisms responsible for its beneficial effects are not well understood. Yamahara et al[8] and Micklefield et al[9] have reported that gastrointestinal motility is enhanced by ginger and its active constituents, but they did not measure gastric emptying. We previously have shown that ginger increases the frequency of antral contractions and accelerates gastric emptying of a low-nutritent liquid in healthy volunteers[10].
There have been few studies on the effects of ginger in patients with functional dyspepsia. We hypothesized that acceleration of gastric emptying in patients with functional dyspepsia might be accompanied by a reduction in upper gastrointestinal symptoms, and that its action on gastric motility could be mediated via increased secretion of ghrelin[11] or motilin[12], or by suppression of glucagon-like peptide-1 (GLP-1)[13].
Eleven patients diagnosed with functional dyspepsia on the basis of Rome III criteria were invited to take part. Patients had persistent or recurrent upper abdominal pain or discomfort, which was characterized by the presence of one or more of early satiety, postprandial fullness, bloating, and nausea. Symptoms had been present for at least 6 wk within the preceding 6 mo, without an identifiable structural or biochemical abnormality to which they could be attributed[1]. Symptoms of retrosternal pain, burning, and regurgitation were considered features of gastroesophageal reflux disease, rather than of functional dyspepsia. Therefore, patients who had predominantly reflux-related symptoms were excluded. Patients were screened by physical examination, laboratory tests (blood picture, fasting glucose, and liver-function tests), abdominal ultrasonography, and upper gastrointestinal endoscopy to exclude other causes of dyspepsia, and none was taking any medication known to affect gastric motility.
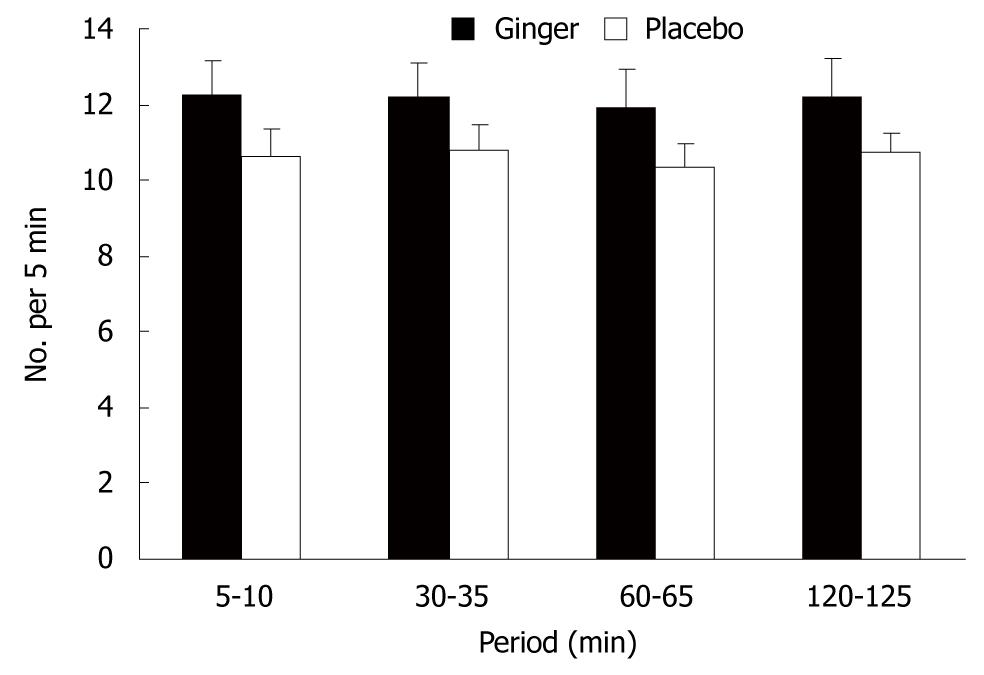
Each subject was studied on two afternoons, separated by at least 7 d, in double-blind randomized order. Following a fast of 8 h for solids and liquids, the patients ingested three capsules that contained a total of 1.2 g ginger root powder (Ginger Root; Nature’s Way Products Inc., Springville, UT, USA), or three identical placebo capsules that contained starch, together with 50 mL water. One hour later, they consumed 500 mL chicken and corn soup (United Kanboo, Taipei, Taiwan), which contained 118.6 kcal (2.6 g protein, 2.6 g fat, 21.2 g carbohydrate). The soup was boiled and subsequently cooled to 37°, and was consumed over 5 min (t = -5 to 0 min). All patients underwent trans-abdominal ultrasound to measure antral area, fundic