Published online Nov 7, 2010. doi: 10.3748/wjg.v16.i41.5247
Revised: July 2, 2010
Accepted: July 9, 2010
Published online: November 7, 2010
AIM: To describe a new surgical technique and evaluate the early results of segmental gastrectomy (SG) with modified D2 lymph node (LN) dissection for early gastric cancer (EGC).
METHODS: Fourteen patients with EGC underwent SG with modified D2 dissection from 2006 to 2008. Their operative results and postoperative courses were compared with those of 17 patients who had distal gastrectomy (DG) for EGC during the same period.
RESULTS: Operating time, blood loss, and hospital stay were similar between the 2 groups. Postoperative complications developed significantly more frequently in the DG group than in the SG group. Mean number of dissected LNs per each station in the SG group was comparable with that in the DG group. Postoperative recovery of body weight was significantly better in the SG group than in the DG group. The incidence of reflux esophagitis and gastritis after surgery was less frequent in the SG group than in the DG group.
CONCLUSION: SG with modified D2 LN dissection may be a new function-preserving gastrectomy that is feasible for treatment of EGC with possible LN involvement.
- Citation: Matsuda T, Kaneda K, Takamatsu M, Aishin K, Awazu M, Okamoto A, Kawaguchi K. Segmental gastrectomy with radical lymph node dissection for early gastric cancer. World J Gastroenterol 2010; 16(41): 5247-5251
- URL: https://www.wjgnet.com/1007-9327/full/v16/i41/5247.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i41.5247
Less invasive surgery for early gastric cancer (EGC) has been more commonly employed in Japan[1-4]. Segmental gastrectomy (SG), which was originally applied for peptic ulcers, is one type of limited gastrectomy available for treating EGC[5]. SG for EGC is generally accompanied only by dissection of the lymph nodes (LNs) adjacent to tumors, although only a few studies regarding the application of SG in EGC have been reported[1-4]. To establish SG with adjacent LN dissection as a valid option for surgical treatment of EGC, it is necessary to demonstrate intraoperatively that there is no lymph node metastasis (N0). However, at present, it is difficult to determine the status of LN metastasis intraoperatively, even using the sentinel node (SN) navigation method[6-8].
The superiority of limited gastrectomy over conventional D2 gastrectomy for EGC in terms of postoperative quality of life seems apparent, as previously reported[1,4,9,10]. In 1995, Sawai et al[11] developed pylorus-preserving gastrectomy (PPG) with D2 dissection by preservation of the infrapyloric artery as a function-preserving gastrectomy. Although this technique is considered to be applicable to submucosal or undifferentiated gastric cancer, there remains a risk of adverse events such as postoperative gastric stasis and reduced capacity of the remnant stomach.
We developed SG with modified D2 dissection and have applied it to EGC that do not meet endoscopic treatment criteria. We report the technique here and evaluate the early results of SG with modified D2 dissection for EGC.
Between April 2006 and November 2008, 35 patients with EGC in the middle or lower third of the stomach which did not meet endoscopic treatment criteria underwent gastrectomy at our institution: 17 patients who underwent SG and 18 patients who underwent conventional distal gastrectomy (DG) with D2 dissection. The criteria for endoscopic treatment is EGC with differentiated histology, invasion limited to the mucosal layer, a diameter smaller than 2 cm, and no ulcer findings. In this study, SG was distinguished from PPG by defining that the remaining pyloric cuff needed to be more than 4 cm in SG. When the distance from the distal edge of the resection to the pylorus was < 4 cm, DG was performed instead of PPG.
A total of 14 patients received SG with modified D2 dissection. The remaining 3 patients who were excluded from this study received SG only with adjacent LN dissection because of intraoperative determination of apparent mucosal cancer with no LN metastasis.
In 3 out of 18 patients in the DG group, SG was converted to DG based on intraoperative findings including suspected T2 tumor or positive LN metastasis.
Patients were followed every 3 mo after surgery. The follow-ups also included an endoscopy and computed tomography 1 year after surgery. The operative and postoperative results were compared between the SG and DG groups.
