Published online Dec 14, 2010. doi: 10.3748/wjg.v16.i46.5838
Revised: August 16, 2010
Accepted: August 23, 2010
Published online: December 14, 2010
AIM: To translate into Greek and validate the chronic liver disease questionnaire (CLDQ).
METHODS: Two hundred and six consecutive adult patients with the diagnosis of a chronic liver disease from 2 general hospitals in Athens were enrolled in the study from May to September 2008. In order to assess their quality of life (QOL) the CLDQ was applied. The instrument was translated from English, back translated and reviewed in focus groups within the framework of a large multicenter study. The measurements that were performed included: 2 independent sample t tests, one-way analysis of variance, reliability coefficients, explanatory factor analysis using a varimax rotation and the principal components method.
RESULTS: One hundred and twenty five (61%) patients were men, half were aged 40-59 years and > 33% were > 60 years old. Among the patients, 48 (23%) were hospitalized and 97 (47%) were cirrhotic according to the Child-Pugh score. The internal consistency of the Greek CLDQ version using Cronbach’s alpha coefficient was found to be 0.93. Exploratory factor analysis identified 7 domains accounting for 65% of the variance of CLDQ items and only partially overlapping with those found in the original version. The area under the receiver operating characteristics curve was calculated at 0.813 and the logistic estimate for the threshold score of 167.50 provided a sensitivity of 74.3% and a specificity of 71.6% for the model.
CONCLUSION: Our data confirmed the validity of the Greek version of the CLDQ in identifying the QOL among patients with chronic liver disease.
- Citation: Kollia Z, Patelarou E, Vivilaki V, Kollia E, Kefou F, Elefsiniotis I, Dourakis SP, Brokalaki H. Translation and validation of the Greek chronic liver disease questionnaire. World J Gastroenterol 2010; 16(46): 5838-5844
- URL: https://www.wjgnet.com/1007-9327/full/v16/i46/5838.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i46.5838
Chronic liver disease encompasses a wide range of illnesses characterized by liver inflammation and progression to cirrhosis. Quality of life (QOL) is a concept that incorporates many aspects of an individual’s experience, general well being and satisfaction, as well as social and physical functioning[1]. Health-related QOL is important when measuring the impact or burden of a chronic disease, such as liver disease, and is highly correlated with fatigue, loss of esteem, depression and disease complications[2]. In the last few decades, the assessment of health-related QOL has become an important outcome measure in clinical research in both gastroenterology and hepatology[3]. Whilst a variety of generic QOL measures have been developed, there is a need to develop specific instruments endowed with sufficient sensitivity to document clinically significant changes over time[4].
The chronic liver disease questionnaire (CLDQ) is a specific health-related QOL instrument designed for patients with liver disease, regardless of the underlying etiology and degree of disease. Its original version was developed first by Younossi et al[5] and has demonstrated appropriate validity and reliability. The CLDQ has already been cross-culturally adapted and validated into different languages in recently published studies[6-10]. Consequently, the aim of this paper was to: (1) report on the development of the Greek version of the CLDQ and on the validation procedures carried out; (2) examine the factorial structure of the Greek CLDQ; and (3) evaluate the sensitivity of the Greek CLDQ in assessing QOL over a range of cut-off scores among liver disease patients.
The CLDQ is a 29-item self-reported scale consisting of statements describing QOL, and is divided into 6 domains including abdominal symptoms, fatigue, systemic symptoms, activity, emotional function and concern. All items refer to the previous 2 wk on a 7-point Likert scale, with 1 corresponding to the maximum frequency labeled as “all of the time” and 7 to the minimum labeled as “none of the time”. Permission to reproduce and validate the CLDQ was provided by Younossi et al[5].
The 29 items of the CLDQ were translated by 2 independent bilingual translators. Another native English speaker who did not have knowledge of the original instrument then back translated the Greek version. The backward translation was sent to a group of English experts for comments. The translated questionnaire was culturally adapted through a cognitive debriefing process that was used to identify any language problems and to assess the degree of respondents understanding of the item’s content that was meant to be elicited[5].
During this stage the reconciled Greek version of the CLDQ was pilot tested among 10 patients. As part of the cultural adaptation process, in-depth interviews were implemented with regard to the respondents understanding of the questionnaire with the purpose of revealing inappropriately interpreted items and translation alternatives. The participants gave their impression on the clarity of each item, the relevance of the content to their situation, the comprehensiveness of the instructions and their ability to complete it on their own. They were also encouraged to make suggestions whenever necessary. Finally, written comments made by the participants were included in the final Greek version of CLDQ.
Consecutive adult patients diagnosed with chronic liver disease, confirmed by laboratory tests, imaging studies and in most cases by liver histology, were asked to participate in our study. Enrolment started in May and ended in September 2008 among patients of the Gastroenterology Clinics of 2 general hospitals in Athens. Inclusion criteria were fluency in the spoken and written Greek language, age > 18 years and the existence of liver disease symptoms during the previous 3 mo. Non-Greek-speaking patients, patients who had undergone liver transplantation, patients with dementia or psychosis, and patients with refractory encephalopathy (grade II and more) were excluded. To asses the severity of liver disease, the patients’ Child-Pugh scores were calculated and patients’ were classified as cirrhotic or not[11]. In total, 220 patients were approached and 206 patients agreed to participate (rate of attendance 93.6%). CLDQs were also completed by healthy participants (n = 208, controls) in order to perform receiver operating characteristics (ROC) analysis. The control group was selected randomly from a list of the Athens county population and was matched with cases by gender, age and educational background. One control was selected for one case participant. Both patients and healthy participants completed the CLDQ in the presence of a nurse.