area and diameter[14], and antral contractions at intervals using an Aloka SSD-2000 CL Ultrasound Machine (Aloka, Tokyo, Japan) with a 3.5-MHz annular array probe. Antral contractions were defined as > 50% change in antral area compared to the relaxed area (ΔA/A)[15], and their frequency as the number of contractions during 5-min periods beginning at 5, 30, 60 and 120 min after soup ingestion. A questionnaire with visual analogue scales (VASs)[16] for symptoms pain, nausea, abdominal discomfort, bloating and abdominal fullness, was administered at 10-min intervals between t = -10 and 90 min. Grading was made on a 100-mm unmarked line between “no symptoms” at one end and “excruciating symptoms” at the other. Venous blood was sampled at t = -10, 30, 60 and 90 min for measurement of blood glucose and plasma peptides. Blood glucose concentrations were determined immediately using a portable blood glucose meter (MediSense Companion 2 meter; MediSense Inc., Waltham, MA, USA). The accuracy of this method has been confirmed using the hexokinase technique[17]. The remainder of the samples was collected into ice-chilled EDTA-treated tubes that contained 400 KIU/mL aprotinin. Plasma was separated and samples stored at -70°C for subsequent analysis of GLP-1, ghrelin and motilin concentrations, using ELISA. Ghrelin