Published online Dec 28, 2010. doi: 10.3748/wjg.v16.i48.6035
Revised: July 12, 2010
Accepted: July 19, 2010
Published online: December 28, 2010
Long term hepatitis B virus (HBV) infection is a major risk factor in pathogenesis of chronic liver diseases, including hepatocellular carcinoma (HCC). The HBV encoded proteins, hepatitis B virus X protein and preS, appear to contribute importantly to the pathogenesis of HCC. Both are associated with oxidative stress, which can damage cellular molecules like lipids, proteins, and DNA during chronic infection. Chronic alcohol use is another important factor that contributes to oxidative stress in the liver. Previous studies reported that treatment with antioxidants, such as curcumin, silymarin, green tea, and vitamins C and E, can protect DNA from damage and regulate liver pathogenesis-related cascades by reducing reactive oxygen species. This review summarizes some of the relationships between oxidative stress and liver pathogenesis, focusing upon HBV and alcohol, and suggests antioxidant therapeutic approaches.
- Citation: Ha HL, Shin HJ, Feitelson MA, Yu DY. Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol 2010; 16(48): 6035-6043
- URL: https://www.wjgnet.com/1007-9327/full/v16/i48/6035.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i48.6035
Hepatocellular carcinoma (HCC) is one of the most frequent tumor types worldwide. It is the fifth most common cancer and the third leading cause of cancer death[1]. There are multiple etiological agents that are associated with the development of HCC, the most frequent being chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, and long-term exposure to the mycotoxin, aflatoxin B1.
HBV is recognized as a major etiological factor in the development of such diseases as fatty liver (steatosis), cirrhosis, hepatocellular adenoma, and HCC[2,3]. The risk of HCC in chronic HBV carriers is more than 100 times greater than in uninfected individuals. In the year 2000, worldwide new cases of HCC had increased to 564 300[4]. More than 80% of these cases occur in developing countries, especially Southeast Asia and sub-Saharan Africa. Some 80%-90% of HCCs develop in cirrhotic liver[5]. After 20-30 years of chronic infection, 20%-30% of patients develop liver cirrhosis. HCC develops at an annual rate of 3%-8% in HBV-infected cirrhotic patients[6].
In the course of chronic infection, fragments of HBV DNA integrate randomly into host DNA. Many of these integrated species encode the hepatitis B virus X protein (HBx) and truncated preS polypeptides, which contribute major steps in hepatocarcinogenesis. HBx binds to the DDB1 subunit of a UV-damaged DNA binding protein[7], the latter of which appears to be important for maintaining the integrity of DNA repair[8]. HBx has also been shown to bind to and functionally inactivate p53[9,10].
Therefore, the HBx and HBs proteins represent the two potential candidate proteins involved in HBV-related hepatocarcinogenesis[11-16]. HCC is also a common complication of alcoholic cirrhosis, although ethanol appears to not be directly carcinogenic[17].
Oxidative stress is a disturbance in the oxidant-antioxidant balance leading to potential cellular damage. Most cells can tolerate a mild degree of oxidative stress, because they have sufficient antioxidant defense capacity and repair systems, which recognize and remove molecules damaged by oxidation. The imbalance can result from a lack of antioxidant capacity caused by disturbances in production and distribution, or by an overabundance of reactive oxygen species (ROS) from other factors. ROS are potential carcinogens because of their roles in mutagenesis, tumor promotion, and progression[18]. If not regulated properly, the excess ROS can damage lipids, protein or DNA, inhibiting normal function[19]. ROS alterations in different signaling pathways may modulate gene expression, cell adhesion, cell metabolism, cell cycle and cell death. These events may induce oxidative DNA damage, which in turn increases chromosomal aberrations associated with cell transformation[20]. ROS may also activate cellular signal pathways, such as those mediated by mitogen-activated protein kinase (MAPK), nuclear factor-κB (NF-κB), phosphatidylinositol 3-kinase (PI3K), p53, β-catenin/Wnt and associated with angiogenesis[21-23]. Importantly, HBx stimulates the activities of MAPK, NF-κB, PI3K, and β-catenin (as well as other pathways) that are thought to contribute importantly to the development of HCC. Perhaps this is why carriers with chronic liver disease (CLD) develop a high incidence of HCC, while asymptomatic carriers do not.
Several in vitro and in vivo observations suggest that oxidative stress and associated damage could represent a common link between different forms of chronic liver injury and hepatic fibrosis. For example, oxidative stress contributing to lipid peroxidation is one of the critical factors involved in the genesis and the progression of nonalcoholic steatohepatitis and liver cancer[24,25]. Viral infection or alcohol abuse greatly increased the highly variable miscoding etheno-modified DNA like epsilondA [1,N(6)-etheno-2'-deoxyadenosine] levels by triggering lipid peroxidation. Patients with chronic hepatitis, liver cirrhosis, and HCC due to HBV infection had more than 20 times higher urinary epsilondA levels[25] compared to uninfected individuals with no liver disease.
Among the mechanisms involved in mediating the process of liver fibrosis, an important role is played by ROS[26]. During the progression of liver injury, hepatic stellate cells (HSCs) become activated, which produce extracellular matrix such as collagen I[27]. Collagen I gene regulation has revealed a complex process involving ROS as a key mediator[28-30]. ROS-sensitive cytokines contribute to HSC activation during inflammation through paracrine signals released from immune cells[31]. The activated HSCs become responsive to platelet-derived growth factor (PDGF) and transforming growth factor (TGF)-β. PDGF facilitates the progression of hepatic fibrosis in human CLD. It increased the accumulation of hydrogen peroxide in HSCs. Specifically PDGF-induced increases in collagen deposition and liver fibrosis is markedly reduced by treatment with the anti-oxidant drug Mn-TBAP[32,33]. TGF-β increases ROS production and decreases the concentration of glutathione (GSH)[34]. In this context, it is important to note that HBx trans-activation activity is stimulated by ROS. Given that HBx is also associated with the development of HCC in both human carriers and in transgenic mice, and that HCC is associated with chronic inflammation, this underscores the importance of inflammation in the context of chronic HBV infection to hepatocarcinogenesis.
Many groups have shown that HBV can induce oxidative stress using HBV transgenic mice or HBV DNA transfection of cells in vitro, while oxidative stress is also common among HBV infected patients with CLD[35-41]. Oxidative stress also precedes the development of HCC in transgenic mice that overproduce and accumulate intracellular HBsAg. Several studies have found that the total peroxide level, a parameter of oxidative stress, is significantly higher in patients with chronic hepatitis compared to asymptomatic carriers, and positively correlated with alanine aminotransferase (ALT) levels, suggesting that oxidative stress plays a critical role in hepatic injury. Oxidative stress is also associated with the severity of the disease. Lipid peroxidation and oxidative DNA damage are enhanced in patients with HBV infection.
