Published online Mar 7, 2010. doi: 10.3748/wjg.v16.i9.1076
Revised: January 4, 2010
Accepted: January 11, 2010
Published online: March 7, 2010
AIM: To investigate the effects of sulfated cholecystokinin octapeptide (CCK-8S) on the contractile activity of guinea-pig proximal colon.
METHODS: Proximal colonic smooth muscle (PCSM) strips were obtained from adult female guinea pigs and contractile response of PCSM strips was recorded using a polyphysiograph. PCSM cells were isolated by enzymatic digestion. Resting potential (RP), action potential (AP), large conductance potassium channel currents (IBKCa) and L-type calcium currents (ICa-L) were recorded by patch-clamp techniques.
RESULTS: (1) CCK-8S (10-7 mol/L) enhanced the mean contractile amplitude of colonic circular muscle and longitudinal muscle (LM) strips by 56.53% ± 11.92% (P = 0.038) and 65.93% ± 12.98% (P = 0.019), respectively, as well as the mean frequency of LM by 31.69% ± 13.58% (P = 0.023), which were significantly attenuated by pretreating strips with devazepide, nifedipine, iberiotoxin, thapsigargin (TG) and BAPTA-AM (BA) respectively; (2) CCK-8S (10-7 mol/L) increased the AP amplitude by 38.6% ± 3.2% (P = 0.015), decreased AP duration by 36.9% ± 8.7% (P = 0.026), and depolarized the RP from -61.3 ± 2.7 mV to -29.8 ± 5.9 mV (P = 0.032); and (3) Compared with the normal control group, CCK-8S (10-7 mol/L) enhanced the peak current of IBKCa by 18.7% ± 2.1% (from 916 ± 183 pA to 1088 ± 226 pA; at +60 mV; P = 0.029), which was inhibited by respective pretreatment with iberiotoxin and devazepide. Additionally, CCK-8S (10-7 mol/L) intensified the peak current of ICa-L by 40% (from 60 ± 8 pA to 84 ± 11 pA; at +10 mV; P = 0.012), compared to the normal control group, which was apparently suppressed by respective pretreatment with nifedipine, devazepide, TG and BA. In the respective presence of heparin and staurosporine, CCK-8S did not significantly enhance IBKCa and ICa-L.
CONCLUSION: The results suggest that CCK-8S promotes guinea-pig proximal colon contraction by CCK1 receptors, following activation of the inositol triphosphate-protein kinase C signal transduction pathway.
- Citation: Zhu J, Chen L, Xia H, Luo HS. Mechanisms mediating CCK-8S-induced contraction of proximal colon in guinea pigs. World J Gastroenterol 2010; 16(9): 1076-1085
- URL: https://www.wjgnet.com/1007-9327/full/v16/i9/1076.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i9.1076
Cholecystokinin (CCK), a gut-brain peptide, plays an important role in regulating a variety of physiological functions such as the stimulation of exocrine and endocrine secretion, mediation of emotion and behavior, and the regulation of gut motor functions[1-3]. Previous studies investigating the targets for CCK in the gastrointestinal tract have focused mainly on the gallbladder and pancreas. However, recent research has shown that CCK and its related peptides are implicated in the pathophysiology of functional digestive diseases, such as irritable bowel syndrome (IBS). Several early reports also have indicated that either an altered release of CCK or abnormal responses to this peptide could be responsible for the symptoms of IBS[3-5].
It has been reported that sulfated CCK octapeptide (CCK-8S) is the predominant and major molecular form of CCK peptides[6,7] and it actions on the gastrointestinal tract through two receptor subtypes, designated as CCK1 and CCK2[3]. Previous studies have shown that some mechanisms could be involved in the effect of CCK on smooth muscle motility in the gastrointestinal tract. First, it has been reported that CCK1 receptors appear to be involved mainly in the control of gut motility and visceral sensation[2,3,8-10]. CCK2 receptors mainly contribute to the release of histamine from enterochromaffin-like cells in the stomach[11], and the stimulation of acid secretion from parietal cells[12,13]. Second, Morton et al[14] have demonstrated that CCK-induced contraction of the human colon and gallbladder smooth muscle is mediated solely through the CCK1 receptor, whereas Fornai et al[3,15] have reported that CCK2 receptors mediate the inhibitory action of the peptide on the contractile activity of human distal colon, via the nitric oxide pathway. Furthermore, activation of protein kinase C (PKC) can enhance L-type calcium current (ICa-L) in a variety of smooth muscles[16]. It has been shown that CCK can activate phospholipase C through binding to its distinct receptors[7,17]. CCK-8S-evoked [Ca2+]i concentration increase in gastric antral smooth muscle cells (SMCs) depends on the release of [Ca2+]i stores that are regulated positively by CCK1 receptors via PKC-mediated phosphorylation of inositol trisphosphate (IP3) type 3 receptor[7]. Released Ca2+ in turn activates ICa-L, which ultimately results in the contraction of the gastric smooth muscle[7]. However, the direct electrophysiological effects of CCK on the contractile activity of the colon and the crosstalk between the CCK-8S-triggered PKC and Ca2+ signaling pathways in colon SMCs remain unclear.
Therefore, in the present study, we investigated the effects of CCK-8S on the contraction of proximal colon smooth muscle strips, resting potential (RP), action potential (AP), large conductance potassium channel current (IBKCa) and ICa-L in smooth muscle cells (SMCs) of proximal colon. In addition, the role of the IP3-mediated protein kinase C (PKC) pathway in the physiological responses of SMCs to CCK-8S was also studied.
Adult female guinea pigs (250-300 g) were obtained by mail from the Experimental Animal Center of Wuhan University and housed under controlled conditions and temperature (25 ± 2°C). The animal-testing protocol used in the present investigation was approved by the Institutional Animal Care and Use Committee of Wuhan University.
The physiological saline solution (CaPSS) contained (in mmol/L): NaCl 135.0, KCl 5.0, CaCl2 2.0, MgCl2 1.2, glucose 10.0, and HEPES 10.0 (adjusted to pH 7.4 with NaOH). The Ca2+-free PSS contained similar ingredients, without CaCl2. Tyrode buffer contained (in mmol/L): NaCl 147.0, KCl 4.0, CaCl2 2.0, NaH2PO4 0.42, Na2HPO4 2.0, MgCl2 1.05, and glucose 5.5 (adjusted to pH 7.4 with NaOH). KB solution contained (in mmol/L): EGTA 0.5, HEPES 10.0, MgCl2 3.0, KCl 50.0, glucose 10.0, L-glutamate 50.0, taurine 20.0, and KH2PO4 20.0 (adjusted to pH 7.4 with NaOH). Digestive solution contained: 0.1% collagenase II, 0.1% trypsin inhibitor and 0.2% BSA dissolved in Ca2+ free-PSS. Internal pipette solution used in experiments investigating APs and RPs contained (in mmol/L): K-aspartate 120.0, HEPES 10.0, EGTA 10.0, Na2ATP 5.0, MgCl2 4.0, and CaCl2 3.0 (adjusted to pH 7.4 with KOH). Internal pipette solution of large conductance potassium channel contained (in mmol/L): KCl 20.0, K-aspartate 110.0, MgCl2·6H2O 1.0, Mg-ATP 5.0, Na2 creatine phosphate 2.5, EGTA 2.5, and 2.0 μg/mL nystatin (adjusted to pH 7.4 with KOH). Internal pipette solution of L-type calcium channel contained (in mmol/L): CsCl 135.0, MgCl2 4.0, HEPES 10.0, Na2-ATP 2.0, EGTA 10.0, tetraethylammonium (TEA) 20.0, and 2.0 μg/mL nystatin (adjusted to pH 7.35 with Tris-HCl). All these chemicals together with CCK-8S, nifedipine, iberiotoxin, thapsigargin (TG) and BAPTA-AM (BA) were purchased from Sigma (St Louis, MO, USA). Devazepide and CI 988 were purchased from Tocris Bioscience (UK).