All participants entering the study provided written informed consent after receiving a complete description of the study and having the opportunity to ask for clarifications. Along with the questionnaires there was a cover letter explaining the purpose of the study, providing the researchers’ affiliation and contact information, and clearly stating that answers would be confidential and anonymity would be guaranteed in the final data reports.
Descriptive characteristics were determined for the sociodemographic variables of the sample and Student t tests were performed on the descriptive characteristics of the study population and the CLDQ score. All P-values were based on 2-sided tests and significance was defined as P < 0.05. The assumptions of normality, homogeneity and independence of the sample were checked. Reliability coefficients as measured by Cronbach’s alpha were calculated for the CLDQ in order to assess reproducibility and consistency of the instrument. The underlying dimensions of the scale were checked with an explanatory factor analysis using a varimax rotation and principal components method as a descriptive method for analyzing grouped data[12]. Factor analysis using principal component analysis with varimax rotation was carried out to determine the dimensional structure of CLDQ using the following criteria: (1) eigenvalue > 1[13]; (2) variables should have a load > 0.50 on only one factor and < 0.40 on more factors; (3) the interpretation of the factor structure should be meaningful; and (4) the screen plot is accurate in the case where the means of communalities are above 0.60[14]. Computations were based on a covariance matrix, as all variables were receiving values from the same measurement scale[15]; Bartlett’s test of sphericity with P < 0.05 and a Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy of 0.6 were used when performing this factor analysis. A factor was considered as important if its eigenvalue exceeded 1.0[13]. As the factor analysis found 2 independent domains, subsequent Cronbach’s alpha measurements were separately performed for each domain, highlighting how the items were grouped together.
The sensitivity and specificity were calculated at several cut-off scores of the CLDQ. A ROC analysis was carried out; this method allows the display of all the pairs of sensitivity and specificity values achievable as the threshold is changed from low to high scores, plotting the true-positive rate (sensitivity) on the vertical axis and the false-positive rate (1-specificity) on the horizontal axis. The area under the ROC curve is a quantitative indicator of the information content of a test and it may be interpreted as an estimate of the probability that a liver disease patient at random will, at each threshold, have a lower test score than a healthy participant.