Mitochondria are a major source of ROS. ROS can form through electron leakage from the mitochondrial respiratory chain[42]. HBx itself targets mitochondria and directly interacts with voltage-dependent anion channel 3. It alters the mitochondrial membrane potential and increases the endogenous ROS level[43-46]. HBx expression also induces oxidative stress through calcium signaling and activates cellular kinases, leading to the activation of transcription factors NF-κB, signal transducer and activator of transcription 3, and others via phosphorylation[47,48]. It is observed that HBV-induced oxidative stress also stimulates the translocation of mitogen-activated protein kinase Raf-1 to mitochondria. This activation involves both the Src- and the PAK-mediated phosphorylation of the Raf-1 activation domain[49]. HBx also induces lipid peroxidation via down-regulation of SeP expression, resulting in increased expression of tumor necrosis factor-α in the human hepatoblastoma cell line, HepG2[50].
Activity of the anti-oxidant enzymes CuZn-SOD and GSH-Px was found to be the lowest in chronically infected patients compared with other groups[51,52]. Detection of an increase in MDA levels, which is a product of lipid peroxidation in HBV infected groups, indicates that oxidative stress is increased in HBV infection[52,53]. After treatment with interferon-α and lamivudine, however, there was a decrease in the products of lipid peroxidation and an increase in the antioxidant enzymes, such as CuZn-SOD and GSH-Px, compared with pretreatment[53].
The marker 8-hydroxydeoxyguanosine (8-OHdG) is useful in estimating DNA damage induced by oxidative stress. Importantly, hepatic 8-OHdG accumulation was detected in patients with chronic hepatitis B[39,54]. Further, HBV replication causes oxidative stress in HepAD38 liver cells, with more than 3 fold increases in the GSSG/GSHtot ratio[37].
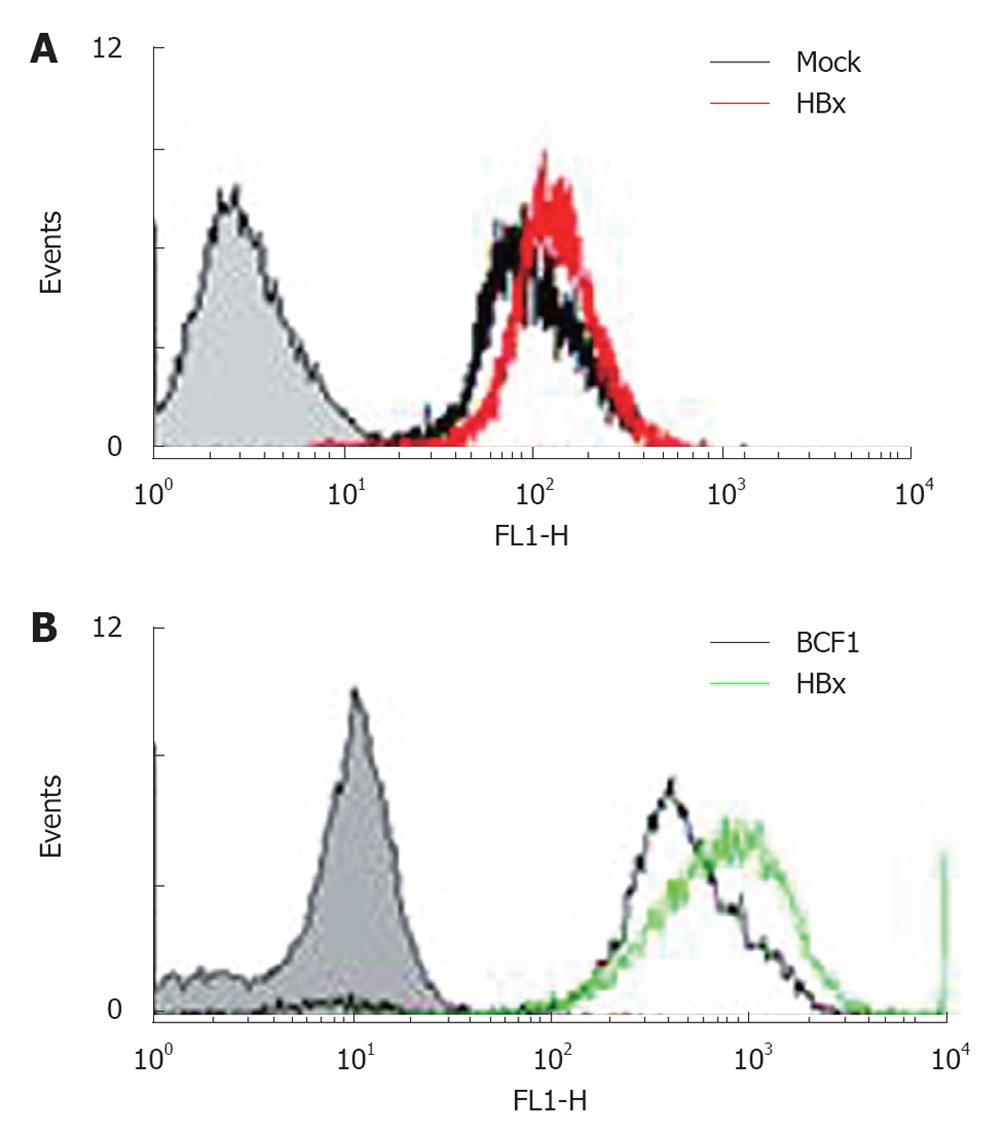
HuH-7 cells carrying the pre-S mutant (a truncated form of preS/S polypeptide) exhibited enhanced levels of ROS and oxidative DNA damage through endoplasmic reticulum (ER) stress pathways. Oxidative DNA damage has also been observed in livers of transgenic mice carrying the pre-S mutant[36]. HepG2-HBx cells and the livers of HBx mice also showed increased ROS levels (Figure 1), mtDNA deletion, and declines in the mitochondrial membrane potential compared to controls (data not shown). Through DNA chip analysis, several ROS-related molecules, such as members of the CYP450 families, were altered in HBx transgenic mice. The cytochrome p450s are a superfamily of hemeproteins that serve as terminal oxidases[55]. A major function of these p450s is to convert compounds into more polar metabolites[56]. Detoxification by cytochrome p450 can also produce ROS[57,58]. CYP2E1, a member of the p450 family that oxidizes ethanol, generates oxidative stress in the mitochondrial compartment of hepatocytes. This has been suggested to play a role in hepatotoxicity, as observed in ALD-related patients[59-61]. In a mouse model of nonalcoholic steatosis, CYP2E1 also plays key roles in ROS production and contributes to the pathogenesis of liver damage[62,63]. Thus, the involvement of mitochondria in the production of free radicals resulting from ethanol metabolism, and the fact that elevated free radical formation stimulates HBx activities, combined with the ability of mitochondria to oxidize ethanol may help to explain the apparent synergistic effects of chronic ethanol intake and HBx expression on the pathogenesis of CLD and HCC.