Guinea pigs were sacrificed by ether, followed by cervical exsanguination. The proximal colon was removed, cleaned and opened along the mesenteric border and then placed in Ca2+-free PSS bubbled with carbogen (95% O2/5% CO2). The smooth muscle strips (3 mm × 10 mm) were obtained after the mucosa and submucosa were excised.
Single SMCs were isolated by enzymatic digestion as described previously[18]. The strips of proximal colon were pinned to the base of the sylgard surface of a Petri dish and the mucosa was carefully dissected away under an anatomical microscope. The tissue was cut into small strips (about 2 mm wide and 5-6 mm long) and placed in Ca2+-free PSS solution that contained 0.12% (w/v) collagenase II supplemented with 0.2% soybean trypsin inhibitor and 0.2% bovine serum albumin (BSA), and incubated for 25 min at 37°C. After completion of digestion, the segments were washed five times in a Ca2+-free PSS solution and then triturated gently with a fire-polished Pasteur pipette to create a cell suspension. Cells were stored at 0-4°C and used within 8 h.
Each fresh smooth muscle strip was mounted in an organ bath and connected to an isometric force transducer (JZJOIH, Chengdu, China). The organ baths contained 10 mL Tyrode’s buffer at 37°C and were constantly warmed by a circulating water jacketed at 37°C, and bubbled with carbogen (95% O2/5% CO2). One end of the strip was fixed to a hook on the bottom of the chamber, while the other end was connected by a thread to an external isometric force transducer at the top. Each muscle strip was placed under a resting preload of 1.0 g and allowed to equilibrate for 60 min, with solution change every 20 min. The frequencies of contraction were calculated by counting the contraction waves per minute. The mean contractile amplitude and frequency of spontaneous contractions were recorded (control values) and compared with the mean contractile amplitude and frequency when exposed to treatment (response values). The results were presented as the changed percentage (changed percentage = 100% × (response value - control value)/control value)[19].
Several drops of cell suspensions were placed in a recording chamber that was mounted with an inverted microscope. Cells were superfused with CaPSS (3 mL/min) after adhering to the coverslip. Membrane potentials were recorded by EPC-10 amplifier (HEKA, Lambrecht, Germany). Cells were patch-clamped in current clamp mode after a 10-min equilibration. The AP stimulus pattern was composed of a 600-pA current step for 8 ms; the holding current of which was 0 pA. After several minutes equilibration, the current-clamp mode was transformed into the whole-cell voltage-clamp mode and the RP was shown in the V-membrane screen. Drugs were added subsequently to the cell suspension to observe relative effects on AP and RP when their values were stable.
Several drops of cell suspensions were placed in a recording chamber that was mounted with an inverted microscope (Olympus, Japan). After adhering to the coverslip, the cells were infused with Tyrode’s buffer (3 mL/min). Pipettes were made using a micropipette puller (PC-10; Narishige, Japan). Typical pipette resistances were 3-5 MΩ. A gigaseal was formed with negative suction. Capacitance was compensated for and the residual capacitance current was removed digitally. Whole-cell currents were recorded by using a nystatin-perforated whole-cell patch-clamp configuration with an EPC-10 amplifier (HEKA, Germany). The effects of CCK-8S at different concentrations were investigated on IBKCa and ICa-L. Data were filtered at 200 Hz, digitized at 10 kHz (filter 1) and 2.9 kHz (filter 2), and stored in the computer for subsequent analyses. All the experiments were conducted at 25 ± 2°C.
Statistical analyses were performed using SPSS for Windows, version 15.0. Results are expressed as the mean ± SD of n strips or cells. Data were compared using Student’s t tests. P < 0.05 was considered statistically significant.
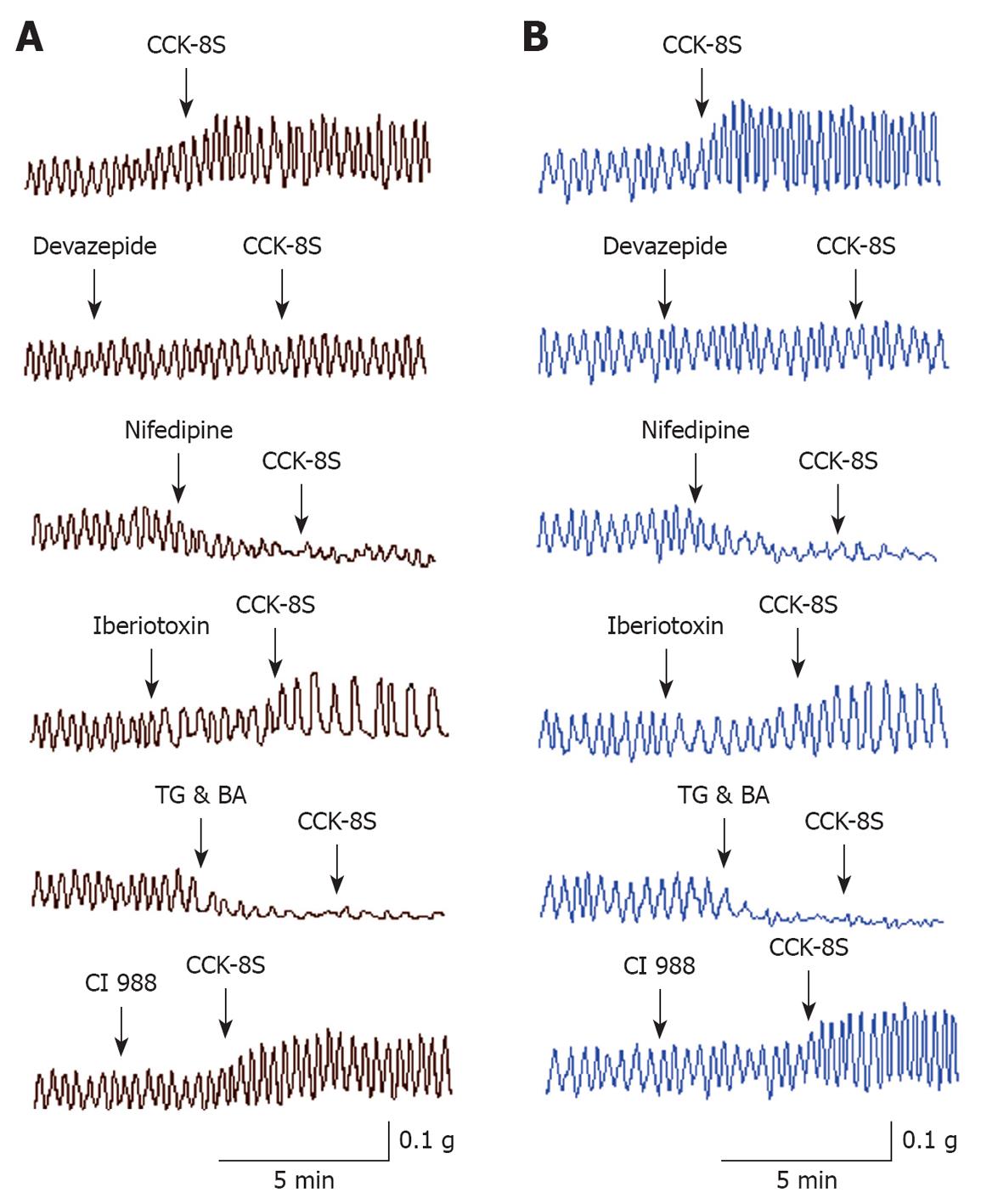
CCK-8S enhanced the resting tension of PCSM strips when applied for 3-5 min (Figure 1). CCK-8S (10-7 mol/L) increased the mean contractile amplitude of circular muscle (CM) and longitudinal muscle (LM) strips by 56.53% ± 11.92% and 65.93% ± 12.98%, respectively, as well as the mean frequency of LM by 31.69% ± 13.58% (n = 15 for each group, P = 0.038, 0.019, 0.023), but CCK-8S had little influence on the frequency of CM (n = 15, P = 0.087) (Figure 1, Table 1). CCK-8S-intensified effects on proximal colonic strips were significantly attenuated when these strips were pretreated with CCK1 receptor antagonist devazepide (10-7 mol/L), L-type calcium channel inhibitor nifedipine (10-5 mol/L), Ca2+-ATPase inhibitor TG (10-5 mol/L), or intracellular calcium chelator BA (10-5 mol/L) (n = 15 for each group, P < 0.05). Pretreating CM and LM strips with iberiotoxin (10-6 mol/L), a selective BKCa channel blocker, did not inhibit the CCK-8S-induced increase in the contractile amplitude of CM and LM strips (n = 15 for each group, P = 0.096, 0.078 vs CCK-8S group), but decreased their frequency (n = 15 for each group, P = 0.036, 0.041 vs CCK-8S group) (Figure 1, Table 1), whereas superfusion with the CCK2 receptor antagonist CI 988 (10-7 mol/L) did not block the CCK-8S-intensified effect on CM and LM strips (n = 15 for each group, P > 0.05) (Figure 1, Table 1).