To make it possible to locate the tumor intraoperatively, proximal and distal margins of the tumor were marked by endoscopic clipping before surgery. After laparotomy, we explored the abdominal cavity and determined if the preoperative diagnosis was correct. If a T2 or T3 tumor was suspected or LN metastases were apparent, DG or total gastrectomy (TG) with D2 dissection was performed. Swollen and/or hard palpable LNs located adjacent to the tumor were excised and immediately examined by frozen section analysis. Infrapyloric LNs (station 6) and those along the common hepatic artery (CHA; station 8a) were also routinely subjected to frozen section analysis. DG or TG with D2 dissection was performed if LN metastasis was proved by frozen section analysis, however, if T1N0 was confirmed SG with modified D2 dissection was carried out.
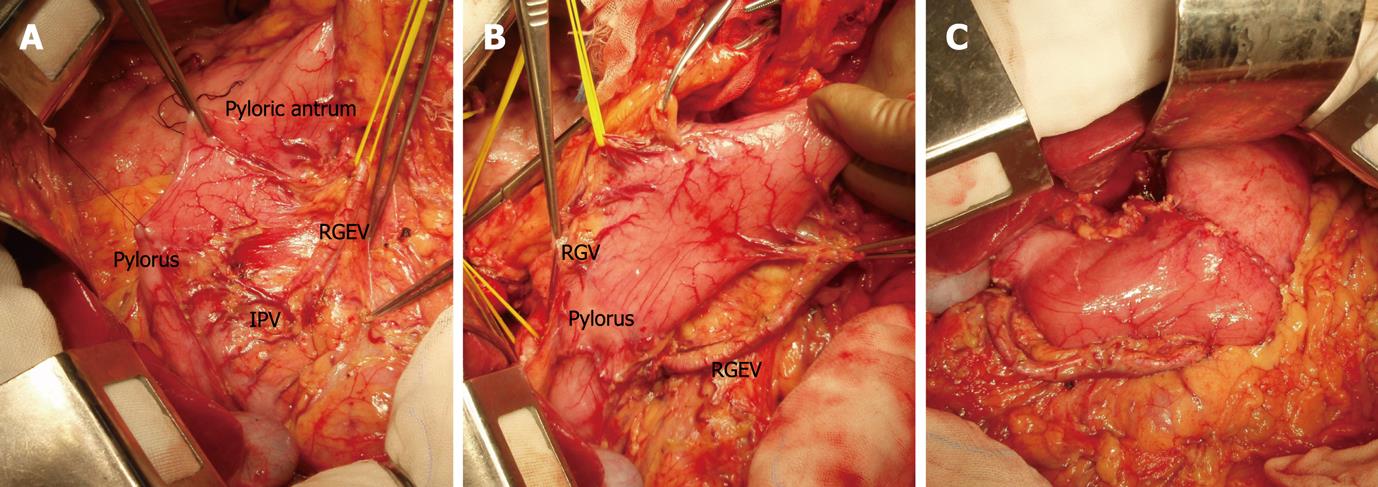
The resection line of the stomach was made 2 cm and 5 cm away from the tumor margin with differentiated and undifferentiated histology, respectively. The greater omentum was preserved, and the gastrocolic ligament corresponding to the resection area of the stomach was dissected, after separation, at least 3 cm away from the gastroepiploic vessels. For dissection of LNs located in station 6 and along the greater curvature (station 4d), right gastroepiploic vessels were skeletonized, allowing excision of adjacent LNs, and divided at the distal resection line of the stomach (Figure 1A). The suprapyloric LNs (station 5) were dissected in the same manner. The right gastric artery was skeletonized and divided distally to the second or third branch (Figure 1B). The left halves of LNs along the proper hepatic artery (station 12a) were removed, and the hepatic and pyloric branches of vagus nerves were preserved. Right paracardial LNs (station 1), LNs along the lesser curvature (station 3), and the left gastric artery (LGA) (station 7) were dissected by dividing the LGA at its origin. The celiac branch of the vagus nerve was also dissected. Dissection of the upper portion of station 1 LNs was occasionally omitted in order to preserve the origin of the hepatic branch of the vagus nerve and the anterior branch of the Latarjet nerve. LNs along the CHA (station 8a, 8p), around the celiac axis (station 9), and along the proximal splenic artery (station 11p) were routinely dissected. LNs along the left gastroepiploic artery (LGEA) (station 4sb) were dissected by dividing the last few branches of the LGEA. After transection of the stomach, the surgical margins were subjected to intraoperative histological examination by frozen section analysis. Reconstruction was performed using hand-sewn end-to-end anastomosis (Figure 1C).