Chronic alcohol consumption has long been associated with progressive liver disease[64,65]. The liver is the major site of ethanol metabolism and thus sustains the most injury from chronic alcohol consumption. In alcohol-related liver disease, free radicals play a part in the pathogenesis of liver damage. Acute and chronic ethanol treatment increases ROS production, lowers cellular antioxidant levels, and enhances oxidative stress in many tissues, especially the liver[66,67]. It induces an accumulation of cysteine, a glutathione precursor/metabolite in the liver, probably due to gamma-glutamyltransferase induction[68]. Acetaldehyde produced by the oxidation of alcohol is able to inhibit the repair of alkylated nucleoproteins, to decrease the activity of several enzymes, and to damage mitochondria. Acetaldehyde also promotes cell death by depleting the concentration of reduced glutathione, by inducing lipid peroxidation, and by increasing the toxic effects of free radicals. Finally, acetaldehyde has been shown to directly stimulate proliferation of HSC and to increase collagen synthesis[69-71].
Chronic ethanol treatment has long been known to depress mitochondrial function[72-74]. The occurrence of DNA fragmentation in peripheral blood lymphocytes reflects a direct genotoxic effect of alcohol, HBV, and/or HCV, and suggests that the same genotoxic effect may operate in the liver and contribute to hepatocarcinogenesis[75].
Alcohol is also metabolized by mitochondrial CYP2E1. Ethanol exposure to VL-17A cells increased CYP2E1, decreased the activity of antigen-trimming enzymes like proteasome peptidase and leucine aminopeptidase (LAP). This defect may potentially result in decreased MHC class I-restricted antigen presentation on virally infected liver cells[68].
Alcohol-induced inflammatory and innate immune responses in Kupffer cells, due to elevated gut-derived plasma endotoxin levels, increase ROS-induced damage, and profibrogenic factors such as acetaldehyde or lipid peroxidation products, contribute to activation of HSCs[76]. Following a fibrogenic stimulus such as alcohol, HSCs transform into activated collagen-producing cells. There is much current interest in the likely synergistic interactions between hepatitis viruses and alcohol, especially with respect to generating oxidative stress.
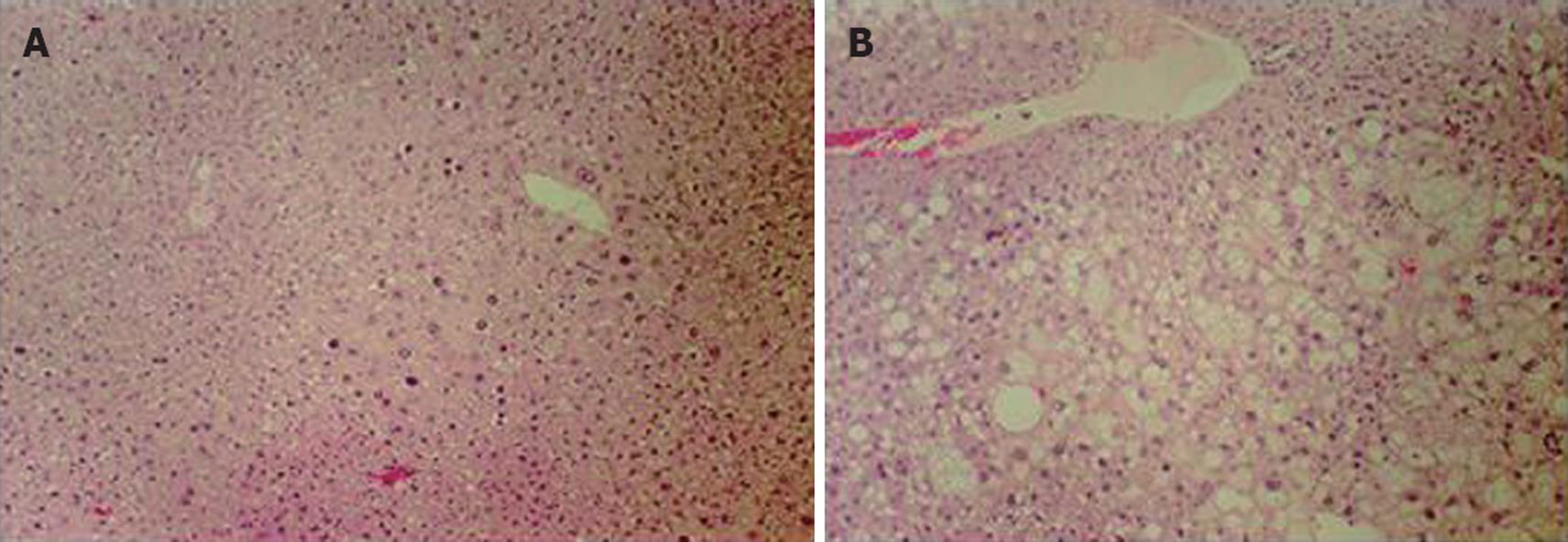
C57BL/6J (control) and HBx transgenic mice 8 mo of age were fed with water or 25% ethanol liquid diets for 12 wk (Table 1). Glutamate oxalate-transferase (GOT) and glutamate-pyruvate-transferase (GPT) levels, both indicators of liver damage, were elevated in control and HBx ethanol-fed groups, but not in the water-fed groups. However, HBx mice showed higher levels of GPT (87.3 ± 35.5 U/L) and GOT (193 ± 83.5 U/L) than wildtype mice (GPT: 61.7 ± 11.5 U/L, GOT: 119 ± 31.9 U/L). This result indicated that HBx transgenic mice developed more severe liver damage from ethanol than control mice. This was confirmed by histological evaluation of the liver, which showed the development of more severe liver injury only in the HBx transgenic mice. Hyperplastic nodules, found in both the water- and ethanol-fed groups of HBx transgenic mice, were more frequent among the ethanol-treated group (Figure 2). Control mice fed ethanol showed mild steatosis (data not shown), but the alcohol-treated HBx transgenic liver had severe steatosis and hepatomegaly compared to the untreated controls (Figure 2). Thus, even moderate ethanol consumption promoted oxidative stress and liver injury in HBx transgenic mice, implying that compromised antioxidant defense promotes alcohol liver injury.
| Groups | Age (mo) | No. of animals | Treatment | Duration (wk) | GOT (U/L) | GPT (U/L) |
| HBx-tg | 8 | 8 | 25% alcohol | 12 | 193 ± 83.5 | 87.3 ± 35.5 |
| 8 | 4 | Normal water | 12 | 60 ± 13.8 | 82 ± 19 | |
| C57BL/6J | 8 | 8 | 25% alcohol | 12 | 119 ± 31.9 | 61.7 ± 11.5 |
| 8 | 9 | Normal water | 12 | 42 ± 11 | 68 ± 6 |
Given that ROS production is a natural process, and that persistent, high levels of ROS could be damaging, the human body has developed antioxidant systems aimed at their neutralization. A variety of enzymatic and nonenzymatic mechanisms have evolved to protect cells against ROS. These include superoxide dismutase (SOD), which detoxifies the superoxide ion, catalase and the GSH peroxidase system, peroxiredoxins, which inactivate hydrogen peroxide (H2O2), and glutathione peroxidase, whose function is to detoxify cellular peroxides. Further, ceruloplasmin and ferritin help remove metals, such as iron, that promote oxidative reactions. There are also nonenzymatic, low-molecular-weight antioxidants, such as GSH, vitamin E, ascorbate (vitamin C), vitamin A, ubiquinone, uric acid, and bilirubin[77,78].