| CM strips | LM strips | |||
| Amplitude | Frequency | Amplitude | Frequency | |
| CCK-8S | 56.53 ± 11.92a | 0.87 ± 1.52 | 65.93 ± 12.98a | 31.69 ± 13.58a |
| Devazepide + CCK-8S | 3.68 ± 1.17 | 1.92 ± 0.83 | 2.09 ± 0.78 | 1.57 ± 1.07 |
| Nifedipine + CCK-8S | -79.26 ± 5.93c | -19.82 ± 3.92c | -78.69 ± 6.42c | -21.58 ± 2.87c |
| Iberiotoxin + CCK-8S | 49.93 ± 11.81 | -36.57 ± 17.35c | 57.47 ± 10.92 | -23.82 ± 5.97c |
| TG & BA + CCK-8S | -98.12 ± 0.72d | -97.42 ± 2.73d | -97.57 ± 1.25d | -96.11 ± 3.26d |
| CI 988 + CCK-8S | 53.29 ± 0.52 | 1.48 ± 0.17 | 57.48 ± 11.27 | 28.96 ± 9.53 |
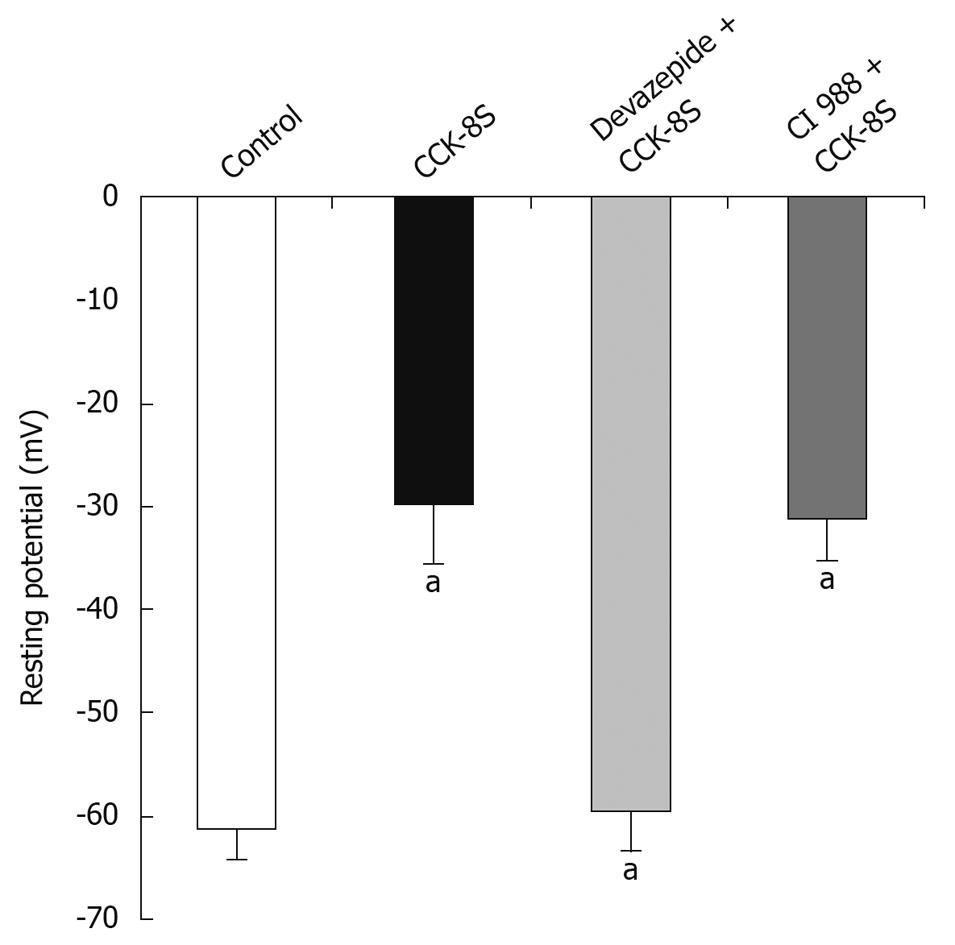
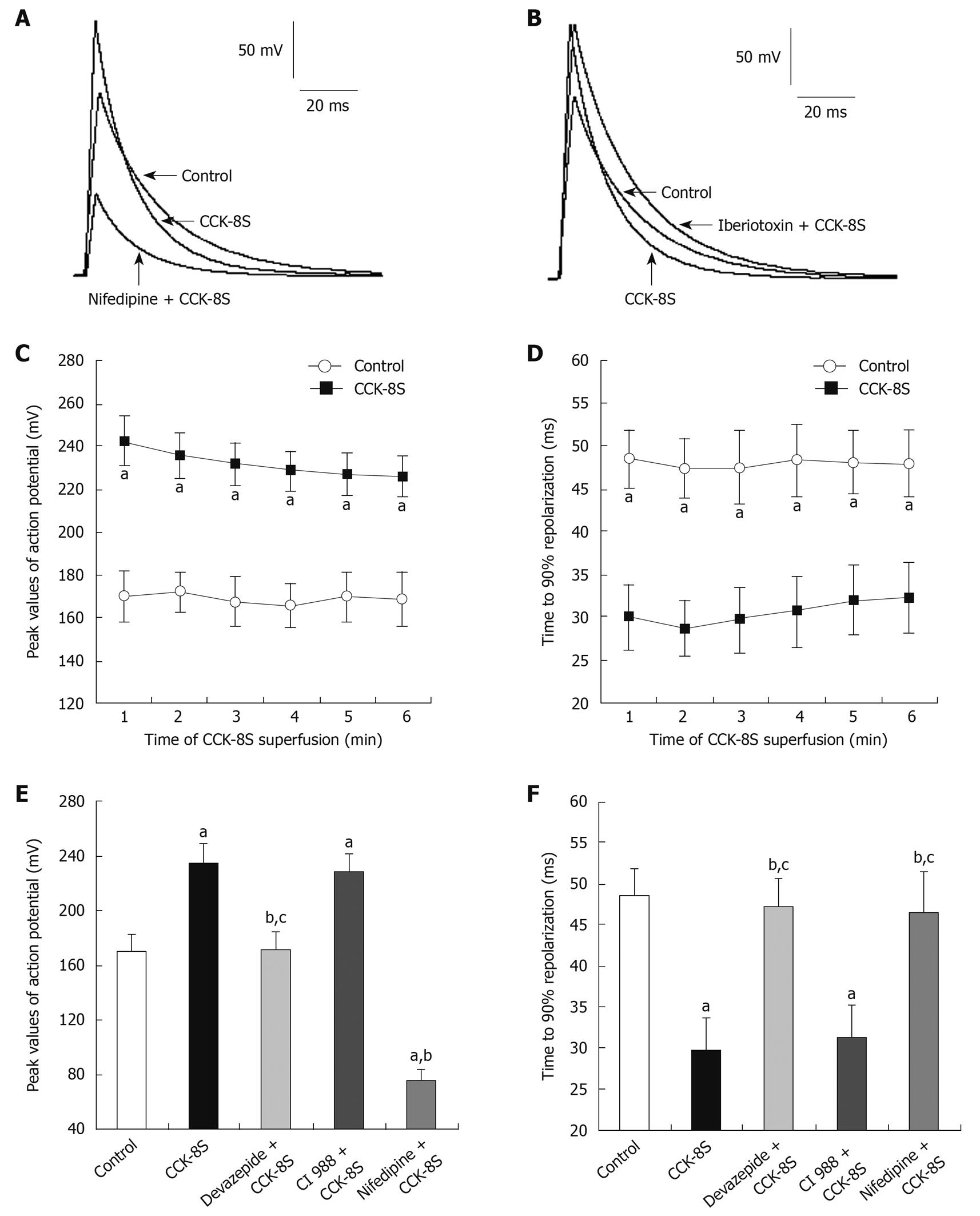
CCK-8S (10-7 mol/L) depolarized RP of SMCs from -61.3 ± 2.7 mV to -29.8 ± 5.9 mV (n = 15, P = 0.032) (Figure 2). In the presence of devazepide (10-7 mol/L), CCK-8S (10-7 mol/L) did not depolarize RP (-59.4 ± 3.8 mV, n = 15, P = 0.065 vs control group) (Figure 2). When SMCs were preincubated with CI 988, CCK-8S (10-7 mol/L) depolarized RP to -32.8 ± 4.2 mV (n = 15, P = 0.029 vs control group) (Figure 2). The AP amplitude was expressed as peak value, and the mean amplitude in the control group (CaPSS) was 169.9 ± 12.3 mV (n = 15) (Figure 3A and D). After the addition of CCK-8S (10-7 mol/L), the AP amplitude was increased by 38.6% ± 3.2% [from 169.9 ± 12.3 mV (in control CaPSS) to 235.5 ± 11.6 mV, n = 15, P = 0.015] (Figure 3A-C and E), and fast repolarization time (repolarizing to 90% of the peak value of AP, T90) was shortened by 36.9% ± 8.7% [from 48.42 ± 3.38 ms (in control CaPSS) to 30.53 ± 4.15 ms, n = 15, P = 0.026] (Figure 3A, B, D and F), which were blocked when SMCs were