Patients’ demographic and medical characteristics are shown in Table 1. Almost 61% of the sample were men and nearly half of the sample (n = 99, 48%) were aged 40-59 years with 33% (n = 67) aged ≥ 60 years old. Seventy four (36%) had a primary level of education, 41% (n = 85) a secondary level and 23% (n = 47) had higher education. The majority of the patients (70%) were married. Regarding the patients’ disease status, 23% were hospitalized while 47% were classified as having severe cirrhotic liver disease based on the Child-Pugh classification. The most common etiology for their liver disease was viral hepatitis (69.5%) and cirrhosis (18%). Table 1 also depicts the differences in the mean total score of CLDQ according to the patients’ characteristics. More specifically, older age, lower educational level and cirrhotic disease were found to be associated with lower CLDQ scores and therefore with lower QOL among patients.
| n (%) | Mean score | P-value | |
| Sex | |||
| Men | 125 (60.7) | 4.77 (1.26) | 0.61 |
| Women | 81 (39.3) | 4.85 (1.13) | |
| Age (yr) | |||
| ≤ 39 | 40 (19.4) | 5.19 (1.15) | 0.02 |
| 40-59 | 99 (48.1) | 4.92 (1.22) | |
| ≥ 60 | 67 (32.5) | 4.40 (1.13) | |
| Educational level | |||
| Primary | 74 (35.9) | 4.55 (1.19) | 0.03 |
| Secondary | 85 (41.3) | 4.84 (1.24) | |
| Higher | 47 (22.8) | 5.13 (1.09) | |
| Family status | |||
| Married | 144 (69.9) | 4.74 (1.22) | 0.27 |
| Non-married | 62 (30.1) | 4.94 (1.17) | |
| Child-Pugh classification | |||
| Class A | 49 (23.8) | 4.71 (1.06) | < 0.001 |
| Class B | 32 (15.5) | 3.82 (1.12) | |
| Class C | 16 (7.8) | 3.36 (0.88) | |
| No Cirrhosis | 109 (52.9) | 5.34 (0.95) | |
| Etiology of liver disease | |||
| Hepatitis B | 78 (37.9) | 5.07 (1.01) | < 0.001 |
| Hepatitis C | 65 (31.6) | 4.97 (1.22) | |
| Autoimmune hepatitis | 12 (5.8) | 4.72 (1.31) | |
| Cirrhosis | 36 (17.5) | 3.92 (1.22) | |
| Other | 15 (7.3) | 4.87 (1.10) |
The mean score of the CLDQ was 4.81 [standard deviation (SD) 2.01] and ranged from 3.17 to 6.41 (Table 2). The communalities for the Greek CLDQs are presented in Table 3. The internal consistency characteristics of the Greek CLDQ showed good reliability as the Cronbach’s alpha was 0.93 for the total scale (Items 1-29). The exploratory factor analysis on the 29 items of the CLDQ revealed 2 orthogonal d (KMO measure of sampling adequacy = 0.886 and Bartlett’s test of sphericity = 3422.25, df = 406, P < 0.001). Factor analysis indicated there are 7 principal domains in the model which explained 64.81% as presented in Table 4. The first domain (F1) included the following items: 2 (fatigue), 8 (decreased strength), 9 (trouble lifting heavy objects) and 11 (decreased level of energy) and this domain was named as “Fatigue”. The second domain (F2) was composed of items 18 (concern about the impact of liver disease on the family), 22 (concern that symptoms will develop into a major symptom), 25 (concern about the condition getting worse) and 28 (concern about never feeling any better). Therefore F2 represented “Concern”. The third factor (F3) included the following items: 1 (abdominal bloating), 5 (abdominal pain) and 17 (abdominal discomfort) and was named as “Abdominal symptoms”. The fourth domain (F4) included the following items: 4 (feeling sleepy during the day) and 13 (drowsiness) and we named this as “Activity”. The fifth factor (F5) was composed of items 12 (unhappiness) and 24 (feeling depressed). Therefore F5 represents “Emotional function”. The sixth domain (F6) was composed of items 20 (unable to fall asleep at night) and 16 (difficulty sleeping at night). Therefore F6 represents “Sleeping disorders”. The seventh domain (F7) was composed of items 10 (anxiety) and 15 (irritability). Therefore F7 represents “Anxiety”. Cronbach’s alpha for the 7 domains ranged from 0.67 (F7) to 0.93 (F2).