Statistical analysis was performed using either the χ2 test or Student’s t-test. A P-value < 0.05 was considered significant.
There were no differences between the 2 groups in terms of sex, age, and tumor characteristics, with the exception of tumor location (Table 1). In 1 patient of the DG group and 2 patients of the SG group, LN metastasis was proven to be positive.
| SG (n = 14) | DG (n = 18) | P | |
| Age (yr, mean ± SD) | 63.9 ± 9.6 | 60.0 ± 12.9 | 0.204 |
| Sex (M/F) | 9/5 | 7/11 | 0.154 |
| Location of tumor | 0.002 | ||
| Upper | 0 | 0 | |
| Middle | 13 | 7 | |
| Lower third of the stomach | 1 | 11 | |
| Depth of invasion | 0.589 | ||
| Mucosa | 4 | 8 | |
| Submucosa | 8 | 9 | |
| Muscularis propria | 1 | 1 | |
| Subserosa | 1 | 0 | |
| Histological type | |||
| Differentiated | 10 | 11 | 0.358 |
| Undifferentiated | 4 | 7 | |
| Lymph node metastasis | 0.401 | ||
| No | 12 | 17 | |
| Yes | 2 | 1 |
Operative data are shown in Table 2. Operating time, blood loss, and hospital stay were similar between the two groups. However, postoperative complications developed significantly more frequently in the DG group than in the SG group. There were no instances of anastomotic leakage, hemorrhage, gastric stasis, wound infection or operative death in either group.
| SG (n = 14) | DG (n = 18) | P | |
| Operating time (min) | 273 ± 65 | 262 ± 46 | 0.589 |
| Blood loss (mL) | 206 ± 124 | 179 ± 101 | 0.511 |
| Hospital stay (d) | 14.9 ± 2.6 | 16.7 ± 5.0 | 0.227 |
| Complications | 0 | 5 | 0.032 |
| Wound infection | 0 | 0 | |
| Anastomotic leakage | 0 | 0 | |
| Hemorrhage | 0 | 0 | |
| Cholecystitis | 0 | 2 | |
| Pancreatitis | 0 | 1 | |
| Pancreatic fistula | 0 | 1 | |
| Adhesive ileus | 0 | 1 | |
| Operative death | 0 | 0 | 1.000 |
The mean number of dissected LNs per station was comparable between the 2 groups (Table 3).
| Lymph node station | SG (n = 14) | DG (n = 18) | P |
| Right paracardial (1) | 2.0 ± 1.3 | 3.1 ± 1.2 | 0.068 |
| Left paracardial (2) | 1.1 ± 1.5 | 0.7 ± 1.6 | 0.462 |
| Along the lesser curvature (3) | 8.7 ± 4.7 | 10.8 ± 7.3 | 0.362 |
| Along the LGEA (4sb) | 1.2 ± 2.1 | 1.4 ± 1.9 | 0.754 |
| Along the RGEA (4d) | 9.2 ± 4.9 | 11.7 ± 4.9 | 0.155 |
| Suprapyloric (5) | 0.3 ± 0.6 | 0.6 ± 0.9 | 0.286 |
| Infrapyloric (6) | 3.7 ± 3.7 | 5.6 ± 2.6 | 0.118 |
| Along the LGA (7) | 3.7 ± 2.4 | 4.3 ± 2.7 | 0.473 |
| Along the CHA (8) | 2.7 ± 1.2 | 3.3 ± 1.5 | 0.232 |
| Along the celiac axis (9) | 2.8 ± 1.6 | 3.3 ± 2.0 | 0.442 |
| Along the proximal SA (11p) | 1.5 ± 1.5 | 2.0 ± 1.7 | 0.412 |
| Along the PHA (12a) | 0.5 ± 1.0 | 0.2 ± 0.5 | 0.246 |
| Along the SMV (14v) | - | 1.2 ± 2.1 | - |
The postoperative course is summarized in Table 4. Postoperative recovery of body weight was significantly better in the SG group than in the DG group. The incidence of reflux esophagitis and gastritis after surgery was less frequent in the SG group than in the DG group.