A CuZn-SOD is present in the cytosol and in the space between the inner and outer mitochondrial membranes, while a manganese-containing SOD is present in the mitochondrial matrix. Both of these enzymes are critical for prevention of ROS-induced toxicity[79].
Catalase is found primarily in peroxisomes; it catalyzes a reaction between two H2O2 molecules, resulting in the formation of water and O2. In addition, catalase can promote the interaction of H2O2 with hydrogen donors so that the H2O2 can be converted to one molecule of water, and the reduced donor becomes oxidized (peroxidatic activity of catalase).
The Prx family has the capacity to decompose H2O2in vivo and in vitro. All Prx enzymes contain a conserved Cys residue that undergoes a cycle of peroxide-dependent oxidation and thiol-dependent reduction during catalysis. Mammalian cells express six isoforms of Prx (Prx I to VI), which are classified into three subgroups (2-Cys, atypical 2-Cys, and 1-Cys) based on the number and position of Cys residues that participate in catalysis. Prx I to Prx IV are members of the 2-Cys Prx subgroup. Prx I and Prx II exist in the cytosol. Prx III, which is synthesized with a mitochondrial targeting sequence, is imported into and matures within mitochondria. Prx IV is a secreted protein[80-83]. Prx V is expressed ubiquitously; it localizes to mitochondria and peroxisomes[84] and possesses antioxidant activity equivalent to that of catalase[85]. All peroxiredoxins have two cysteine residues, but Prx VI has only one at position 47. Prx VI is the only peroxiredoxin whose target is glutathione rather than thioredoxin. It is mostly cytosolic.
As discussed above, oxidative stress plays a central role in HBV- and alcohol-induced liver damage. There are several possible strategies for preventing this stress[34]. Among them is the addition of antioxidant agents to antiviral drugs for patients with chronic hepatitis B.
For example, curcuminoids, the main yellow pigments in Curcuma longa (turmeric), have been used widely and for a long time in the treatment of sprains and inflammation[86]. Curcumin is the main component of turmeric, and two minor components are also present as curcuminoids. Curcuminoids possess antioxidant activity[87]. They protect DNA against oxidative attack, thereby lowering the risk for mutations and other genetic damage[88,89]. They also activate detoxification enzymes such as glutathione S-transferase[90]. Curcumins can down-regulate NF-κB, a nuclear transcription factor and critical upstream regulator of genes that control acute and chronic inflammation cascades[91,92]. Curcumin exerts beneficial effects in animal models of liver injury and cirrhosis[93,94]. Curcumin prevents alcohol-induced liver disease in rats by blocking activation of NF-κB[95] and by induction of HO-1[96]. Curcumin inhibits the fibrogenic progression of murine steatohepatitis[97]. It inhibits extracellular matrix formation by enhancing HSC matrix metalloproteinase expression via PPARγ and suppresses connective tissue growth factor expression[98]. CLL extract also represses HBV replication by enhancing the level of p53 protein[99].
Silymarin is a purified extract from milk thistle [Silybum marianum (L.) Gaertn], composed of a mixture of four isomeric flavonolignans: silibinin (its main, active component), isosilibinin, silydianin, and silychristin. This extract has been used as a remedy for almost 2000 years[100] and continues to be used as a medicine for many types of acute and chronic liver diseases. Silybin is an effective antioxidant, conserving GSH in liver cells while stabilizing the liver cell membranes against oxidative attack[100,101].
Inhibition of liver fibrogenesis in clinical trials, and promotion of liver regeneration[102,103] have been inconsistent with these treatments. In clinical trials among patients with viral hepatitis[104], alcoholic liver damage[105], and/or other liver diseases, silymarin and silybin lowered liver enzymes and (at times) improved antioxidant status, but did not consistently improve symptoms[104,105]. It is routinely used in the clinic as a hepatoprotectant. Silymarin exerts beneficial effects on the early stages of chronic liver disease, preventing and delaying the onset of HBV-related liver carcinogenesis[106-110].
Mechanistically, the anti-inflammatory and anticancer effects of silybin and the other flavonolignans are related to the potent inhibition of NF-κB. Silybin is a potent inhibitor of NF-κB activation, as induced by a variety of anti-inflammatory agents[111].
Green tea, a product of the plant Camellia sinensis (family Theaceae), contains polyphenols, specifically catechins of the flavan-3-ol class and their gallate derivatives. They are potent antioxidant and anti-inflammatory agents[112]. The flavan-3-ol structure makes them efficient scavengers of superoxide, singlet oxygen, nitric oxide, and peroxynitrite[113]. They up-regulate antioxidant and other detoxifying enzymes and protect DNA from oxidative damage[114-116]. Like other flavonoids, the green tea catechins can down-regulate NF-κB and AP-1, both of which may promote chronic inflammation and carcinogenesis when abnormally activated[117].
When treated with natural green tea extract, cells supporting HBV replication had reduced virus gene expression and reduced cell growth[118].
Vitamin C is essential to a healthy diet as well as a highly effective antioxidant. It is a substrate for ascorbate peroxidase. Vitamin E is a fat-soluble antioxidant that is the major antioxidant found in lipid-phase membranes. It blocks the production of ROS formed when fat undergoes oxidation[119]. Several studies have clearly shown that serum levels of vitamin E are significantly reduced in patients with alcoholic liver disease[120,121]. Vitamin E levels also negatively correlate with production of oxidative stress products and directly correlate with the extent of liver damage[122]. Therefore, maintenance of normal concentrations of vitamin E seems to be essential to prevent lipid peroxidation induced by alcohol consumption. Works from several laboratories have indicated that mitochondrial damage may present a common early event in cell injury[123]. Mitochondrial damage was prevented by vitamin E[124]. Vitamin E or C alone or in combination can facilitate scavenging free radicals generated in liver tissue[125]. Pretreatment with vitamin C against imidacloprid-induced oxidative liver stress in mice is better than post-treatment administration[126]. Pretreatment with vitamin E reduced the degree of oxidative stress[90], although this vitamin produced only slight changes in hepatic injury[127]. In the mouse model, vitamin E supplementation restored alcohol-induced redox status, reduced apoptosis, and prevented oxidative stress[128]. In addition, vitamin E in doses of 600 mg daily was effective in suppressing HBV replication and normalizing ALT in a significant proportion of chronically infected patients with CLD[129]. In this context, it will be important to determine whether anti-oxidants reduce HBxAg expression and/or function in cultured cells, or promote the resolution of CLD in human carriers and/or among human carriers with CLD who are also chronic alcoholics. If so, then anti-oxidant treatments may reduce the risk for progressive CLD lesions ultimately resulting in HCC, and/or eliminate the synergy between HBV and chronic alcoholism in the pathogenesis of alcoholic liver disease.