| CLDQ item | mean ± SD | |
| Q1 | Abdominal bloating | 5.08 ± 2.01 |
| Q2 | Tiredness or fatigue | 3.98 ± 2.14 |
| Q3 | Bodily pain | 5.22 ± 2.03 |
| Q4 | Sleepiness during the day | 4.96 ± 1.89 |
| Q5 | Abdominal pain | 5.67 ± 1.84 |
| Q6 | Shortness of breath | 5.42 ± 2.02 |
| Q7 | Not eating enough | 4.78 ± 2.35 |
| Q8 | Decreased strength | 4.50 ± 2.21 |
| Q9 | Trouble in carrying or lifting heavy objects | 4.59 ± 2.49 |
| Q10 | Anxiety | 3.40 ± 2.29 |
| Q11 | Decreased energy | 4.50 ± 2.25 |
| Q12 | Unhappiness | 4.67 ± 2.02 |
| Q13 | Drowsiness | 5.04 ± 1.87 |
| Q14 | Bothered by a limitation of the diet | 3.17 ± 2.50 |
| Q15 | Irritability | 4.33 ± 2.12 |
| Q16 | Difficulty in sleeping at night | 4.91 ± 2.15 |
| Q17 | Abdominal discomfort | 5.16 ± 2.05 |
| Q18 | Worries about the impact of the liver disease | 3.95 ± 2.22 |
| Q19 | Mood swings | 4.55 ± 2.01 |
| Q20 | Difficulty falling asleep at night | 5.07 ± 2.15 |
| Q21 | Muscle cramps | 5.50 ± 1.87 |
| Q22 | Worries that symptoms will develop into major problems | 4.21 ± 2.05 |
| Q23 | Dry mouth | 5.17 ± 2.06 |
| Q24 | Depression | 4.74 ± 2.04 |
| Q25 | Worries that the condition is getting worse | 4.28 ± 2.12 |
| Q26 | Problems | 5.75 ± 1.74 |
| Q27 | Itching | 5.78 ± 1.91 |
| Q28 | Worries about never feeling any better | 4.46 ± 2.22 |
| Q29 | Concerned about the availability of a liver in the case of a transplant | 6.41 ± 1.42 |
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Q17 | Q18 | Q19 | Q20 | Q21 | Q22 | Q23 | Q24 | Q25 | Q26 | Q27 | Q28 | Q29 | |
| Q1 | 1.00 | 0.45 | 0.42 | 0.22 | 0.45 | 0.37 | 0.29 | 0.35 | 0.34 | 0.30 | 0.41 | 0.20 | 0.18 | 0.21 | 0.21 | 0.31 | 0.59 | 0.26 | 0.23 | 0.36 | 0.16 | 0.22 | 0.28 | 0.20 | 0.29 | 0.25 | 0.36 | 0.31 | 0.16 |
| Q2 | 0.45 | 1.00 | 0.56 | 0.34 | 0.42 | 0.57 | 0.52 | 0.76 | 0.67 | 0.30 | 0.75 | 0.34 | 0.42 | 0.22 | 0.30 | 0.43 | 0.43 | 0.40 | 0.44 | 0.41 | 0.21 | 0.35 | 0.33 | 0.31 | 0.38 | 0.30 | 0.32 | 0.39 | 0.13 |
| Q3 | 0.42 | 0.56 | 1.00 | 0.39 | 0.37 | 0.33 | 0.28 | 0.50 | 0.41 | 0.29 | 0.52 | 0.37 | 0.42 | 0.12 | 0.18 | 0.32 | 0.39 | 0.24 | 0.38 | 0.34 | 0.21 | 0.12 | 0.37 | 0.30 | 0.18 | 0.20 | 0.24 | 0.18 | 0.10 |
| Q4 | 0.22 | 0.34 | 0.39 | 1.00 | 0.28 | 0.34 | 0.29 | 0.33 | 0.30 | 0.26 | 0.40 | 0.24 | 0.72 | 0.17 | 0.16 | 0.32 | 0.22 | 0.20 | 0.33 | 0.28 | 0.28 | 0.23 | 0.29 | 0.19 | 0.26 | 0.30 | 0.14 | 0.26 | 0.31 |
| Q5 | 0.45 | 0.42 | 0.37 | 0.28 | 1.00 | 0.41 | 0.32 | 0.40 | 0.36 | 0.18 | 0.43 | 0.18 | 0.26 | 0.24 | 0.17 | 0.35 | 0.50 | 0.34 | 0.24 | 0.28 | 0.14 | 0.20 | 0.27 | 0.17 | 0.26 | 0.17 | 0.23 | 0.22 | 0.22 |
| Q6 | 0.36 | 0.57 | 0.33 | 0.34 | 0.41 | 1.00 | 0.49 | 0.58 | 0.57 | 0.24 | 0.61 | 0.20 | 0.32 | 0.35 | 0.28 | 0.48 | 0.47 | 0.39 | 0.33 | 0.47 | 0.29 | 0.28 | 0.32 | 0.29 | 0.29 | 0.21 | 0.32 | 0.35 | 0.20 |
| Q7 | 0.29 | 0.52 | 0.28 | 0.29 | 0.32 | 0.49 | 1.00 | 0.54 | 0.50 | 0.36 | 0.60 | 0.38 | 0.28 | 0.28 | 0.34 | 0.40 | 0.39 | 0.41 | 0.40 | 0.39 | 0.31 | 0.36 | 0.19 | 0.36 | 0.39 | 0.26 | 0.27 | 0.39 | 0.12 |