| SG (n = 14) | DG (n = 18) | P | |
| Gastric stasis | 0 | 0 | 1.000 |
| Dumping syndrome | 1 | 1 | 0.854 |
| Body weight change ratio (%) | 96.8 ± 6.1 | 90.4 ± 7.0 | 0.012 |
| Laboratory data | |||
| Lymphocytes (/mm3) | 2007 ± 437 | 1977 ± 646 | 0.885 |
| Total protein (g/dL) | 6.8 ± 0.3 | 7.0 ± 0.3 | 0.156 |
| Total cholesterol (mg/dL) | 215 ± 34 | 192 ± 32 | 0.063 |
| Endoscopic examination | |||
| Reflux gastritis | 1 | 5 | 0.138 |
| Reflux esophagitis | 0 | 4 | 0.059 |
No recurrence or death was observed in either group during a median follow-up period of 32.8 mo. One patient in the SG group developed colon cancer during the follow-up period and subsequently underwent curative resection.
SG has not been previously used to treat EGC, since it was first reported by Wangensteen et al[5] as an operation for peptic ulcer. In 1999, Ohwada et al[2] modified the procedure and reported its use for treatment of EGC of the middle third of the stomach. However, previous studies concerning the use of SG for the treatment of EGC are limited, primarily due to difficulty in diagnosing LN metastasis accurately during surgery, and it being performed with very limited LN dissection. Deterioration of the radicality caused by omitting or limiting LN dissection should be avoided, because EGC can be treated with conventional D2 gastrectomy with excellent prognosis. Therefore, SG with limited LN dissection is not recommended unless T1N0 is confirmed.
On the other hand, a method for accurate intraoperative diagnosis of LN metastasis has yet to be established. Recently, SN biopsy has been used to assist intraoperative diagnosis of LN metastasis during surgical treatment of EGC. The SN concept seems feasible for treatment of EGC as described previously, however, several important issues including optimal tracer, method of injection, and false negative cases, still need to be resolved[6-8].
Our modified D2 dissection was considered to be comparable with conventional D2 dissection, because dissected stations in our SG corresponded with those in conventional D2 dissection. The mean number of dissected LNs of station 1 tended to be smaller in the SG group than in the DG group. This will be because dissection of the upper half of station 1 was occasionally omitted in order to preserve the anterior branches of the vagus nerve along the lesser curvature of the proximal remnant stomach, which may help maintain postoperative motility of this portion. The influence of limited dissection of station 1 on the oncological outcome is unclear, however, tumor recurrence resulting from incomplete dissection of station 1 has not been seen so far. Even in 3 patients whose LN metastasis was positive in the DG or SG group, no recurrence has been observed. In 1 of 2 patients whose LN metastasis was positive in the SG group, positive LN metastasis was proved after surgery in station 7 which belongs to the second tier. In the other 2 patients, LN metastasis was positive in station 3. These results may support the quality and effectiveness of our modified D2 dissection and the impact of D2 dissection.