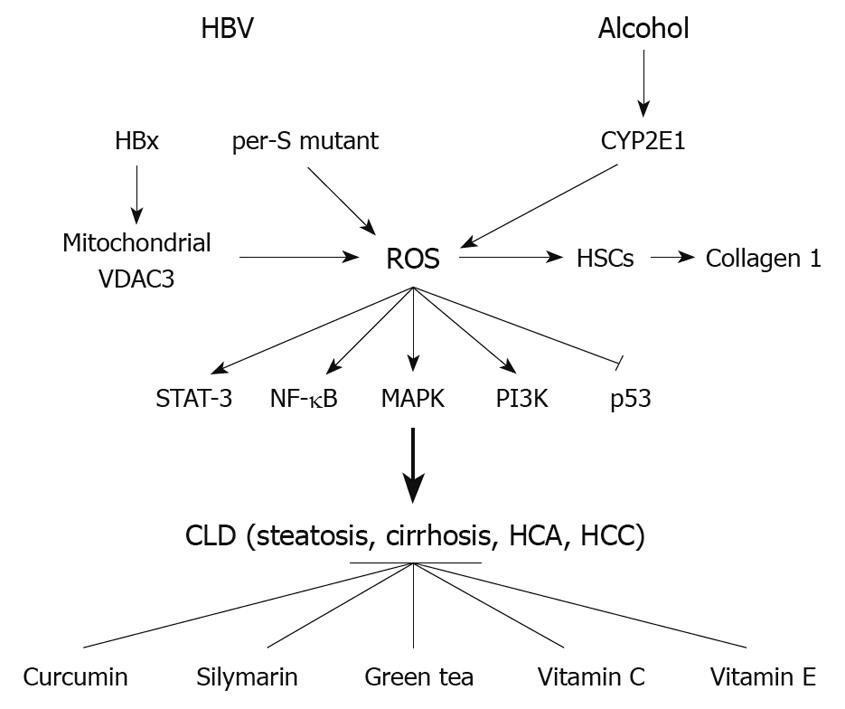
In summary (Figure 3), HBV and alcohol-induced liver injury are multi-step processes involving several mechanisms. The ability of HBV and alcohol to induce oxidative stress and the role of ROS in HBV- or alcohol-triggered liver damage is an important area of research, particularly because that information could be of major therapeutic value in protecting the liver. As basic information continues to emerge regarding the role of oxidative stress in disease development and the mechanisms underlying ROS-related cellular toxicity, these findings will lead to more rational antioxidant therapeutic approaches.
Peer reviewer: Thomas Bock, PhD, Professor, Department of Molecular Pathology, Institute of Pathology, University Hospital of Tuebingen, D-72076 Tuebingen, Germany
S- Editor Wang JL L- Editor O'Neill M E- Editor Lin YP
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [Cited in This Article: ] |
| 2. | Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129-1133. [Cited in This Article: ] |
| 3. | Ito K, Arai M, Imazeki F, Yonemitsu Y, Bekku D, Kanda T, Fujiwara K, Fukai K, Sato K, Itoga S. Risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Scand J Gastroenterol. 2010;45:243-249. [Cited in This Article: ] |
| 4. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [Cited in This Article: ] |
| 5. | Caldwell S, Park SH. The epidemiology of hepatocellular cancer: from the perspectives of public health problem to tumor biology. J Gastroenterol. 2009;44 Suppl 19:96-101. [Cited in This Article: ] |
| 6. | Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23:47-58. [Cited in This Article: ] |
| 7. | Becker SA, Lee TH, Butel JS, Slagle BL. Hepatitis B virus X protein interferes with cellular DNA repair. J Virol. 1998;72:266-272. [Cited in This Article: ] |
| 8. | Kazantsev A, Mu D, Nichols AF, Zhao X, Linn S, Sancar A. Functional complementation of xeroderma pigmentosum complementation group E by replication protein A in an in vitro system. Proc Natl Acad Sci USA. 1996;93:5014-5018. [Cited in This Article: ] |
| 9. | Ueda H, Ullrich SJ, Gangemi JD, Kappel CA, Ngo L, Feitelson MA, Jay G. Functional inactivation but not structural mutation of p53 causes liver cancer. Nat Genet. 1995;9:41-47. [Cited in This Article: ] |
| 10. | Wang XW, Forrester K, Yeh H, Feitelson MA, Gu JR, Harris CC. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci USA. 1994;91:2230-2234. [Cited in This Article: ] |
| 11. | Zhang X, Zhang H, Ye L. Effects of hepatitis B virus X protein on the development of liver cancer. J Lab Clin Med. 2006;147:58-66. [Cited in This Article: ] |
| 12. | Chan HL, Sung JJ. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis. 2006;26:153-161. [Cited in This Article: ] |
| 13. | Koike K, Tsutsumi T, Fujie H, Shintani Y, Kyoji M. Molecular mechanism of viral hepatocarcinogenesis. Oncology. 2002;62 Suppl 1:29-37. [Cited in This Article: ] |
| 14. | Yu DY, Moon HB, Son JK, Jeong S, Yu SL, Yoon H, Han YM, Lee CS, Park JS, Lee CH. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J Hepatol. 1999;31:123-132. [Cited in This Article: ] |
| 15. | Zhu H, Wang Y, Chen J, Cheng G, Xue J. Transgenic mice expressing hepatitis B virus X protein are more susceptible to carcinogen induced hepatocarcinogenesis. Exp Mol Pathol. 2004;76:44-50. [Cited in This Article: ] |
| 16. | Hildt E, Munz B, Saher G, Reifenberg K, Hofschneider PH. The PreS2 activator MHBs(t) of hepatitis B virus activates c-raf-1/Erk2 signaling in transgenic mice. EMBO J. 2002;21:525-535. [Cited in This Article: ] |
| 17. | Nalpas B, Feitelson M, Bréchot C, Rubin E. Alcohol, hepatotropic viruses, and hepatocellular carcinoma. Alcohol Clin Exp Res. 1995;19:1089-1095. [Cited in This Article: ] |
| 18. | Dröge W. Oxidative stress and aging. Adv Exp Med Biol. 2003;543:191-200. [Cited in This Article: ] |
| 19. | Perry G, Raina AK, Nunomura A, Wataya T, Sayre LM, Smith MA. How important is oxidative damage? Lessons from Alzheimer's disease. Free Radic Biol Med. 2000;28:831-834. [Cited in This Article: ] |
| 20. | Choi J, Ou JH. Mechanisms of liver injury. III. Oxidative stress in the pathogenesis of hepatitis C virus. Am J Physiol Gastrointest Liver Physiol. 2006;290:G847-G851. [Cited in This Article: ] |
| 21. | Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1-15. [Cited in This Article: ] |
| 22. | Tien Kuo M, Savaraj N. Roles of reactive oxygen species in hepatocarcinogenesis and drug resistance gene expression in liver cancers. Mol Carcinog. 2006;45:701-709. [Cited in This Article: ] |
| 23. | Czaja MJ. Cell signaling in oxidative stress-induced liver injury. Semin Liver Dis. 2007;27:378-389. [Cited in This Article: ] |
| 24. | Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128-133. [Cited in This Article: ] |
| 25. | Nair J, Srivatanakul P, Haas C, Jedpiyawongse A, Khuhaprema T, Seitz HK, Bartsch H. High urinary excretion of lipid peroxidation-derived DNA damage in patients with cancer-prone liver diseases. Mutat Res. 2010;683:23-28. [Cited in This Article: ] |
| 26. | Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297-306. [Cited in This Article: ] |
| 27. | Okuno M, Kojima S, Akita K, Matsushima-Nishiwaki R, Adachi S, Sano T, Takano Y, Takai K, Obora A, Yasuda I. Retinoids in liver fibrosis and cancer. Front Biosci. 2002;7:d204-d218. [Cited in This Article: ] |
| 28. | Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98-105. [Cited in This Article: ] |
| 29. | Urtasun R, Conde de la Rosa L, Nieto N. Oxidative and nitrosative stress and fibrogenic response. Clin Liver Dis. 2008;12:769-790, viii. [Cited in This Article: ] |
| 30. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [Cited in This Article: ] |
| 31. | Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129-140. [Cited in This Article: ] |
| 32. | Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, Kawata S. NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology. 2005;41:1272-1281. [Cited in This Article: ] |