| Q8 | 0.35 | 0.76 | 0.50 | 0.33 | 0.40 | 0.58 | 0.54 | 1.00 | 0.78 | 0.31 | 0.87 | 0.44 | 0.39 | 0.28 | 0.26 | 0.41 | 0.43 | 0.42 | 0.44 | 0.42 | 0.20 | 0.35 | 0.28 | 0.38 | 0.42 | 0.32 | 0.32 | 0.40 | 0.13 |
| Q9 | 0.34 | 0.67 | 0.41 | 0.30 | 0.36 | 0.57 | 0.50 | 0.78 | 1.00 | 0.28 | 0.77 | 0.32 | 0.35 | 0.32 | 0.18 | 0.39 | 0.48 | 0.30 | 0.37 | 0.38 | 0.19 | 0.24 | 0.32 | 0.21 | 0.31 | 0.23 | 0.36 | 0.32 | 0.09 |
| Q10 | 0.30 | 0.30 | 0.29 | 0.26 | 0.18 | 0.24 | 0.36 | 0.31 | 0.28 | 1.00 | 0.37 | 0.41 | 0.29 | 0.16 | 0.45 | 0.30 | 0.23 | 0.36 | 0.48 | 0.32 | 0.18 | 0.27 | 0.24 | 0.36 | 0.35 | 0.29 | 0.08 | 0.29 | 0.09 |
| Q11 | 0.41 | 0.75 | 0.52 | 0.40 | 0.43 | 0.61 | 0.60 | 0.87 | 0.77 | 0.37 | 1.00 | 0.39 | 0.40 | 0.31 | 0.31 | 0.42 | 0.43 | 0.42 | 0.47 | 0.43 | 0.26 | 0.38 | 0.29 | 0.36 | 0.42 | 0.33 | 0.32 | 0.40 | 0.13 |
| Q12 | 0.20 | 0.34 | 0.37 | 0.24 | 0.18 | 0.20 | 0.38 | 0.44 | 0.32 | 0.41 | 0.39 | 1.00 | 0.30 | 0.23 | 0.35 | 0.36 | 0.29 | 0.34 | 0.63 | 0.36 | 0.08 | 0.35 | 0.24 | 0.68 | 0.44 | 0.22 | 0.16 | 0.41 | 0.10 |
| Q13 | 0.18 | 0.42 | 0.42 | 0.72 | 0.26 | 0.32 | 0.28 | 0.39 | 0.35 | 0.30 | 0.40 | 0.30 | 1.00 | 0.10 | 0.15 | 0.30 | 0.28 | 0.23 | 0.36 | 0.34 | 0.28 | 0.15 | 0.32 | 0.26 | 0.25 | 0.40 | 0.20 | 0.25 | 0.18 |
| Q14 | 0.21 | 0.22 | 0.12 | 0.17 | 0.24 | 0.35 | 0.28 | 0.28 | 0.32 | 0.16 | 0.31 | 0.23 | 0.10 | 1.00 | 0.14 | 0.22 | 0.29 | 0.36 | 0.12 | 0.28 | 0.06 | 0.20 | 0.22 | 0.14 | 0.23 | 0.07 | 0.17 | 0.27 | 0.17 |
| Q15 | 0.21 | 0.30 | 0.18 | 0.16 | 0.17 | 0.28 | 0.34 | 0.29 | 0.18 | 0.45 | 0.31 | 0.35 | 0.15 | 0.14 | 1.00 | 0.34 | 0.20 | 0.46 | 0.43 | 0.35 | 0.13 | 0.33 | 0.04 | 0.32 | 0.37 | 0.21 | 0.12 | 0.33 | 0.13 |
| Q16 | 0.31 | 0.43 | 0.32 | 0.32 | 0.35 | 0.48 | 0.40 | 0.41 | 0.39 | 0.30 | 0.42 | 0.36 | 0.30 | 0.22 | 0.34 | 1.00 | 0.36 | 0.30 | 0.40 | 0.84 | 0.28 | 0.17 | 0.32 | 0.38 | 0.20 | 0.25 | 0.22 | 0.24 | 0.24 |
| Q17 | 0.59 | 0.43 | 0.39 | 0.22 | 0.50 | 0.47 | 0.39 | 0.43 | 0.48 | 0.23 | 0.43 | 0.29 | 0.28 | 0.29 | 0.20 | 0.36 | 1.00 | 0.36 | 0.31 | 0.38 | 0.22 | 0.28 | 0.39 | 0.29 | 0.36 | 0.19 | 0.37 | 0.35 | 0.12 |
| Q18 | 0.26 | 0.40 | 0.24 | 0.20 | 0.34 | 0.39 | 0.41 | 0.42 | 0.30 | 0.34 | 0.42 | 0.34 | 0.23 | 0.36 | 0.46 | 0.30 | 0.36 | 1.00 | 0.40 | 0.33 | 0.15 | 0.51 | 0.11 | 0.38 | 0.53 | 0.13 | 0.19 | 0.53 | 0.20 |
| Q19 | 0.23 | 0.44 | 0.38 | 0.33 | 0.24 | 0.33 | 0.40 | 0.44 | 0.37 | 0.48 | 0.47 | 0.63 | 0.36 | 0.12 | 0.43 | 0.40 | 0.31 | 0.40 | 1.00 | 0.42 | 0.14 | 0.36 | 0.27 | 0.60 | 0.45 | 0.20 | 0.24 | 0.45 | 0.15 |
| Q20 | 0.36 | 0.41 | 0.34 | 0.28 | 0.28 | 0.47 | 0.39 | 0.42 | 0.38 | 0.32 | 0.43 | 0.36 | 0.34 | 0.28 | 0.35 | 0.84 | 0.38 | 0.33 | 0.42 | 1.00 | 0.30 | 0.22 | 0.34 | 0.39 | 0.22 | 0.29 | 0.26 | 0.27 | 0.21 |
| Q21 | 0.16 | 0.21 | 0.21 | 0.28 | 0.14 | 0.29 | 0.31 | 0.20 | 0.19 | 0.18 | 0.26 | 0.08 | 0.28 | 0.06 | 0.13 | 0.28 | 0.22 | 0.15 | 0.14 | 0.30 | 1.00 | 0.14 | 0.25 | 0.21 | 0.15 | 0.15 | 0.23 | 0.13 | 0.18 |
| Q22 | 0.22 | 0.35 | 0.12 | 0.23 | 0.20 | 0.28 | 0.36 | 0.35 | 0.24 | 0.27 | 0.38 | 0.35 | 0.15 | 0.20 | 0.33 | 0.17 | 0.28 | 0.51 | 0.36 | 0.22 | 0.14 | 1.00 | 0.16 | 0.43 | 0.81 | 0.15 | 0.10 | 0.74 | 0.15 |