pretreated with 10-7 mol/L devazepide (171.2 ± 13.4 mV, 47.18 ± 3.45 ms, n = 15, P = 0.006 vs CCK-8S group, P = 0.074 vs control group) (Figure 3E and F). In contrast, CI 988 did not inhibit the CCK-8S-evoked effect on AP amplitude and T90 (228.8 ± 12.9 mV, 31.26 ± 3.97 ms, n = 15, P = 0.083 0.092 vs CCK-8S group) (Figure 3E and F). After SMCs were incubated with nifedipine (10-5 mol/L), the enhancement of AP amplitude by CCK-8S was inhibited (75.6 ± 8.3 mV, n = 15, P = 0.039 vs CCK-8S group) (Figure 3A and E). In addition, superfusion with iberiotoxin (10-6 mol/L) significantly attenuated the effect of CCK-8S on T90 (46.54 ± 4.88 ms, n = 15, P = 0.026 vs CCK-8S group; P = 0.079 vs control group) (Figure 3B and F).
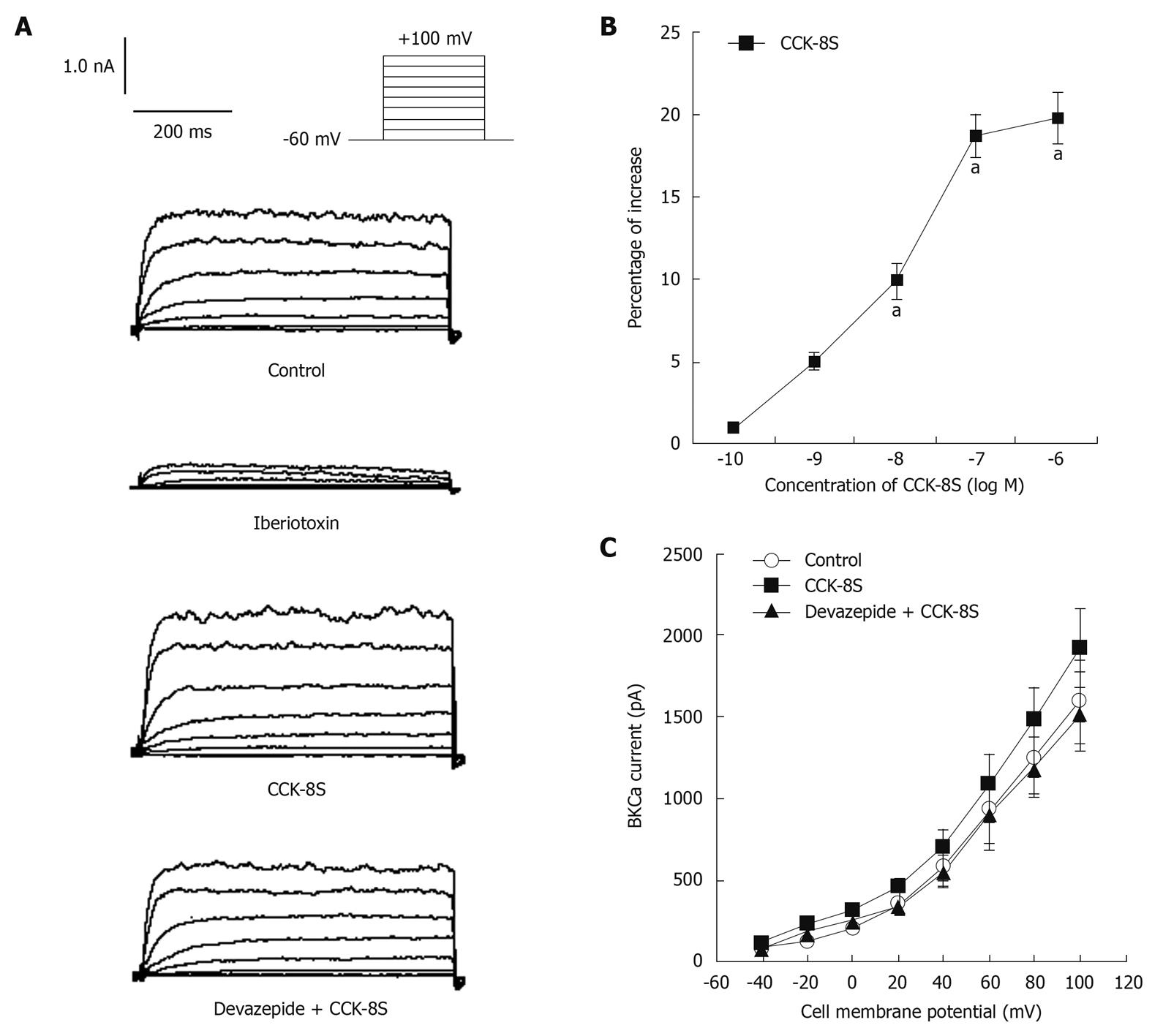
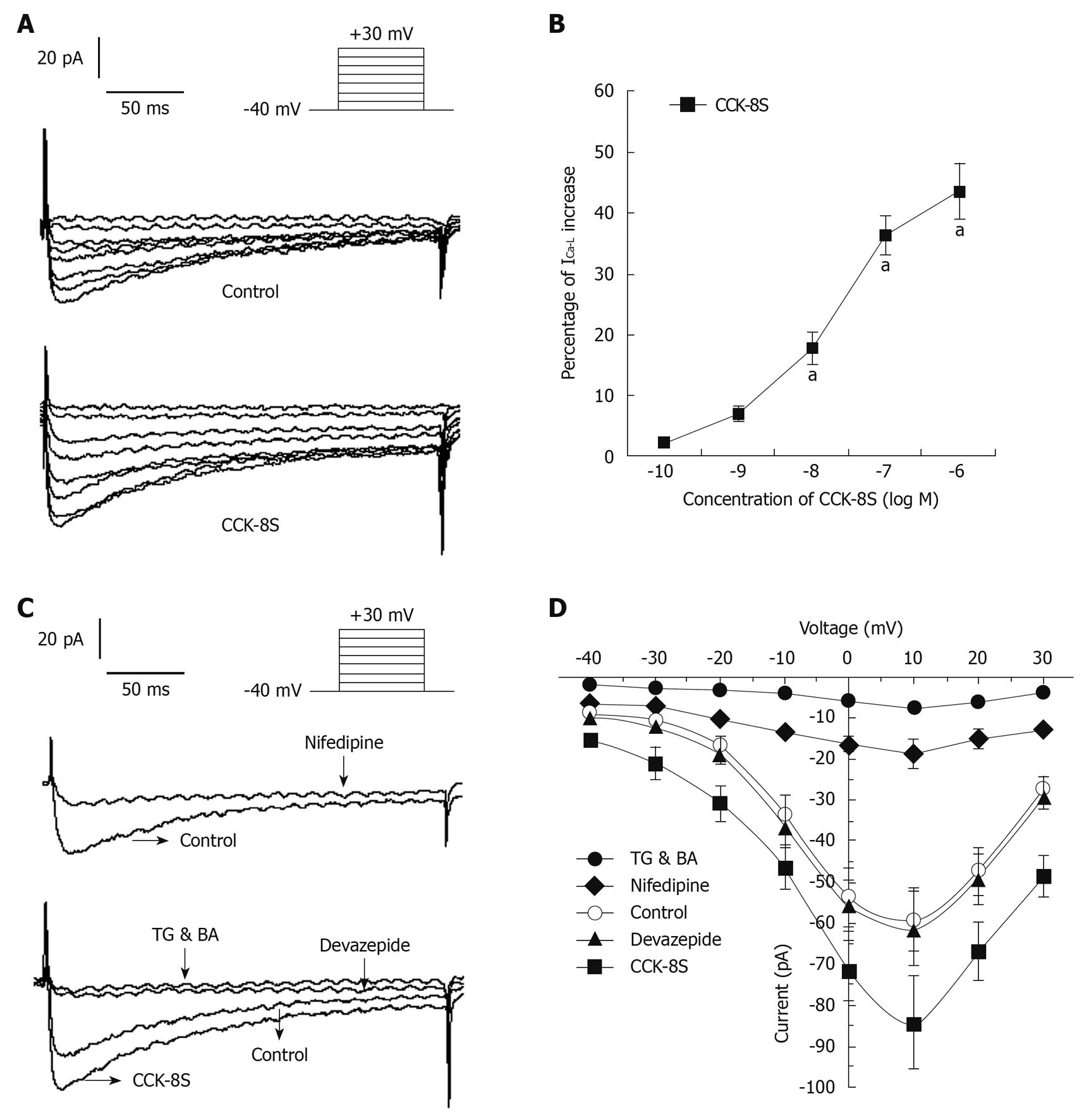
With nystatin-perforated whole-cell voltage-clamp recordings, IBKCa was evoked by using a depolarizing step pulse from a holding potential of -60 mV to +100 mV for 400 ms, with an interpulse interval of 10 s (Figure 4A). To reduce the amount of non-Ca2+-dependent delayed rectifier K+ currents through inactivation, cells were held at 0 mV for at least 2 min before being subjected to step depolarization[18,19], and superfused with 3 mmol/L 4-aminopyridine in the extracellular solutions[18,20]. The application of iberiotoxin (10-5 mol/L) markedly blocked the inward current by 79% (at +60 mV, 192 ± 37 pA) (Figure 4A), which demonstrated that this current was IBKCa. CCK-8S increased IBKCa in a concentration-dependent manner (n = 8, P < 0.05, at 10-8, 10-7 and 10-6 mol/L; EC50 = 3.5 × 10-8 mol/L; Figure 4B). Compared with the CaPSS control group, CCK-8S (10-7 