| 33. | De Minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys. 2007;462:266-272. [Cited in This Article: ] |
| 34. | Liu RM, Gaston Pravia KA. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med. 2010;48:1-15. [Cited in This Article: ] |
| 35. | Demirdag K, Yilmaz S, Ozdarendeli A, Ozden M, Kalkan A, Kilic SS. Levels of plasma malondialdehyde and erythrocyte antioxidant enzyme activities in patients with chronic hepatitis B. Hepatogastroenterology. 2003;50:766-770. [Cited in This Article: ] |
| 36. | Hsieh YH, Su IJ, Wang HC, Chang WW, Lei HY, Lai MD, Chang WT, Huang W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis. 2004;25:2023-2032. [Cited in This Article: ] |
| 37. | Severi T, Ying C, Vermeesch JR, Cassiman D, Cnops L, Verslype C, Fevery J, Arckens L, Neyts J, van Pelt JF. Hepatitis B virus replication causes oxidative stress in HepAD38 liver cells. Mol Cell Biochem. 2006;290:79-85. [Cited in This Article: ] |
| 38. | Yang F, Yin Y, Wang F, Zhang L, Wang Y, Sun S. An altered pattern of liver apolipoprotein A-I isoforms is implicated in male chronic hepatitis B progression. J Proteome Res. 2010;9:134-143. [Cited in This Article: ] |
| 39. | Niu D, Zhang J, Ren Y, Feng H, Chen WN. HBx genotype D represses GSTP1 expression and increases the oxidative level and apoptosis in HepG2 cells. Mol Oncol. 2009;3:67-76. [Cited in This Article: ] |
| 40. | Chrobot AM, Szaflarska-Szczepanik A, Drewa G. Antioxidant defense in children with chronic viral hepatitis B and C. Med Sci Monit. 2000;6:713-718. [Cited in This Article: ] |
| 41. | Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95. [Cited in This Article: ] |
| 42. | Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639-645. [Cited in This Article: ] |
| 43. | Lee YI, Hwang JM, Im JH, Lee YI, Kim NS, Kim DG, Yu DY, Moon HB, Park SK. Human hepatitis B virus-X protein alters mitochondrial function and physiology in human liver cells. J. Biol Chem. 2004;279:15460-15471. [Cited in This Article: ] |
| 44. | Rahmani Z, Huh KW, Lasher R, Siddiqui A. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J Virol. 2000;74:2840-2846. [Cited in This Article: ] |
| 45. | Takada S, Shirakata Y, Kaneniwa N, Koike K. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene. 1999;18:6965-6973. [Cited in This Article: ] |
| 46. | Lim W, Kwon SH, Cho H, Kim S, Lee S, Ryu WS, Cho H. HBx targeting to mitochondria and ROS generation are necessary but insufficient for HBV-induced cyclooxygenase-2 expression. J Mol Med. 2010;88:359-369. [Cited in This Article: ] |
| 47. | Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004;78:12725-12734. [Cited in This Article: ] |
| 48. | Waris G, Huh KW, Siddiqui A. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-kappa B via oxidative stress. Mol Cell Biol. 2001;21:7721-7730. [Cited in This Article: ] |
| 49. | Chen J, Siddiqui A. Hepatitis B virus X protein stimulates the mitochondrial translocation of Raf-1 via oxidative stress. J Virol. 2007;81:6757-6760. [Cited in This Article: ] |
| 50. | Yi YS, Park SG, Byeon SM, Kwon YG, Jung G. Hepatitis B virus X protein induces TNF-alpha expression via down-regulation of selenoprotein P in human hepatoma cell line, HepG2. Biochim Biophys Acta. 2003;1638:249-256. [Cited in This Article: ] |
| 51. | Abel S, De Kock M, van Schalkwyk DJ, Swanevelder S, Kew MC, Gelderblom WC. Altered lipid profile, oxidative status and hepatitis B virus interactions in human hepatocellular carcinoma. Prostaglandins Leukot Essent Fatty Acids. 2009;81:391-399. [Cited in This Article: ] |
| 52. | Tsai SM, Lin SK, Lee KT, Hsiao JK, Huang JC, Wu SH, Ma H, Wu SH, Tsai LY. Evaluation of redox statuses in patients with hepatitis B virus-associated hepatocellular carcinoma. Ann Clin Biochem. 2009;46:394-400. [Cited in This Article: ] |
| 53. | Acar A, Görenek L, Aydin A, Eyigün CP, Eken A, Sayal A, Pahsa A. [Investigation of oxidative stress and antioxidant defense in patients with hepatitis B virus infection and the effect of interferon-alpha plus lamivudine combination therapy on oxidative stress]. Mikrobiyol Bul. 2009;43:411-23. [Cited in This Article: ] |
| 54. | Fujita N, Sugimoto R, Ma N, Tanaka H, Iwasa M, Kobayashi Y, Kawanishi S, Watanabe S, Kaito M, Takei Y. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J Viral Hepat. 2008;15:498-507. [Cited in This Article: ] |
| 55. | Guengerich FP, Beaune PH, Umbenhauer DR, Churchill PF, Bork RW, Dannan GA, Knodell RG, Lloyd RS, Martin MV. Cytochrome P-450 enzymes involved in genetic polymorphism of drug oxidation in humans. Biochem Soc Trans. 1987;15:576-578. [Cited in This Article: ] |
| 56. | Lewis DF, Pratt JM. The P450 catalytic cycle and oxygenation mechanism. Drug Metab Rev. 1998;30:739-786. [Cited in This Article: ] |
| 57. | Blanck J, Ristau O, Zhukov AA, Archakov AI, Rein H, Ruckpaul K. Cytochrome P-450 spin state and leakiness of the monooxygenase pathway. Xenobiotica. 1991;21:121-135. [Cited in This Article: ] |
| 58. | White RE. The involvement of free radicals in the mechanisms of monooxygenases. Pharmacol Ther. 1991;49:21-42. [Cited in This Article: ] |
| 59. | Lytton SD, Helander A, Zhang-Gouillon ZQ, Stokkeland K, Bordone R, Aricò S, Albano E, French SW, Ingelman-Sundberg M. Autoantibodies against cytochromes P-4502E1 and P-4503A in alcoholics. Mol Pharmacol. 1999;55:223-233. [Cited in This Article: ] |
| 60. | Eliasson E, Kenna JG. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol Pharmacol. 1996;50:573-582. [Cited in This Article: ] |
| 61. | Bourdi M, Chen W, Peter RM, Martin JL, Buters JT, Nelson SD, Pohl LR. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chem Res Toxicol. 1996;9:1159-1166. [Cited in This Article: ] |
| 62. | Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067-1075. [Cited in This Article: ] |
| 63. | Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135-G1139. [Cited in This Article: ] |