| Q23 | 0.27 | 0.33 | 0.37 | 0.29 | 0.27 | 0.32 | 0.19 | 0.28 | 0.32 | 0.24 | 0.29 | 0.24 | 0.32 | 0.22 | 0.04 | 0.32 | 0.39 | 0.11 | 0.27 | 0.34 | 0.25 | 0.16 | 1.00 | 0.26 | 0.15 | 0.28 | 0.32 | 0.14 | 0.17 |
| Q24 | 0.20 | 0.31 | 0.30 | 0.19 | 0.17 | 0.29 | 0.36 | 0.38 | 0.21 | 0.36 | 0.36 | 0.68 | 0.26 | 0.14 | 0.32 | 0.38 | 0.29 | 0.38 | 0.60 | 0.39 | 0.21 | 0.43 | 0.26 | 10.0 | 0.50 | 0.19 | 0.18 | 0.49 | 0.09 |
| Q25 | 0.29 | 0.38 | 0.18 | 0.26 | 0.25 | 0.29 | 0.39 | 0.42 | 0.31 | 0.35 | 0.42 | 0.44 | 0.25 | 0.23 | 0.37 | 0.20 | 0.36 | 0.53 | 0.45 | 0.22 | 0.15 | 0.81 | 0.15 | 0.50 | 1.00 | 0.15 | 0.11 | 0.86 | 0.08 |
| Q26 | 0.25 | 0.30 | 0.20 | 0.30 | 0.17 | 0.21 | 0.26 | 0.32 | 0.23 | 0.29 | 0.33 | 0.22 | 0.40 | 0.07 | 0.21 | 0.25 | 0.19 | 0.13 | 0.20 | 0.29 | 0.15 | 0.15 | 0.28 | 0.19 | 0.15 | 1.00 | 0.04 | 0.19 | 0.13 |
| Q27 | 0.36 | 0.32 | 0.24 | 0.14 | 0.23 | 0.32 | 0.27 | 0.32 | 0.36 | 0.08 | 0.32 | 0.16 | 0.20 | 0.17 | 0.12 | 0.22 | 0.37 | 0.19 | 0.24 | 0.26 | 0.23 | 0.10 | 0.32 | 0.18 | 0.11 | 0.04 | 1.00 | 0.12 | 0.17 |
| Q28 | 0.31 | 0.39 | 0.18 | 0.26 | 0.22 | 0.35 | 0.39 | 0.40 | 0.32 | 0.29 | 0.40 | 0.41 | 0.25 | 0.27 | 0.33 | 0.24 | 0.35 | 0.53 | 0.45 | 0.27 | 0.13 | 0.74 | 0.14 | 0.49 | 0.86 | 0.19 | 0.12 | 10.0 | 0.13 |
| Q29 | 0.16 | 0.13 | 0.10 | 0.31 | 0.22 | 0.20 | 0.12 | 0.13 | 0.09 | 0.09 | 0.13 | 0.10 | 0.18 | 0.17 | 0.13 | 0.24 | 0.12 | 0.20 | 0.15 | 0.21 | 0.18 | 0.15 | 0.17 | 0.09 | 0.08 | 0.13 | 0.19 | 0.13 | 1.00 |
| Factors | Rotation sums of squared loadings | |||||
| Rescaled loadings | Eigen values | % of variance | Cumulative variance | Cronbach’s alpha | ||
| Factor I | Question 2 | 0.72 | ||||
| Question 8 | 0.84 | |||||
| Question 9 | 0.84 | 4.08 | 14.07 | 14.07 | 0.91 | |
| Question 11 | 0.82 | |||||
| Question 18 | 0.55 | |||||
| Factor II | Question 22 | 0.85 | ||||
| Question 25 | 0.87 | 3.24 | 11.07 | 25.24 | 0.93 | |
| Question 28 | 0.85 | |||||
| Factor III | Question 1 | 0.79 | ||||
| Question 5 | 0.57 | 2.78 | 9.57 | 34.81 | 0.80 | |
| Question 17 | 0.74 | |||||
| Factor IV | Question 4 | 0.83 | 2.49 | 8.6 | 43.41 | 0.91 |
| Question 13 | 0.8 | |||||
| Factor V | Question 12 | 0.79 | 2.42 | 8.35 | 51.76 | 0.79 |
| Question 24 | 0.73 | |||||
| Factor VI | Question 16 | 0.79 | 2.11 | 7.27 | 59.03 | 0.84 |
| Question 20 | 0.77 | |||||
| Factor VII | Question 10 | 0.72 | 1.68 | 5.78 | 64.81 | 0.67 |
| Question 15 | 0.73 | |||||
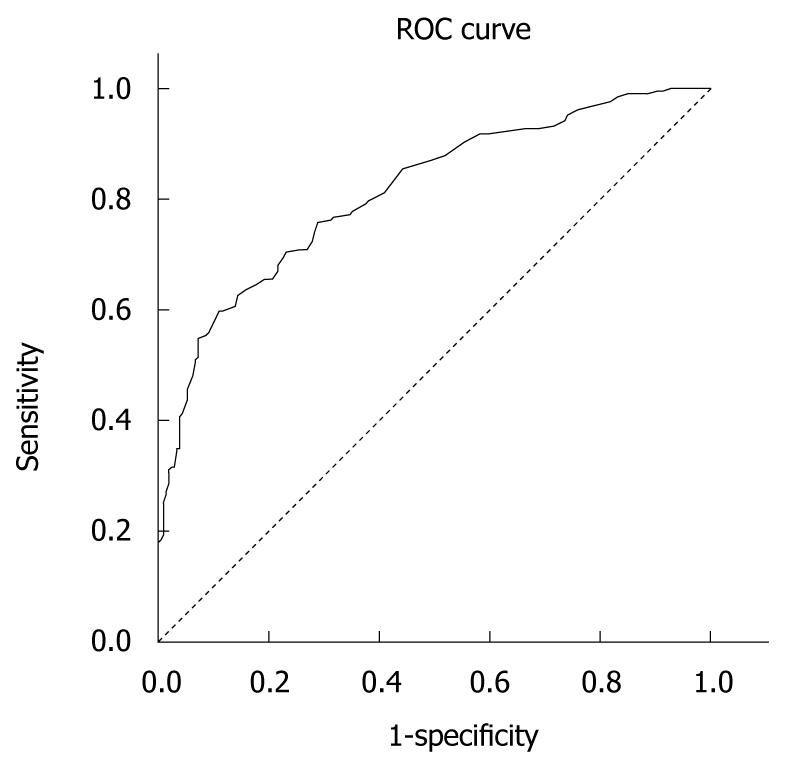
The Greek version of the CLDQ was well accepted by the patients. It was easily and very quickly (approximately 10 min) completed. The questions appeared to be relevant, reasonable, unambiguous and clear. Therefore, face validity was considered to be very good. The overall accuracy of the Greek CLDQ, as an instrument for assessing QOL among liver disease patients can be described as the area under its ROC curve calculated as 0.813 (SD = 0.021, Asymp. Sig < 0.0001). Table 5 presents the sensitivity and specificity values for the different cut-off values of the ROC analysis. A 167.50 cut-off score of the CLDQ provided the best sensitivity (74.3%) and specificity (71.6%). Figure 1 depicts the accuracy of the Greek CLDQ for assessing the level of QOL among patients with liver disease.