In our procedures, 2-5 LNs were subjected to frozen section analysis. Station 8a was routinely examined by frozen section analysis. The frequency of LN metastasis in station 8a is relatively high even in EGC, and stations 7, 8a, and 11p are sometimes SN stations although they belong to the second tier[6,8,12-14]. Once the tumor advances to stage T2 or T3, lymphatic vessels can be obstructed by cancerous invasion, and metastasis in distant LNs can occur even in the absence of involvement of LNs adjacent to tumors. In the case where station 7 LN was proved positive after SG, intraoperative frozen section analysis detected no metastasis of stations 3 and 8a. Therefore, it is not recommended to limit LN dissection even if frozen section analysis reveals no LN involvement. Frozen section analysis with LN sampling was performed by us in order to rule out patients with positive LN metastasis rather than to confirm N0.
Unexpectedly, postoperative gastric stasis has not been observed in our patient population thus far. The precise mechanisms of development of gastric stasis after limited gastrectomy such as PPG remain unclear[15-18]. The main causes of gastric stasis are generally thought to include damage to the vagus nerve, insufficient blood supply to the pyloric region, or tonic and phasic contractions of the pylorus. In our procedure, not only the blood supply but also the venous drainage of the pyloric region is more sufficient than in PPG. These findings may have contributed to good maintenance of pyloric motility.
The present study demonstrated the safety and effectiveness of our procedures. This procedure could be a new function-preserving gastrectomy that is feasible for treatment of EGC with possible LN involvement. However, further studies including a larger number of patients and longer follow-up periods are essential for a more definitive conclusion.
Less invasive surgery for early gastric cancer (EGC) has been more commonly employed based on the background of an increase in EGC. Although it has been reported that pylorus-preserving gastrectomy can be performed with D2 dissection, there has been no report on segmental gastrectomy with D2 dissection.
A sentinel node navigation method has been developed for accurate intraoperative determination of lymph node (LN) metastasis during surgery for EGC.
This is the first report to demonstrate that segmental gastrectomy can be performed with D2 dissection for treatment of EGC.
The superiority of function-preserving gastrectomy over conventional D2 gastrectomy for EGC in terms of postoperative quality of life is apparent. However, limited LN dissection accompanying function-preserving gastrectomy may reduce the radicality of gastrectomy for EGC. This procedure may be applicable to EGC with possible LN involvement.
Segmental gastrectomy is one of the limited gastrectomies in which the central one third portion of the stomach is resected. The sentinel node is generally defined as the lymph node which receives the lymphatic flow first from the tumor.
This is an interesting small series and I think it has value. I would like more information concerning the pathological outcomes before accepting the conclusions and suggestions in the discussion.
Peer reviewer: Robert V Rege, MD, Department of Surgery, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, Texas, TX 75390-9031, United States
S- Editor Tian L L- Editor Cant MR E- Editor Lin YP
| 1. | Furukawa H, Hiratsuka M, Imaoka S, Ishikawa O, Kabuto T, Sasaki Y, Kameyama M, Ohigashi H, Nakano H, Yasuda T. Phase II study of limited surgery for early gastric cancer: segmental gastric resection. Ann Surg Oncol. 1999;6:166-170. [Cited in This Article: ] |
| 2. | Ohwada S, Nakamura S, Ogawa T, Izumi M, Tanahashi Y, Sato Y, Ikeya T, Iino Y, Morishita Y. Segmental gastrectomy for early cancer in the mid-stomach. Hepatogastroenterology. 1999;1229-1233. [Cited in This Article: ] |