| 64. | Ronis MJ, Korourian S, Blackburn ML, Badeaux J, Badger TM. The role of ethanol metabolism in development of alcoholic steatohepatitis in the rat. Alcohol. 2010;44:157-169. [Cited in This Article: ] |
| 65. | Shepard BD, Tuma DJ, Tuma PL. Chronic ethanol consumption induces global hepatic protein hyperacetylation. Alcohol Clin Exp Res. 2010;34:280-291. [Cited in This Article: ] |
| 66. | Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141-154. [Cited in This Article: ] |
| 67. | Bondy SC, Guo SX. Effect of ethanol treatment on indices of cumulative oxidative stress. Eur J Pharmacol. 1994;270:349-355. [Cited in This Article: ] |
| 68. | Osna NA, White RL, Todero S, McVicker BL, Thiele GM, Clemens DL, Tuma DJ, Donohue TM Jr. Ethanol-induced oxidative stress suppresses generation of peptides for antigen presentation by hepatoma cells. Hepatology. 2007;45:53-61. [Cited in This Article: ] |
| 69. | Svegliati-Baroni G, Ridolfi F, Di Sario A, Saccomanno S, Bendia E, Benedetti A, Greenwel P. Intracellular signaling pathways involved in acetaldehyde-induced collagen and fibronectin gene expression in human hepatic stellate cells. Hepatology. 2001;33:1130-1140. [Cited in This Article: ] |
| 70. | Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9-19. [Cited in This Article: ] |
| 71. | Kato J, Sato Y, Inui N, Nakano Y, Takimoto R, Takada K, Kobune M, Kuroiwa G, Miyake S, Kohgo Y. Ethanol induces transforming growth factor-alpha expression in hepatocytes, leading to stimulation of collagen synthesis by hepatic stellate cells. Alcohol Clin Exp Res. 2003;27:58S-63S. [Cited in This Article: ] |
| 72. | Cunningham CC, Coleman WB, Spach PI. The effects of chronic ethanol consumption on hepatic mitochondrial energy metabolism. Alcohol Alcohol. 1990;25:127-136. [Cited in This Article: ] |
| 73. | Boveris A, Fraga CG, Varsavsky AI, Koch OR. Increased chemiluminescence and superoxide production in the liver of chronically ethanol-treated rats. Arch Biochem Biophys. 1983;227:534-541. [Cited in This Article: ] |
| 74. | Kukiełka E, Dicker E, Cederbaum AI. Increased production of reactive oxygen species by rat liver mitochondria after chronic ethanol treatment. Arch Biochem Biophys. 1994;309:377-386. [Cited in This Article: ] |
| 75. | Grossi S, Sumberaz A, Gosmar M, Mattioli F, Testino G, Martelli A. DNA damage in peripheral blood lymphocytes of patients with cirrhosis related to alcohol abuse or to hepatitis B and C viruses. Eur J Gastroenterol Hepatol. 2008;20:22-25. [Cited in This Article: ] |
| 76. | Cubero FJ, Urtasun R, Nieto N. Alcohol and liver fibrosis. Semin Liver Dis. 2009;29:211-221. [Cited in This Article: ] |
| 77. | Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic Res. 1999;31:261-272. [Cited in This Article: ] |
| 78. | Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139-162. [Cited in This Article: ] |
| 79. | Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515-18517. [Cited in This Article: ] |
| 80. | Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35-41. [Cited in This Article: ] |
| 81. | Watabe S, Kohno H, Kouyama H, Hiroi T, Yago N, Nakazawa T. Purification and characterization of a substrate protein for mitochondrial ATP-dependent protease in bovine adrenal cortex. J Biochem. 1994;115:648-654. [Cited in This Article: ] |
| 82. | Jin DY, Chae HZ, Rhee SG, Jeang KT. Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation. J Biol Chem. 1997;272:30952-30961. [Cited in This Article: ] |
| 83. | Matsumoto A, Okado A, Fujii T, Fujii J, Egashira M, Niikawa N, Taniguchi N. Cloning of the peroxiredoxin gene family in rats and characterization of the fourth member. FEBS Lett. 1999;443:246-250. [Cited in This Article: ] |
| 84. | Seo MS, Kang SW, Kim K, Baines IC, Lee TH, Rhee SG. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J Biol Chem. 2000;275:20346-20354. [Cited in This Article: ] |
| 85. | Knoops B, Clippe A, Bogard C, Arsalane K, Wattiez R, Hermans C, Duconseille E, Falmagne P, Bernard A. Cloning and characterization of AOEB166, a novel mammalian antioxidant enzyme of the peroxiredoxin family. J Biol Chem. 1999;274:30451-30458. [Cited in This Article: ] |
| 86. | Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75:787-809. [Cited in This Article: ] |
| 87. | Strimpakos AS, Sharma RA. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal. 2008;10:511-545. [Cited in This Article: ] |
| 88. | Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631-1652. [Cited in This Article: ] |
| 89. | Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120-125. [Cited in This Article: ] |
| 90. | Subudhi U, Das K, Paital B, Bhanja S, Chainy GB. Supplementation of curcumin and vitamin E enhances oxidative stress, but restores hepatic histoarchitecture in hypothyroid rats. Life Sci. 2009;84:372-379. [Cited in This Article: ] |
| 91. | Sarkar FH, Li Y, Wang Z, Kong D. NF-kappaB signaling pathway and its therapeutic implications in human diseases. Int Rev Immunol. 2008;27:293-319. [Cited in This Article: ] |
| 92. | Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995;270:24995-25000. [Cited in This Article: ] |
| 93. | Park EJ, Jeon CH, Ko G, Kim J, Sohn DH. Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J Pharm Pharmacol. 2000;52:437-440. [Cited in This Article: ] |
| 94. | Bruck R, Ashkenazi M, Weiss S, Goldiner I, Shapiro H, Aeed H, Genina O, Helpern Z, Pines M. Prevention of liver cirrhosis in rats by curcumin. Liver Int. 2007;27:373-383. [Cited in This Article: ] |
| 95. | Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93:321-329. [Cited in This Article: ] |
| 96. | Bao W, Li K, Rong S, Yao P, Hao L, Ying C, Zhang X, Nussler A, Liu L. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J Ethnopharmacol. 2010;128:549-553. [Cited in This Article: ] |
| 97. | Zamara E, Galastri S, Aleffi S, Petrai I, Aragno M, Mastrocola R, Novo E, Bertolani C, Milani S, Vizzutti F. Prevention of severe toxic liver injury and oxidative stress in MCP-1-deficient mice. J Hepatol. 2007;46:230-238. [Cited in This Article: ] |