| Threshold scores | Sensitivity (%) | Specificity (%) |
| 147.50 | 57.8 | 99.0 |
| 157.50 | 64.6 | 82.2 |
| 167.50 | 74.3 | 71.6 |
| 175.50 | 85.4 | 55.8 |
| 185.50 | 92.2 | 37.0 |
| 194.50 | 98.5 | 16.8 |
CLDQ is a non-generic, disease-specific instrument for assessing QOL among liver disease patients. Our validation study provided a Cronbach’s alpha equal to 0.93 and the factor analysis identified 7 domains with Cronbach’s alpha ranging from 0.67 to 0.93 and included “Fatigue”, “Concern”, “Abdominal symptoms”, “Emotional function”, “Sleeping disorders”, “Anxiety” and “Activity”. Those domains explained 65% of the total variance. The ROC analysis presented the highest sensitivity and specificity at the overall score of 167.50, which can be considered as the cut-off score under which QOL can be assessed accurately.
CLDQ has already been validated in many countries including Spain, Italy, Germany, Lithuania and Thailand and has shown remarkable reproducibility and comparability[6-10]. The overall Cronbach’s alpha for the Greek CLDQ was found to be the same as those reported by the Spanish, Lithuanian and Thailand validation teams whereas the German validation study reported a Cronbach’s alpha ranging from 0.69 to 0.95 among different domains. The cumulative variance of the Greek validated questionnaire is similar to the Spanish (68%) and Italian (65.4%) versions.
In comparing the structure of the Greek CLDQ with those of other countries we identified both discrepancies and similarities. The factor analysis of the Greek questionnaire revealed significance in 18 out of the 29 items included in the original one. The Greek validated version of CLDQ revealed a 7 domain structure similar to the Spanish validated questionnaire as opposed to the 6 domains of the original U.S. version[5] and the Italian version[10]. Additionally, the Greek version did not include questions regarding systemic symptoms which are included in the original version and in the existing validated versions of the questionnaire (Italian and Spanish)[9,10]. Sleepiness and drowsiness constitute a new domain in the Greek validation which was named “Activity” where as in both the Spanish[9] and in the original validation[5] they are included under the domain “Fatigue” and in the Italian version[10] under the domain “Somatic symptoms”. “Anxiety” (including anxiety and irritability items) also consist of a separate domain in our validation whereas in the other validation studies these items are included in the domain of “Emotional symptoms”. The “Fatigue” domain included the definitions of “felt drowsy” and “felt sleepy during the day” in the original and Spanish version[9] whereas in the Greek version these items are included in a new domain which was named as “Sleeping disorders”. Only the factor comprising items that explored patient concern is common among the different versions.
The ROC analysis confirmed the effectiveness of CLDQ in assessing health-related QOL in the range of cut-off scores proposed. In our study, the high sensitivity (74.3%) of the 167.50 score allows the use of this cut-off score in the clinical assessment of QOL. If a health professional would like to use the Greek CLDQ for QOL assessment then these different cut-offs should be used. It is very important for the CLDQ to be used as a diagnostic tool in clinical practice which may allow health care professionals to understand the impact of health care interventions on the patient’s everyday life, rather than the effects of treatment on their bodies[16]. Additional, the Greek CLDQ’s use provides a basis for the holistic view of the patient and therefore may help facilitate a dialogue with patients with low QOL.
In general, this is the first study to validate the Greek CLDQ which is recommended to be incorporated into research and clinical practice to allow international comparison of the results of separate national studies[4]. An important strength of our study is that this is the first study to perform a ROC analysis which provided us with a cut-off score for assessing QOL accurately among patients with liver disease. Our findings also revealed a different structure of the questionnaire after the factor analysis which underlines the necessity of cultural validation and adaptation of the questionnaire before its use in specific countries. A limitation of this validation study was that there was no test-retest, because it may have resulted in a low correlation due to an actual change in the QOL symptoms. Additionally, the high percentage of patients with a cirrhotic liver disease might have affected the CLDQ score as the complications associated with cirrhosis, such as hepatic encephalopathy, have been shown to negatively influence physical and mental domains of QOL[17]. On the other hand, the exclusion of the patients with hepatic encephalopathy of grade II or more might have reduced this error. In addition, the ethnic and cultural background of the patients may have had an effect on the score of CLDQ whereas previous studies have reported no differences in the CLDQ score among different ethnic groups[8,17].
The Greek version of the CLDQ has shown a satisfactory reliability and the factor analysis indicated 7 factors that were of interest. We can therefore assert that it is a reliable and valid tool for identifying QOL among liver disease patients and it can be used by health professionals in their clinical practice to improve assessment of patients with low scores. Our findings, however, need to be confirmed by future cross-sectional and cohort studies.