mol/L) enhanced peak IBKCa depolarized to +60 mV by about 18.7% ± 2.1% (from 916 ± 183 pA to 1088 ± 226 pA, n = 8, P = 0.029) (Figure 4A). Although this enhancement effect was blocked by pretreatment with 10-7 mol/L, devazepide (908 ± 109 pA, n = 8, P = 0.012 vs CCK-8S group, P = 0.083 vs control group) (Figure 4A), the CCK2 receptor antagonist CI 988 (10-7 mol/L) had no effect (1052 ± 196 pA, n = 8, P = 0.098 vs CCK-8S group).
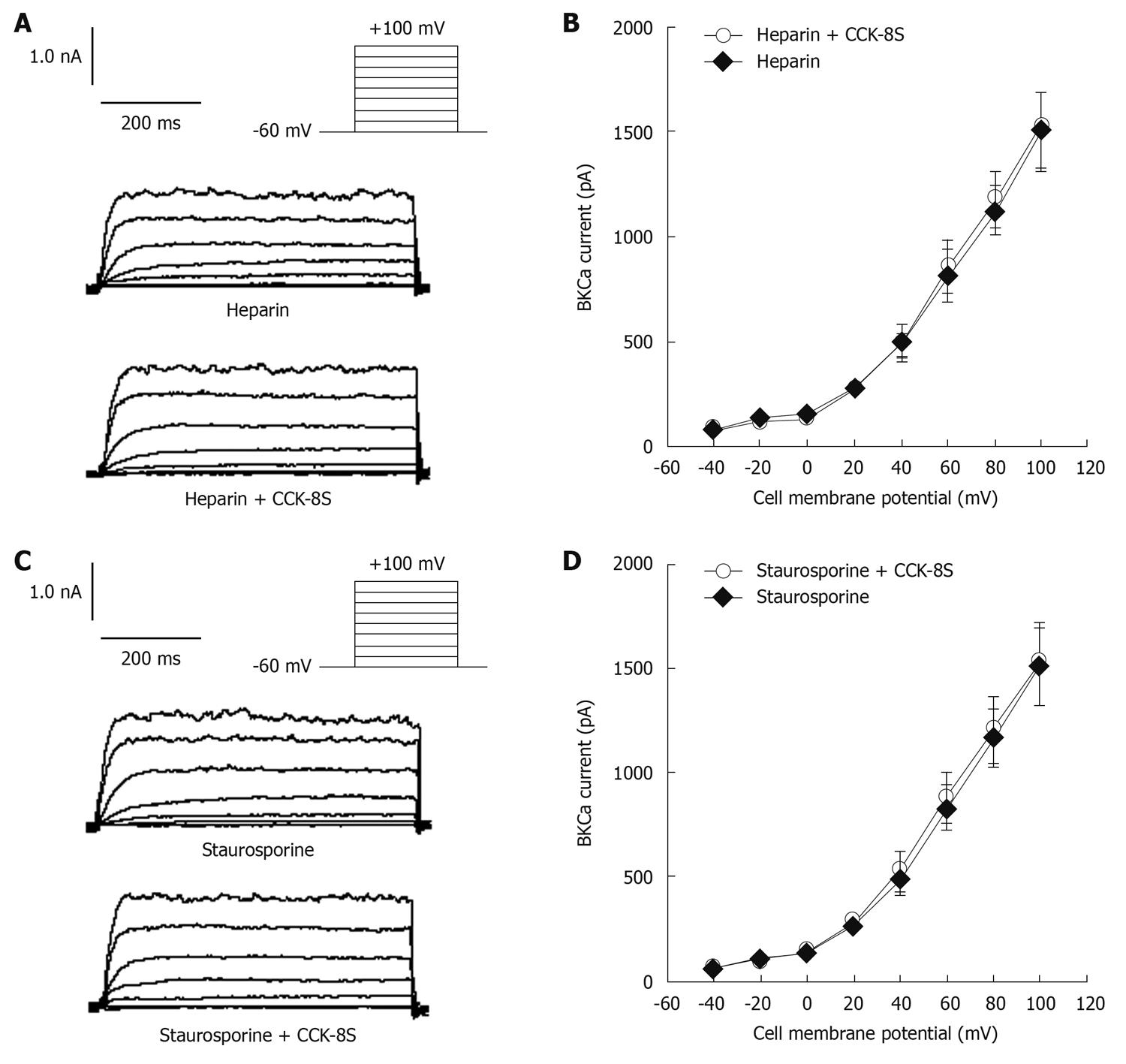
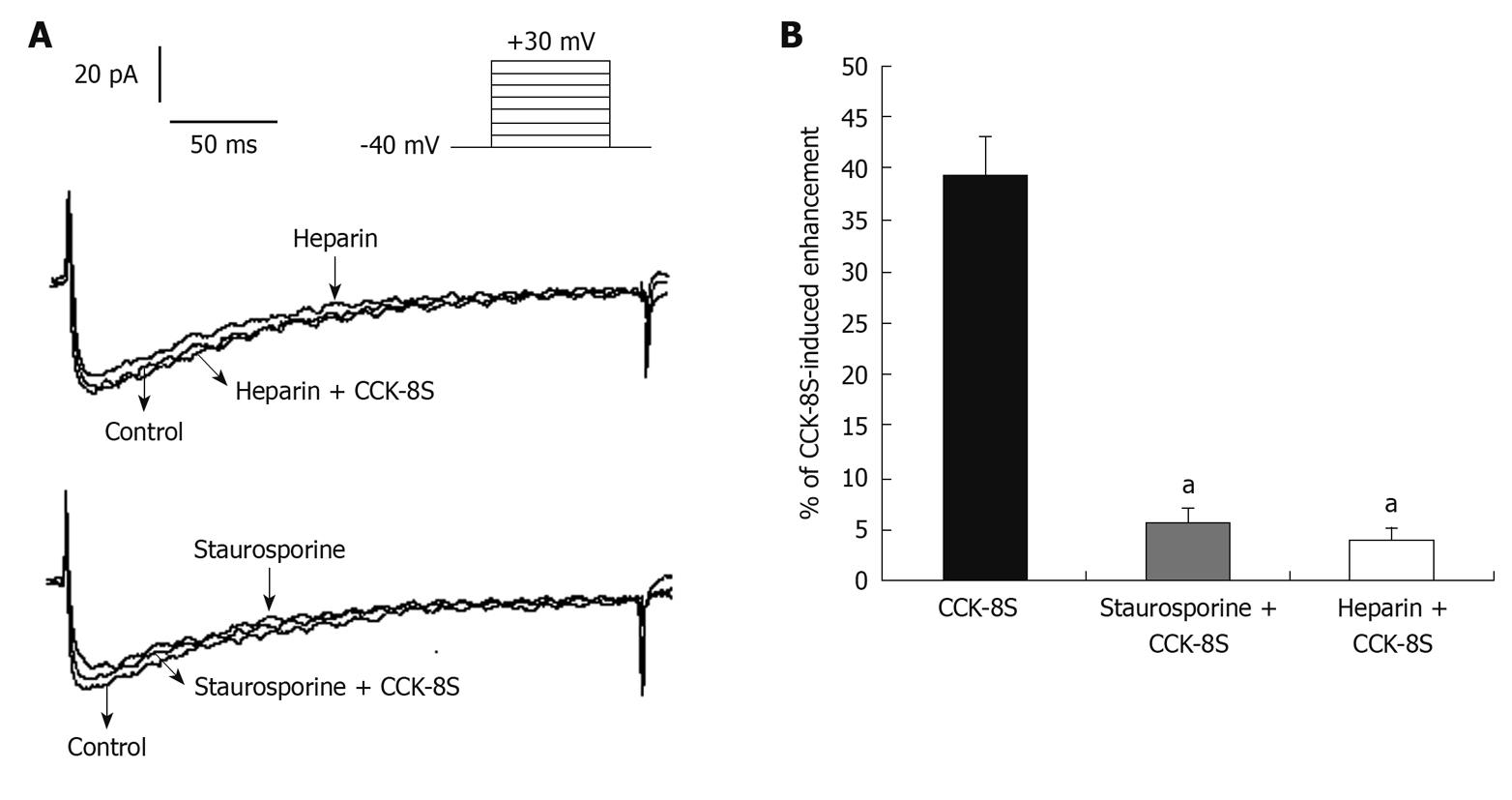
When heparin (10-6 mol/L), an inhibitor of IP3 receptors, was added to the pipette solution, IBKCa in cells depolarized to +60 mV was 813 ± 126 pA. CCK-8S (10-7 mol/L) did not enhance IBKCa (879 ± 117 pA, n = 8, P = 0.074 vs control group, P = 0.016 vs CCK-8S group) (Figure 5A and B). To investigate whether the CCK-8S-intensified effect on IBKCa was mediated by the PKC pathway, SMCs were pretreated with the PKC inhibitor staurosporine (10-6 mol/L), and IBKCa in cells depolarized to +60 mV was 835 ± 112 pA. CCK-8S (10-7 mol/L ) had no effect on IBKCa (887 ± 120 pA; n = 8; P = 0.069 vs control group; P = 0.041 vs CCK-8S group; Figure 5C and D).
With nystatin-perforated whole-cell voltage-clamp recordings, ICa-L was evoked by using a depolarizing step pulse from a holding potential of -40 mV to +30 mV for 400 ms, with an interpulse interval of 10 ms (Figure 6A). ICa-L reached a maximum value at around +10 mV and the inward current was markedly blocked by 80% by nifedipine (10-5 mol/L), which indicated that this current was ICa-L (Figure 6C). CCK-8S increased the amplitude of ICa-L in a concentration-dependent manner (n = 8, P < 0.05, at 10-8, 10-7 