| 3. | Shinohara T, Ohyama S, Muto T, Kato Y, Yanaga K, Yamaguchi T. Clinical outcome of high segmental gastrectomy for early gastric cancer in the upper third of the stomach. Br J Surg. 2006;93:975-980. [Cited in This Article: ] |
| 4. | Ishikawa K, Arita T, Ninomiya S, Bandoh T, Shiraishi N, Kitano S. Outcome of segmental gastrectomy versus distal gastrectomy for early gastric cancer. World J Surg. 2007;31:2204-2207. [Cited in This Article: ] |
| 5. | Wangensteen OH. Segmental gastric resection for peptic ulcer; method permitting restoration of anatomic continuity. J Am Med Assoc. 1952;149:18-23. [Cited in This Article: ] |
| 6. | Ichikura T, Chochi K, Sugasawa H, Yaguchi Y, Sakamoto N, Takahata R, Kosuda S, Mochizuki H. Individualized surgery for early gastric cancer guided by sentinel node biopsy. Surgery. 2006;139:501-507. [Cited in This Article: ] |
| 7. | Tajima Y, Yamazaki K, Masuda Y, Kato M, Yasuda D, Aoki T, Kato T, Murakami M, Miwa M, Kusano M. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg. 2009;249:58-62. [Cited in This Article: ] |
| 8. | Ichikura T, Sugasawa H, Sakamoto N, Yaguchi Y, Tsujimoto H, Ono S. Limited gastrectomy with dissection of sentinel node stations for early gastric cancer with negative sentinel node biopsy. Ann Surg. 2009;249:942-947. [Cited in This Article: ] |
| 9. | Shimoyama S, Seto Y, Yasuda H, Mafune K, Kaminishi M. Concepts, rationale, and current outcomes of less invasive surgical strategies for early gastric cancer: data from a quarter-century of experience in a single institution. World J Surg. 2005;29:58-65. [Cited in This Article: ] |
| 10. | Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 2008;95:1131-1135. [Cited in This Article: ] |
| 11. | Sawai K, Takahashi T, Fujioka T, Minato H, Taniguchi H, Yamaguchi T. Pylorus-preserving gastrectomy with radical lymph node dissection based on anatomical variations of the infrapyloric artery. Am J Surg. 1995;170:285-288. [Cited in This Article: ] |
| 12. | Xu YY, Huang BJ, Sun Z, Lu C, Liu YP. Risk factors for lymph node metastasis and evaluation of reasonable surgery for early gastric cancer. World J Gastroenterol. 2007;13:5133-5138. [Cited in This Article: ] |
| 13. | Kojima N, Yonemura Y, Bando E, Morimoto K, Kawamura T, Yun HY, Ito I, Kameya T, Hayashi I. Optimal extent of lymph node dissection for T1 gastric cancer, with special reference to the distribution of micrometastasis, and accuracy of preoperative diagnosis for wall invasion. Hepatogastroenterology. 2008;55:1112-1117. [Cited in This Article: ] |
| 14. | Tokunaga M, Ohyama S, Hiki N, Fukunaga T, Yamada K, Sano T, Yamaguchi T. Investigation of the lymphatic stream of the stomach in gastric cancer with solitary lymph node metastasis. World J Surg. 2009;33:1235-1239. [Cited in This Article: ] |
| 15. | Ohwada S, Sato Y, Oriuchi N, Nakamura S, Tanahashi Y, Izumi M, Ogawa T, Takeyoshi I, Ikeya T, Iino Y. Gastric emptying after segmental gastrectomy for early cancer in the middle part of the stomach. Hepatogastroenterology. 1999;46:2081-2085. [Cited in This Article: ] |
| 16. | Nakabayashi T, Mochiki E, Garcia M, Haga N, Suzuki T, Asao T, Kuwano H. Pyloric motility after pylorus-preserving gastrectomy with or without the pyloric branch of the vagus nerve. World J Surg. 2002;26:577-583. [Cited in This Article: ] |
| 17. | Nunobe S, Hiki N, Fukunaga T, Tokunaga M, Ohyama S, Seto Y, Yamaguchi T. Laparoscopy-assisted pylorus-preserving gastrectomy: preservation of vagus nerve and infrapyloric blood flow induces less stasis. World J Surg. 2007;31:2335-2340. [Cited in This Article: ] |
| 18. | Tomita R. Gastric emptying function in patients 5 years after pylorus-preserving distal gastrectomy with or without preserving pyloric and hepatic branches of the vagal nerve for early gastric cancer. World J Surg. 2009;33:2119-2126. [Cited in This Article: ] |