was measured by a commercial ELISA kit (Phoenix Pharmaceuticals Inc., Burlingame, CA, USA); intra- and interassay coefficients of variation (CV) were < 5% and < 9%, respectively; motilin and GLP-1 were also measured by a commercial ELISA kit from Phoenix Pharmaceuticals.
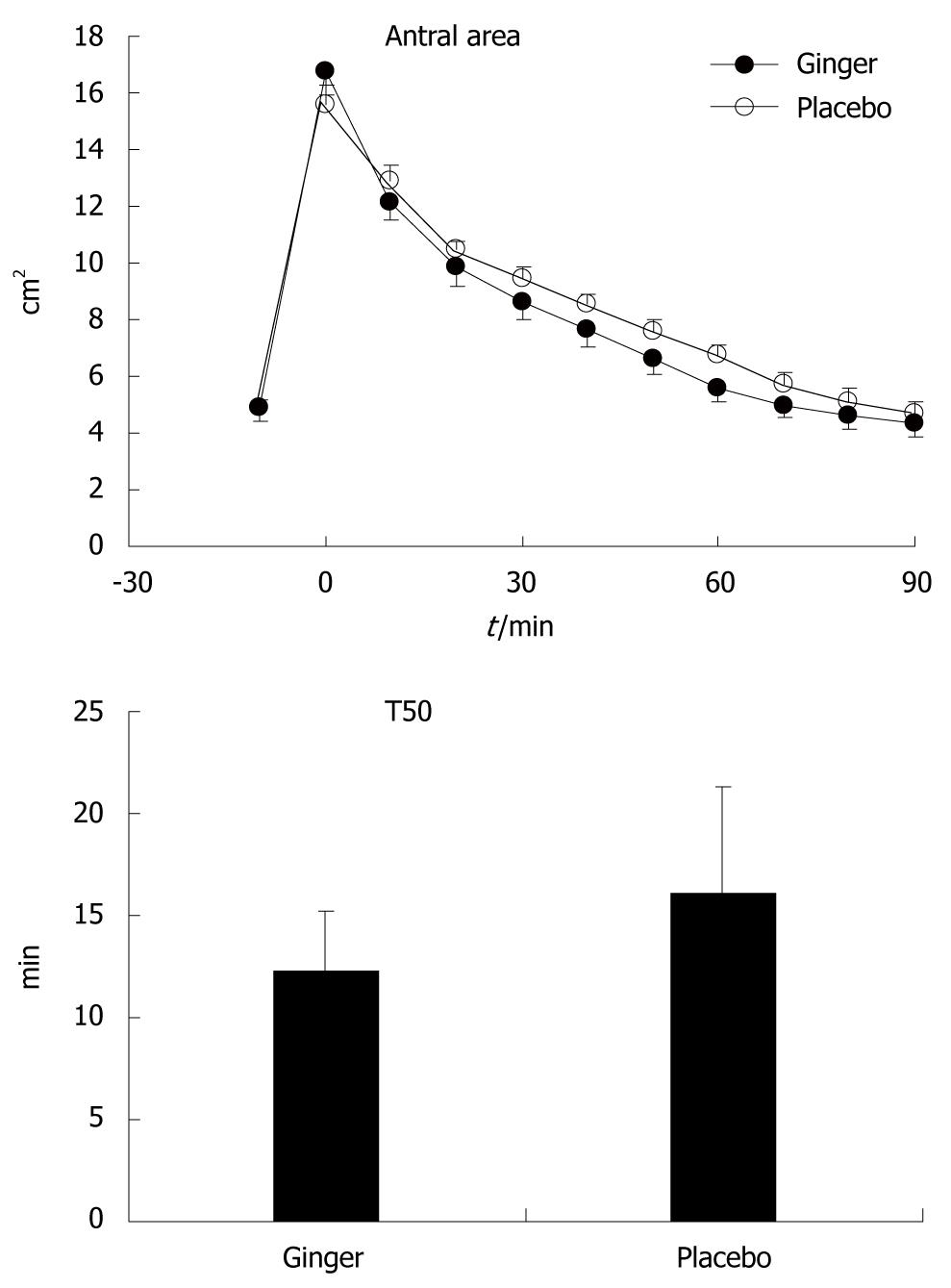
Gastric half-emptying time (T50) was defined as the time for antral area to decrease to half the maximum increase above baseline, and was calculated by linear interpolation between time points[10]. The values on the two study days were compared using the Wilcoxon signed rank test. Antral area, fundic area and diameter, frequency of antral contractions and gastrointestinal sensation scores were compared using repeated measures ANOVA. Results are shown as median and range for T50, and mean ± SD for other variables. P < 0.05 was considered significant.
All subjects tolerated the study well.
Gastric emptying was more rapid after ginger than placebo [T50: 12.3 (8.5-17.0) min vs 16.1 (8.3-22.6) min, P≤ 0.05]. There was a trend for smaller antral area after ginger, although this did not reach statistical significance (P = 0.13) (Figure 1).
There was a trend for more antral contractions after ginger compared to placebo (P = 0.06) (Figure 2).
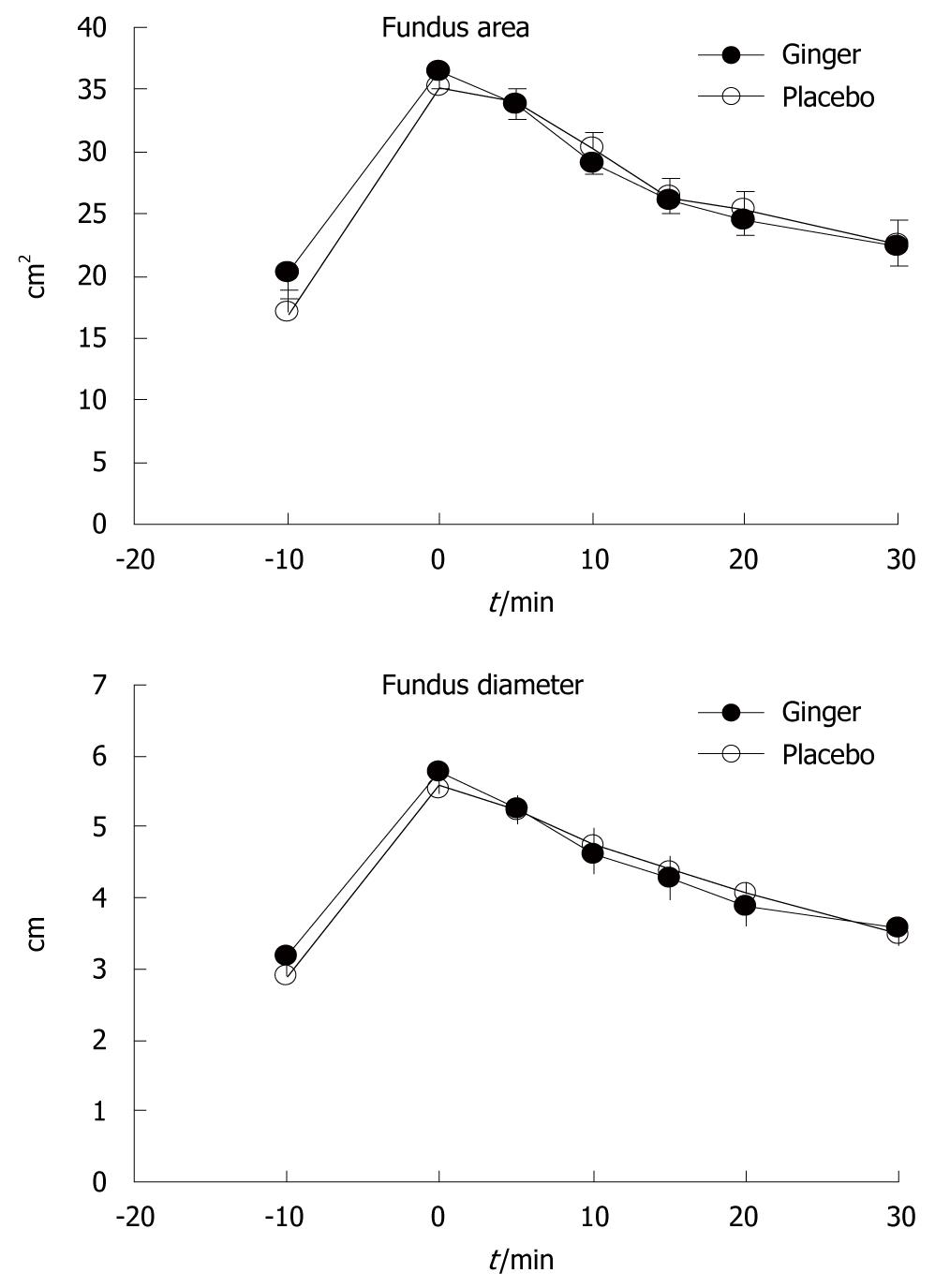