and 10-6 mol/L; EC50 = 3.2 × 10-8 mol/L; Figure 6B). When CCK-8S (10-7 mol/L) was applied for 3-6 min, the amplitude of ICa-L was augmented by about 40% (from 60 ± 8 pA to 84 ± 11 pA), compared to the normal controls (at +10 mV, n = 8, P = 0.012) (Figure 6A). Although this enhancement effect was completely inhibited by 10-7 mol/L CCK1 receptor antagonist devazepide (61 ± 9 pA; at +10 mV, n = 8, P = 0.023 vs CCK-8S group, P = 0.079 vs control group) (Figure 6C), the CCK2 receptor antagonist CI 988 (10-7 mol/L) had no effect (84 ± 11 pA, n = 10, P = 0.079 vs CCK-8S group). CCK-8S-intensified ICa-L was reduced by 91.7 ± 5.6% by 10-5 mol/L TG and BA (7 ± 5 pA, at +10 mV, n = 8, P = 0.006 vs CCK-8S group) (Figure 6C). The current-voltage relationships are shown in Figure 6D. The peak current amplitudes of ICa-L under each condition were normalized relative to the maximum control current amplitude.
When 10-6 mol/L heparin was added to the pipette solution, CCK-8S (10-7 mol/L) had no effect on ICa-L (63 ± 12 pA, at +10 mV, n = 8, P = 0.183 vs control group, P = 0.042 vs CCK-8S group) (Figure 7). When SMCs were pretreated with 10-6 mol/L PKC inhibitor staurosporine for 10 min, CCK-8S (10-7 mol/L) did not increase ICa-L (65 ± 10 pA, at +10 mV, n = 8, P = 0.215 vs control group; P = 0.032 vs CCK-8S group) (Figure 7).
In the present study, we demonstrated that: (1) CCK-8S prompted the contraction of guinea pig PCSM strips and intensified IBKCa and ICa-L by CCK1 receptors via activation of the IP3-PKC signal transduction pathway; and (2) CCK-8S increased AP amplitude through enhancing ICa-L, and accelerated fast repolarization of AP by increasing IBKCa.