| 98. | Ma C, Liu WY, Cui Q, Gu CH, Dou YW, Zhao R, Chen M, Zheng X. [Effects of intensive insulin therapy on plasma nitric oxide and endothelin-1 levels in patients undergoing cardiac surgery under cardiopulmonary bypass]. Zhonghua Waike Zazhi. 2008;46:443-445. [Cited in This Article: ] |
| 99. | Kim HJ, Yoo HS, Kim JC, Park CS, Choi MS, Kim M, Choi H, Min JS, Kim YS, Yoon SW. Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J Ethnopharmacol. 2009;124:189-196. [Cited in This Article: ] |
| 100. | Wang XG, Lin B, Kidder JM, Telford S, Hu LT. Effects of environmental changes on expression of the oligopeptide permease (opp) genes of Borrelia burgdorferi. J Bacteriol. 2002;184:6198-6206. [Cited in This Article: ] |
| 101. | Shaker E, Mahmoud H, Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol. 2010;48:803-806. [Cited in This Article: ] |
| 102. | Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos). Altern Med Rev. 2005;10:193-203. [Cited in This Article: ] |
| 103. | Makeham MA, Dovey SM, County M, Kidd MR. An international taxonomy for errors in general practice: a pilot study. Med J Aust. 2002;177:68-72. [Cited in This Article: ] |
| 104. | Mayer KE, Myers RP, Lee SS. Silymarin treatment of viral hepatitis: a systematic review. J Viral Hepat. 2005;12:559-567. [Cited in This Article: ] |
| 105. | Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491-504. [Cited in This Article: ] |
| 106. | Wu YF, Fu SL, Kao CH, Yang CW, Lin CH, Hsu MT, Tsai TF. Chemopreventive effect of silymarin on liver pathology in HBV X protein transgenic mice. Cancer Res. 2008;68:2033-2042. [Cited in This Article: ] |
| 107. | Jacobs BP, Dennehy C, Ramirez G, Sapp J, Lawrence VA. Milk thistle for the treatment of liver disease: a systematic review and meta-analysis. Am J Med. 2002;113:506-515. [Cited in This Article: ] |
| 108. | Quaglia MG, Bossù E, Donati E, Mazzanti G, Brandt A. Determination of silymarine in the extract from the dried silybum marianum fruits by high performance liquid chromatography and capillary electrophoresis. J Pharm Biomed Anal. 1999;19:435-442. [Cited in This Article: ] |
| 109. | Gazák R, Walterová D, Kren V. Silybin and silymarin--new and emerging applications in medicine. Curr Med Chem. 2007;14:315-338. [Cited in This Article: ] |
| 110. | Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035-2063. [Cited in This Article: ] |
| 111. | Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163:6800-6809. [Cited in This Article: ] |
| 112. | Coyle CH, Philips BJ, Morrisroe SN, Chancellor MB, Yoshimura N. Antioxidant effects of green tea and its polyphenols on bladder cells. Life Sci. 2008;83:12-18. [Cited in This Article: ] |
| 113. | Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15:1840-1850. [Cited in This Article: ] |
| 114. | Khan SG, Katiyar SK, Agarwal R, Mukhtar H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: possible role in cancer chemoprevention. Cancer Res. 1992;52:4050-4052. [Cited in This Article: ] |
| 115. | Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, Wei Z, Liang Y, Wang K, Zhang ZQ. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262-268. [Cited in This Article: ] |
| 116. | Chou FP, Chu YC, Hsu JD, Chiang HC, Wang CJ. Specific induction of glutathione S-transferase GSTM2 subunit expression by epigallocatechin gallate in rat liver. Biochem Pharmacol. 2000;60:643-650. [Cited in This Article: ] |
| 117. | Zhao B, Guo Q, Xin W. Free radical scavenging by green tea polyphenols. Methods Enzymol. 2001;335:217-231. [Cited in This Article: ] |
| 118. | Xu J, Wang J, Deng F, Hu Z, Wang H. Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antiviral Res. 2008;78:242-249. [Cited in This Article: ] |
| 119. | Herrera E, Barbas C. Vitamin E: action, metabolism and perspectives. J Physiol Biochem. 2001;57:43-56. [Cited in This Article: ] |
| 120. | Bjørneboe GE, Johnsen J, Bjørneboe A, Bache-Wiig JE, Mørland J, Drevon CA. Diminished serum concentration of vitamin E in alcoholics. Ann Nutr Metab. 1988;32:56-61. [Cited in This Article: ] |
| 121. | Leo MA, Rosman AS, Lieber CS. Differential depletion of carotenoids and tocopherol in liver disease. Hepatology. 1993;17:977-986. [Cited in This Article: ] |
| 122. | Masalkar PD, Abhang SA. Oxidative stress and antioxidant status in patients with alcoholic liver disease. Clin Chim Acta. 2005;355:61-65. [Cited in This Article: ] |
| 123. | Fulda S, Scaffidi C, Susin SA, Krammer PH, Kroemer G, Peter ME, Debatin KM. Activation of mitochondria and release of mitochondrial apoptogenic factors by betulinic acid. J Biol Chem. 1998;273:33942-33948. [Cited in This Article: ] |
| 124. | Sakurai K, Cederbaum AI. Oxidative stress and cytotoxicity induced by ferric-nitrilotriacetate in HepG2 cells that express cytochrome P450 2E1. Mol Pharmacol. 1998;54:1024-1035. [Cited in This Article: ] |
| 125. | Zaidi SM, Al-Qirim TM, Banu N. Effects of antioxidant vitamins on glutathione depletion and lipid peroxidation induced by restraint stress in the rat liver. Drugs R D. 2005;6:157-165. [Cited in This Article: ] |
| 126. | El-Gendy KS, Aly NM, Mahmoud FH, Kenawy A, El-Sebae AK. The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol. 2010;48:215-221. [Cited in This Article: ] |
| 127. | Bansal AK, Bansal M, Soni G, Bhatnagar D. Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact. 2005;156:101-111. [Cited in This Article: ] |
| 128. | Kaur J, Shalini S, Bansal MP. Influence of vitamin E on alcohol-induced changes in antioxidant defenses in mice liver. Toxicol Mech Methods. 2010;20:82-89. [Cited in This Article: ] |
| 129. | Andreone P, Fiorino S, Cursaro C, Gramenzi A, Margotti M, Di Giammarino L, Biselli M, Miniero R, Gasbarrini G, Bernardi M. Vitamin E as treatment for chronic hepatitis B: results of a randomized controlled pilot trial. Antiviral Res. 2001;49:75-81. [Cited in This Article: ] |