Fundus dimensions did not differ between the two study days (Figure 3).
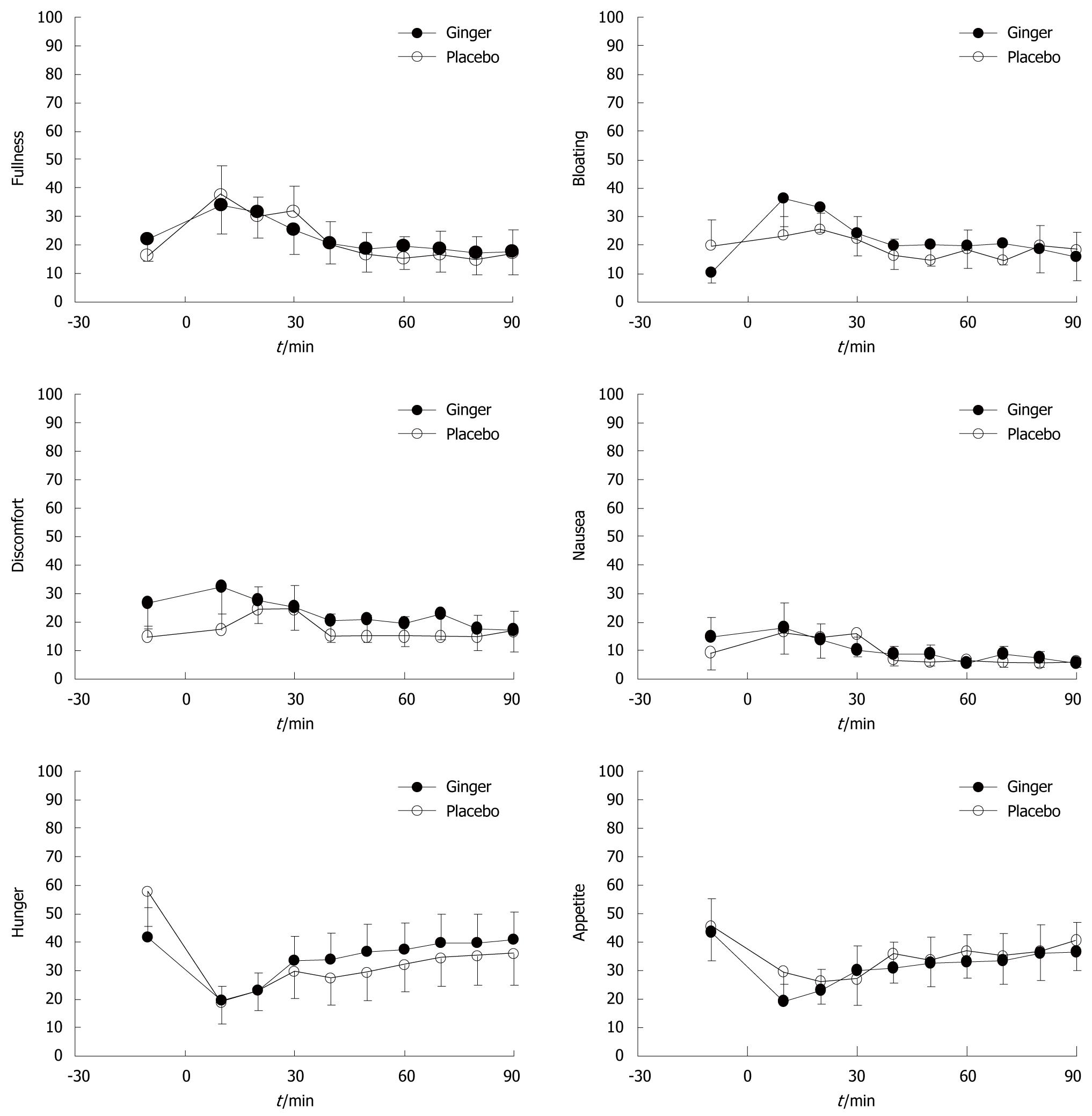
Soup ingestion was associated with increased fullness and bloating, and decreased hunger and appetite scores, but without any difference between ginger and placebo. There were no significant changes in nausea or abdominal discomfort from baseline, or any differences in these sensations between study days (Figure 4).
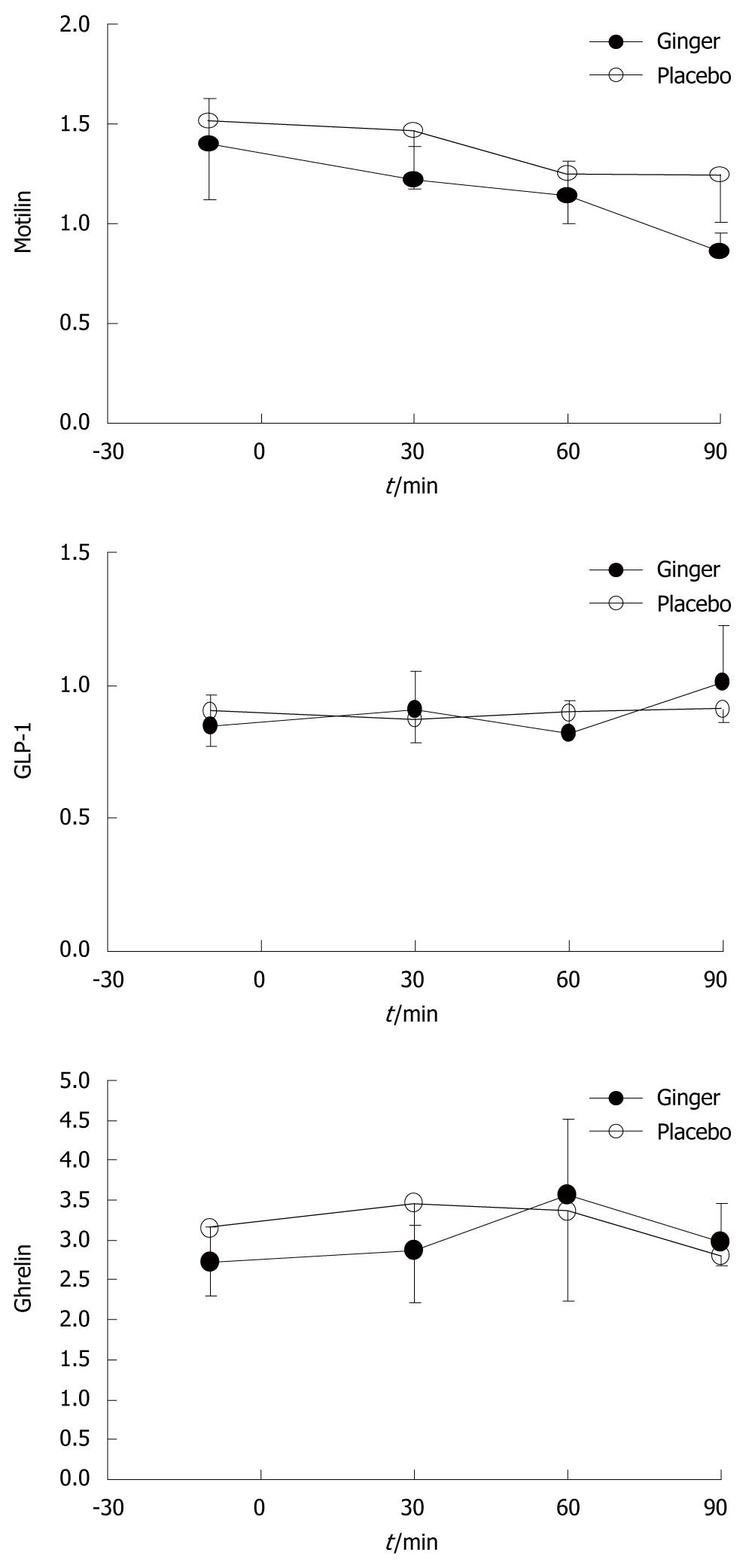
There were no differences in plasma concentrations of motilin, ghrelin, or GLP-1 between the two study days (Figure 5).
In this study, we demonstrated that ginger increased the rate of gastric emptying in patients with functional dyspepsia when compared to placebo, and that this was associated with a trend for an increased frequency of gastric antral contractions, but no change in dimensions of the fundus. This was consistent with our previous study in healthy volunteers[10]. Despite more rapid emptying, ginger had no effect on gastrointestinal symptom scores in our patients.
About 40% of patients with functional dyspepsia have abnormally delayed gastric emptying[18], and prokinetic medications have often been used in the treatment of this condition[19]. We did not select our dyspeptic patients on the basis of delayed gastric emptying, and as a group, their rate of gastric emptying was comparable to the healthy volunteers that we had studied previously using the same technique[10]. It is possible that ginger could have improved symptoms in a more selected group of patients who had delayed emptying, and in particular, those with an abnormally wide antrum, a feature that has been associated particularly with bloating[20].
It is also possible that the lack of symptomatic improvement with ginger was related to the low-nutrient nature of the soup meal. Although this meal was associated with increases in fullness and bloating, the changes were modest. A meal with a higher caloric load, particularly one that contained more lipid[21], might have provoked more symptoms, from which it would be possible to demonstrate an improvement with ginger. Similarly, although we used the same dose of ginger as Lien et al[22], who reported a reduction in nausea induced by circular vection, our subjects reported low ratings for nausea throughout the study, thus, it would be difficult to demonstrate an effect of ginger if one existed. Stadelmann et al[23] have reported that a combination of peppermint oil and ginger extract for 4 wk improved gastrointestinal symptom scores when compared to placebo, but the relative contribution of each active agent is unclear.