CCK has been implicated in the pathophysiology of functional digestive diseases, such as IBS[1-3] and exerts its effects by two specific receptors, designated as CCK1 and CCK2, which are expressed in the colon and have a high affinity for CCK-8S[3,8-15,21,22]. However, it remains unknown which receptor plays a more important role in mediating colon contractility. Our results showed that CCK-8S not only promoted the mean contractility and frequency of longitudinal PCSM strips, but also the mean contractility of circular PCSM strips. Devazepide and CI 988 are CCK antagonists that are selective for the CCK1 and CCK2 receptor, respectively, and are used widely in scientific research[23,24]. Pretreatment with CCK1 receptor antagonist devazepide markedly abolished CCK-8S-intensified contraction of PCSM strips, whereas CCK2 receptor antagonist CI 988 had little effect, thus indicating that CCK1 instead of CCK2 receptor plays a major role in mediating the motility of the proximal colon. It is consistent with the effect of CCK-8S on the gastric antral smooth muscle[7] and human ascending colonic smooth muscle[14].
Elevation in [Ca2+]i concentration is an important function in the regulation of cell contraction and can be accomplished by release from internal calcium stores, extracellular Ca2+ influx across the plasma membrane, or both[25,26]. Ca2+-ATPase inhibitor TG and [Ca2+]i chelator BA are used to deplete [Ca2+]i and are often used in experiments to explore the role of intracellular calcium stores and signaling pathways[7,27,28], whereas nifedipine is often used to examine the role of extracellular Ca2+ influx[7]. We found that CCK-8S-evoked contraction of PCSM was significantly antagonized by pretreatment not only with CCK1 receptor antagonist devazepide, but also with nifedipine, TG and BA, but CCK2 receptor antagonist CI 988 had little effect, thus indicating that CCK-8S acts on the CCK1 receptor and enhances extracellular Ca2+ influx by L-type calcium channels and promotes Ca2+ release from intracellular calcium stores by intracellular signaling pathways.
APs are responsible for the contractile activity of colon SMCs. The upstroke of the AP is mainly the result of calcium entry through L-type calcium channels, and the number of APs within a certain period of time can be regarded as an indicator of contractility of gastrointestinal SMCs[18,29]. In this study, we demonstrated that CCK-8S enhanced AP amplitude and shortened the period of fast repolarization, by increasing AP generation as well as depolarizing RP. All these effects could be inhibited by pretreatment with CCK1 receptor antagonist devazepide, but CCK2 receptor antagonist CI 988 had little influence. It is also well known that contraction of gastrointestinal SMCs results from the close interaction between the two mechanisms: an initial peak caused by calcium release from stores and a plateau phase caused by calcium influx[30-33]. Based on these studies, we found that CCK-8S intensified ICa-L in a concentration-dependent manner, which was significantly attenuated by pretreatment with devazepide and nifedipine, but CI 988 exerted little effect, indicating that extracellular calcium entry through L-type calcium channels is essential for contraction of PCSM cells, and the increase in ICa-L induced by CCK-8S through CCK1 receptors can depolarize membrane potentials, which may contribute to the decrease in RP. We also found that the depletion of [Ca2+]i by the Ca2+-ATPase inhibitor TG and [Ca2+]i chelator BA completely blocked CCK-8S-intensified ICa-L and contraction of PCSM. Moreover, the characteristic of the current-voltage curve was not significantly altered. Thus, we can suppose that [Ca2+]i stores are primarily responsible for the CCK-8S-induced contraction, and this can lead to depolarization of the membrane, which in turn leads to calcium influx through L-type calcium channels, to cause a further increase in [Ca2+]i and, ultimately, contraction of PCSMCs.
Large-conductance calcium activated potassium channels have been identified in guinea pig colonic SMCs, and are associated with fast repolarization (repolarizing to 90% of the peak value of AP, T90)[18,30]. We thus studied whether CCK-8S could also affect IBKCa. We showed that CCK-8S shortened the time to T90 and augmented IBKCa in a concentration-dependent manner, whereas this effect was inhibited by potassium channel blocker iberiotoxin, and the CCK1 receptor antagonist devazepide, but the CCK2 receptor antagonist CI 988 exerted little effect. Furthermore, the characteristics of the current-voltage curve were not apparently altered. Our results demonstrated that CCK-8S shortened the refractory period and accelerated the repolarization of the AP by increasing IBKCa, mediated by activation of CCK1 receptors, which may enhance the contractile frequency and ultimately result in a synergistic effect on the contraction.
It is well established that IP3 and PKC play an important role in the regulation of smooth muscle movement. Activation of phosphatidylinositol (4,5) bisphosphate can generate IP3, which binds to IP3 receptors in the sarcoplasmic reticulum (SR) and then induces the release of Ca2+ from the intracellular calcium stores, which immediately activates PKC to exert its biological effects[7,33]. Likewise, serotonin promotes contraction of colonic myocytes, mostly as a result of Ca2+ release from SR following activation of the IP3 pathway[18]. In addition, CCK-induced gallbladder muscle contraction can be blocked by the PKC inhibitor staurosporine[33]. In agreement with these results, we demonstrated that heparin, an inhibitor of IP3 receptors, blocked CCK-8S-intensified ICa-L and IBKCa in isolated PCSMCs. When cells were pretreated with the PKC inhibitor staurosporine, CCK-8S had no effect on ICa-L and IBKCa. These results may further indicate that CCK-8S regulates ICa-L and IBKCa by activating the IP3-PKC signal transduction pathway. However, the exact mechanism by which CCK-8S enhances the activity of IP3-mediated PKC has not been elucidated.
In conclusion, CCK-8S evokes contraction of the proximal colon in guinea pigs, mainly by promoting Ca2+ release from the SR and increasing Ca2+ influx through L-type calcium channels, via the IP3-PKC signal transduction pathway by activation of CCK1 receptors. In addition, the decrease in AP duration is caused by the acceleration of fast repolarization of AP by increased IBKCa, and the increase in AP amplitude results from enhancement of ICa-L, which both ultimately contribute to the contraction induced by CCK-8S.
Cholecystokinin (CCK) acts as a hormone and neurotransmitter in the gastrointestinal tract, and regulates gut motility via two receptor subtypes, which have been characterized as CCK1 and CCK2, although the direct electrophysiological effect of CCK on the contractile activity of colon remains undetermined. The authors investigated how CCK regulates colon contraction through CCK receptors.
CCK has been implicated in the pathophysiology of functional digestive diseases. In this study, the authors demonstrated the electrophysiological mechanisms that mediate CCK octapeptide (CCK-8S)-induced contraction and the relationship between the CCK-8S-triggered protein kinase C (PKC) and Ca2+ signaling pathways in colon smooth muscle cells.
The present study has proved that: (1) CCK-8S elicited stimulant effects on the motor activity of guinea pig proximal colon through CCK1 receptors, following activation of the inositol trisphosphate (IP3)-PKC signal transduction pathway; and (2) the decrease in action potential (AP) duration was caused by acceleration of fast repolarization of the AP by increased large conductance potassium channel currents, and the increase in AP amplitude was caused by enhancement of L-type calcium current, which both ultimately contribute to the contraction induced by CCK-8S. This is believed to be the first report on the electrophysiological mechanisms of proximal colon contraction evoked by CCK-8S in guinea pigs.
The results of this study indicate that CCK stimulates proximal colon contraction through CCK1 receptors following activation of the IP3-PKC signal transduction pathway, which could be useful in further study of functional digestive diseases, such as irritable bowel syndrome.
This appears to be a well executed piece of work. The authors have established clearly that CCK-8S stimulates the contraction of colonic circular and longitudinal muscle through the CCK1 receptor, and that enhanced Ca effects are involved.