Chronic liver disease questionnaire (CLDQ) is a disease specific instrument for assessing quality of life (QOL) among liver disease patients. In the last few decades, the assessment of QOL related to chronic liver disease has become an important outcome measure in clinical research.
The CLDQ has already been cross-culturally adapted and validated into different languages. In the current study, the authors aimed to translate and validate the Greek version of the CLDQ.
The authors’ findings revealed a different structure of the questionnaire after the factor analysis, which underlines the necessity of cultural validation and adaptation of the questionnaire before its use in specific countries. This is the first study to validate the Greek CLDQ which therefore should be incorporated into research and clinical practice so as to allow international comparison of the results of separate national studies. An important strength of the study is that this is the first study to perform a receiver operating characteristics (ROC) analysis which provided us with a cut-off score for accurately assessing QOL among patients with liver disease.
The Greek version of the CLDQ has shown a satisfactory reliability. The authors can therefore assert that it is a reliable and valid tool for identifying QOL among liver disease patients and it can be used by health professionals in their clinical practice to improve assessment of patients with low scores.
ROC curve: a graphical plot of the sensitivity, or true positives, vs (1-specificity), or false positives, for a binary classification system as its discrimination threshold is varied.
Dr. Zoi Kollia and colleagues validated the Greek version of the CLDQ to assess QOL factors for patients with chronic liver diseases. The establishment of QOL measures or indices is very important to assess what physical or mental status a patient is in and to compare multinational patients. This study provides an important clue to assess QOL of patients with chronic liver diseases.
Peer reviewers: Akihito Tsubota, Assistant Professor, Institute of Clinical Medicine and Research, Jikei University School of Medicine, 163-1 Kashiwa-shita, Kashiwa, Chiba 277-8567, Japan; Assy Nimer, MD, Assistant Professor, Liver Unit, Ziv Medical Centre, Box 1008, Safed 13100, Israel
S- Editor Wang JL L- Editor Cant MR E- Editor Lin YP
| 1. | Glise H, Wilklund I. Health-related quality of life and gastrointestinal disease. J Gastroenterol Hepatol. 2002;17 Suppl:S72-S84. [Cited in This Article: ] |
| 2. | Simpson KJ, Finlayson ND. Clinical evaluation of liver disease. Baillieres Clin Gastroenterol. 1995;9:639-659. [Cited in This Article: ] |
| 3. | Yacavone RF, Locke GR 3rd, Provenzale DT, Eisen GM. Quality of life measurement in gastroenterology: what is available? Am J Gastroenterol. 2001;96:285-297. [Cited in This Article: ] |
| 4. | Cirrincione R, Taliani G, Caporaso N, Mele A. Quality of life assessment in chronic liver disease. Hepatogastroenterology. 2002;49:813-816. [Cited in This Article: ] |
| 5. | Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45:295-300. [Cited in This Article: ] |
| 6. | Sobhonslidsuk A, Silpakit C, Kongsakon R, Satitpornkul P, Sripetch C. Chronic liver disease questionnaire: translation and validation in Thais. World J Gastroenterol. 2004;10:1954-1957. [Cited in This Article: ] |
| 7. | Sumskiene J, Sumskas L, Petrauskas D, Kupcinskas L. Disease-specific health-related quality of life and its determinants in liver cirrhosis patients in Lithuania. World J Gastroenterol. 2006;12:7792-7797. [Cited in This Article: ] |
| 8. | Häuser W, Schnur M, Steder-Neukamm U, Muthny FA, Grandt D. Validation of the German version of the Chronic Liver Disease Questionnaire. Eur J Gastroenterol Hepatol. 2004;16:599-606. [Cited in This Article: ] |
| 9. | Ferrer M, Córdoba J, Garin O, Olivé G, Flavià M, Vargas V, Esteban R, Alonso J. Validity of the Spanish version of the Chronic Liver Disease Questionnaire (CLDQ) as a standard outcome for quality of life assessment. Liver Transpl. 2006;12:95-104. [Cited in This Article: ] |
| 10. | Rucci P, Taliani G, Cirrincione L, Alberti A, Bartolozzi D, Caporaso N, Colombo M, Coppola R, Chiaramonte M, Craxi A. Validity and reliability of the Italian version of the Chronic Liver Disease Questionnaire (CLDQ-I) for the assessment of health-related quality of life. Dig Liver Dis. 2005;37:850-860. [Cited in This Article: ] |
| 11. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [Cited in This Article: ] |
| 12. | Tabachnick B, Fidell L. Using multivariate statistics. 3rd ed. NY: Addison- Wesley- Longman 2007; . [Cited in This Article: ] |
| 13. | Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20:141-151. [Cited in This Article: ] |
| 14. | Hakstian AR, Rogers WT, Cattell RB. The behaviour of numbers factors rules with simulated data. Multivariate Behav Res. 1982;17:193-219. [Cited in This Article: ] |
| 15. | Morrison DF. Multivariate statistical methods. 2nd edition. New York: McGraw-Hill 1976; . [Cited in This Article: ] |
| 16. | Addington-Hall J, Kalra L. Who should measure quality of life? BMJ. 2001;322:1417-1420. [Cited in This Article: ] |
| 17. | Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci. 2003;48:1622-1626. [Cited in This Article: ] |