The mechanism by which ginger could enhance antral contractions and gastric emptying is not clear. We could not demonstrate any modulation of gut-derived hormones that are known to affect gastric motility, including motilin, ghrelin or GLP-1. It would be of interest to examine whether ginger affects the plasma concentrations or the actions of cholecystokinin, because this hormone is reported to be elevated in patients with functional dyspepsia[21]. Abdel-Aziz et al[24] have reported the potential for ginger to act on the 5-HT3 receptor ion-channel complex, by binding the serotonin binding site, and Shibata et al[25] have reported that a component of Dai-Kenchu-Tou (which contains ginger) stimulated gastric motility through cholinergic and -5HT3 receptors in dogs. The limitation of this study is that our observation was limited to 90 min of gastric emptying, and a single dose of ginger would not have been adequate for treatment of dyspepsia symptoms in patients with functional dyspepsia, especially as this disease is chronic and recurrent. Therefore, it is difficult to draw any clear conclusion. Further proper clinical trials of several weeks’ treatment with ginger capsules seem to be necessary before starting trials in subgroups of patients with functional dyspepsia.
In summary, we confirmed that the acceleration of gastric emptying by ginger that we initially demonstrated in healthy volunteers extended to patients with functional dyspepsia. Further studies could be indicated in specific subgroups of patients (e.g. those with predominant bloating or nausea, or those with known delayed gastric emptying), to determine whether this can be a useful therapeutic approach.
Pharmacological therapy for patients with functional dyspepsia remains unsatisfactory. There have been few studies on the effects of ginger in patients with functional dyspepsia.
Ginger (Zingiber officinale) has been used to treat a number of medical conditions, including those that affect the digestive tract. In this study, the authors demonstrated that the effect of ginger could be a potential mechanism for mediating gastric motility.
The authors had previously shown that ginger increases the frequency of antral contractions and accelerates gastric emptying of a low-nutrient liquid in healthy volunteers. However, the actual effect of ginger on patients with functional dyspepsia is still unknown, and this is believed to be the first study to explore this issue. Furthermore, current in vivo studies suggest that ginger is an effective therapy for patients with functional dyspepsia.
By understanding how ginger works on gastric motility, this study might represent a future strategy for therapeutic intervention in patients with delayed gastric emptying.
The acceleration of gastric emptying by ginger that we initially demonstrated in healthy volunteers extends to patients with functional dyspepsia.
Overall, this preliminary study of 11 patients with functional dyspepsia seems well designed. The data were appropriately analyzed and indicate that ginger has an impact on symptoms and gastric emptying. A large study needs to be undertaken to demonstrate its efficacy convincingly.
Peer reviewer: Ted Dinan, Professor, Department of Psychiatry, Cork University Hospital, Wilton, Cork, C1, Ireland
S- Editor Sun H L- Editor Kerr C E- Editor Ma WH
| 1. | Clouse RE, Mayer EA, Aziz Q, Drossman DA, Dumitrascu DL, Mönnikes H, Naliboff BD. Functional abdominal pain syndrome. Gastroenterology. 2006;130:1492-1497. [Cited in This Article: ] |
| 2. | Holtmann G, Talley NJ. Functional dyspepsia. Current treatment recommendations. Drugs. 1993;45:918-930. [Cited in This Article: ] |
| 3. | Talley NJ, McNeil D, Hayden A, Piper DW. Randomized, double-blind, placebo-controlled crossover trial of cimetidine and pirenzepine in nonulcer dyspepsia. Gastroenterology. 1986;91:149-156. [Cited in This Article: ] |
| 4. | Moayyedi P, Delaney BC, Vakil N, Forman D, Talley NJ. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329-1337. [Cited in This Article: ] |