Peer reviewer: Leonard R Johnson, Professor, Department of Physiology, University Tennessee College of Medicine, 894 Union Ave, Memphis, TN 38163, United States
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
| 1. | Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15:731-755. [Cited in This Article: ] |
| 2. | Miyasaka K, Funakoshi A. Cholecystokinin and cholecystokinin receptors. J Gastroenterol. 2003;38:1-13. [Cited in This Article: ] |
| 3. | Fornai M, Colucci R, Antonioli L, Crema F, Buccianti P, Chiarugi M, Baschiera F, Ghisu N, Tuccori M, Blandizzi C. Cholecystokinin CCK2 receptors mediate the peptide's inhibitory actions on the contractile activity of human distal colon via the nitric oxide pathway. Br J Pharmacol. 2007;151:1246-1253. [Cited in This Article: ] |
| 4. | Sjölund K, Ekman R, Lindgren S, Rehfeld JF. Disturbed motilin and cholecystokinin release in the irritable bowel syndrome. Scand J Gastroenterol. 1996;31:1110-1114. [Cited in This Article: ] |
| 5. | Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499-1506. [Cited in This Article: ] |
| 6. | Wu T, Wang HL. The excitatory effect of cholecystokinin on rat neostriatal neurons: ionic and molecular mechanisms. Eur J Pharmacol. 1996;307:125-132. [Cited in This Article: ] |
| 7. | Si XM, Huang L, Paul SC, An P, Luo HS. Signal transduction pathways mediating CCK-8S-induced gastric antral smooth muscle contraction. Digestion. 2006;73:249-258. [Cited in This Article: ] |
| 8. | Sternini C, Wong H, Pham T, De Giorgio R, Miller LJ, Kuntz SM, Reeve JR, Walsh JH, Raybould HE. Expression of cholecystokinin A receptors in neurons innervating the rat stomach and intestine. Gastroenterology. 1999;117:1136-1146. [Cited in This Article: ] |
| 9. | Noble F, Wank SA, Crawley JN, Bradwejn J, Seroogy KB, Hamon M, Roques BP. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol Rev. 1999;51:745-781. [Cited in This Article: ] |
| 10. | Varga G, Bálint A, Burghardt B, D'Amato M. Involvement of endogenous CCK and CCK1 receptors in colonic motor function. Br J Pharmacol. 2004;141:1275-1284. [Cited in This Article: ] |
| 11. | Waldum HL, Kleveland PM, Sandvik AK, Brenna E, Syversen U, Bakke I, Tømmerås K. The cellular localization of the cholecystokinin 2 (gastrin) receptor in the stomach. Pharmacol Toxicol. 2002;91:359-362. [Cited in This Article: ] |
| 12. | Kulaksiz H, Arnold R, Göke B, Maronde E, Meyer M, Fahrenholz F, Forssmann WG, Eissele R. Expression and cell-specific localization of the cholecystokinin B/gastrin receptor in the human stomach. Cell Tissue Res. 2000;299:289-298. [Cited in This Article: ] |
| 13. | Ochi Y, Horie S, Maruyama T, Watanabe K, Yano S. Necessity of intracellular cyclic AMP in inducing gastric acid secretion via muscarinic M3 and cholecystokinin2 receptors on parietal cells in isolated mouse stomach. Life Sci. 2005;77:2040-2050. [Cited in This Article: ] |
| 14. | Morton MF, Harper EA, Tavares IA, Shankley NP. Pharmacological evidence for putative CCK(1) receptor heterogeneity in human colon smooth muscle. Br J Pharmacol. 2002;136:873-882. [Cited in This Article: ] |
| 15. | Fornai M, Colucci R, Antonioli L, Baschiera F, Ghisu N, Tuccori M, Gori G, Blandizzi C, Del Tacca M. CCK2 receptors mediate inhibitory effects of cholecystokinin on the motor activity of guinea-pig distal colon. Eur J Pharmacol. 2007;557:212-220. [Cited in This Article: ] |
| 16. | Wu ZX, Yu BP, Xia H, Xu L. Emodin increases Ca(2+) influx through L-type Ca(2+) channel in guinea pig gallbladder smooth muscle. Eur J Pharmacol. 2008;595:95-99. [Cited in This Article: ] |
| 17. | Montero M, Lobatón CD, Gutierrez-Fernández S, Moreno A, Alvarez J. Modulation of histamine-induced Ca2+ release by protein kinase C. Effects on cytosolic and mitochondrial [Ca2+] peaks. J Biol Chem. 2003;278:49972-49979. [Cited in This Article: ] |
| 18. | Xu L, Yu BP, Chen JG, Luo HS. Mechanisms mediating serotonin-induced contraction of colonic myocytes. Clin Exp Pharmacol Physiol. 2007;34:120-128. [Cited in This Article: ] |
| 19. | Xu L, Chen J, Yu B, Dong W, Chen K, Luo H, Zhu Y. Effect of progesterone on calcium activated potassium currents and intracellular calcium in guinea pig colon myocytes. Methods Find Exp Clin Pharmacol. 2005;27:475-482. [Cited in This Article: ] |
| 20. | Zhu Y, Golden CM, Ye J, Wang XY, Akbarali HI, Huizinga JD. ERG K+ currents regulate pacemaker activity in ICC. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1249-G1258. [Cited in This Article: ] |
| 21. | Kisfalvi K, Rácz G, Zsirka-Klein A, Pelosini I, Scarpignato C, Varga G. Different affinity states of CCK(1) receptors on pancreatic acini and gastric smooth muscle in the rat. J Physiol Paris. 2001;95:391-398. [Cited in This Article: ] |
| 22. | Patterson LM, Zheng H, Ward SM, Berthoud HR. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res. 2001;305:11-23. [Cited in This Article: ] |
| 23. | Hill DR, Woodruff GN. Differentiation of central cholecystokinin receptor binding sites using the non-peptide antagonists MK-329 and L-365,260. Brain Res. 1990;526:276-283. [Cited in This Article: ] |
| 24. | Singh L, Field MJ, Hughes J, Menzies R, Oles RJ, Vass CA, Woodruff GN. The behavioural properties of CI-988, a selective cholecystokininB receptor antagonist. Br J Pharmacol. 1991;104:239-245. [Cited in This Article: ] |
| 25. | Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201-279. [Cited in This Article: ] |
| 26. | Sawisky GR, Chang JP. Intracellular calcium involvement in pituitary adenylate cyclase-activating polypeptide stimulation of growth hormone and gonadotrophin secretion in goldfish pituitary cells. J Neuroendocrinol. 2005;17:353-371. [Cited in This Article: ] |
| 27. | Rogers TB, Inesi G, Wade R, Lederer WJ. Use of thapsigargin to study Ca2+ homeostasis in cardiac cells. Biosci Rep. 1995;15:341-349. [Cited in This Article: ] |
| 28. | Billman GE. Intracellular calcium chelator, BAPTA-AM, prevents cocaine-induced ventricular fibrillation. Am J Physiol. 1993;265:H1529-H1535. [Cited in This Article: ] |
| 29. | Kuriyama H, Kitamura K, Nabata H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissues. Pharmacol Rev. 1995;47:387-573. [Cited in This Article: ] |
| 30. | Burdyga T, Wray S. Sarcoplasmic reticulum function and contractile consequences in ureteric smooth muscles. Novartis Found Symp. 2002;246:208-217; discussion 217-220, 221-227. [Cited in This Article: ] |
| 31. | Haddock RE, Hill CE. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J Physiol. 2002;545:615-627. [Cited in This Article: ] |
| 32. | Ogura T, Kinnamon SC. IP(3)-Independent release of Ca(2+) from intracellular stores: A novel mechanism for transduction of bitter stimuli. J Neurophysiol. 1999;82:2657-2666. [Cited in This Article: ] |
| 33. | Yu P, Chen Q, Xiao Z, Harnett K, Biancani P, Behar J. Signal transduction pathways mediating CCK-induced gallbladder muscle contraction. Am J Physiol. 1998;275:G203-G211. [Cited in This Article: ] |