| 5. | Laine L, Schoenfeld P, Fennerty MB. Therapy for Helicobacter pylori in patients with nonulcer dyspepsia. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;134:361-369. [Cited in This Article: ] |
| 6. | Johns Cupp M. Toxicology and clinical pharmacology of herbal products. Totowa, New Jersey (USA): Humana Press 2000; 123-129. [Cited in This Article: ] |
| 7. | Capasso F, Gaginella TS, Grandolini G, Izzo AA. Phytotherapy. A quick reference to herbal medicine SpringerVerlag, Heidelberg (Germany), 2003. . [Cited in This Article: ] |
| 8. | Yamahara J, Huang QR, Li YH, Xu L, Fujimura H. Gastrointestinal motility enhancing effect of ginger and its active constituents. Chem Pharm Bull (Tokyo). 1990;38:430-431. [Cited in This Article: ] |
| 9. | Micklefield GH, Redeker Y, Meister V, Jung O, Greving I, May B. Effects of ginger on gastroduodenal motility. Int J Clin Pharmacol Ther. 1999;37:341-346. [Cited in This Article: ] |
| 10. | Wu KL, Rayner CK, Chuah SK, Changchien CS, Lu SN, Chiu YC, Chiu KW, Lee CM. Effects of ginger on gastric emptying and motility in healthy humans. Eur J Gastroenterol Hepatol. 2008;20:436-440. [Cited in This Article: ] |
| 11. | Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, Janssens J, Peeters T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327-333. [Cited in This Article: ] |
| 12. | Luiking YC, Akkermans LM, van der Reijden AC, Peeters TL, van Berge-Henegouwen GP. Differential effects of motilin on interdigestive motility of the human gastric antrum, pylorus, small intestine and gallbladder. Neurogastroenterol Motil. 2003;15:103-111. [Cited in This Article: ] |
| 13. | Wishart JM, Horowitz M, Morris HA, Jones KL, Nauck MA. Relation between gastric emptying of glucose and plasma concentrations of glucagon-like peptide-1. Peptides. 1998;19:1049-1053. [Cited in This Article: ] |
| 14. | Gilja OH, Lunding J, Hausken T, Gregersen H. Gastric accommodation assessed by ultrasonography. World J Gastroenterol. 2006;12:2825-2829. [Cited in This Article: ] |
| 15. | Hausken T, Odegaard S, Berstad A. Antroduodenal motility studied by real-time ultrasonography. Effect of enprostil. Gastroenterology. 1991;100:59-63. [Cited in This Article: ] |
| 16. | Sepple CP, Read NW. Gastrointestinal correlates of the development of hunger in man. Appetite. 1989;13:183-191. [Cited in This Article: ] |
| 17. | Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993;36:857-862. [Cited in This Article: ] |
| 18. | Talley NJ, Locke GR 3rd, Lahr BD, Zinsmeister AR, Tougas G, Ligozio G, Rojavin MA, Tack J. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55:933-939. [Cited in This Article: ] |
| 19. | Chen SL, Ji JR, Xu P, Cao ZJ, Mo JZ, Fang JY, Xiao SD. Effect of domperidone therapy on nocturnal dyspeptic symptoms of functional dyspepsia patients. World J Gastroenterol. 2010;16:613-617. [Cited in This Article: ] |
| 20. | Hausken T, Berstad A. Wide gastric antrum in patients with non-ulcer dyspepsia. Effect of cisapride. Scand J Gastroenterol. 1992;27:427-432. [Cited in This Article: ] |
| 21. | Pilichiewicz AN, Feltrin KL, Horowitz M, Holtmann G, Wishart JM, Jones KL, Talley NJ, Feinle-Bisset C. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. Am J Gastroenterol. 2008;103:2613-2623. [Cited in This Article: ] |
| 22. | Lien HC, Sun WM, Chen YH, Kim H, Hasler W, Owyang C. Effects of ginger on motion sickness and gastric slow-wave dysrhythmias induced by circular vection. Am J Physiol Gastrointest Liver Physiol. 2003;284:G481-G489. [Cited in This Article: ] |
| 23. | Stadelmann O, Kohler S, Kieser M, Stolte M. Pfefferminzol/Ingwerextrakt bei funktioneller Dyspepsie. Randomisierte plazebokontrollierte Wirksamkeits und Vertraglichkeitsstudie mit Kombinationspraparat. Leber Magen Darm. 1999;29:1-8. [Cited in This Article: ] |
| 24. | Abdel-Aziz H, Windeck T, Ploch M, Verspohl EJ. Mode of action of gingerols and shogaols on 5-HT3 receptors: binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur J Pharmacol. 2006;530:136-143. [Cited in This Article: ] |