Published online Sep 14, 2010. doi: 10.3748/wjg.v16.i34.4272
Revised: May 25, 2010
Accepted: June 1, 2010
Published online: September 14, 2010
AIM: To study the beneficial effects of triterpene α,β-amyrin and the underlying mechanisms in an experimental pancreatitis model.
METHODS: Acute pancreatitis was induced in five groups of rats (n = 8) by L-arginine (2 × 2.5 g/kg, intraperitoneal, 1 h apart) and 1 h later, they received a single oral dose of α,β-amyrin (10, 30 and 100 mg/kg), methylprednisolone (30 mg/kg) and vehicle (3% Tween 80). A saline (0.9% NaCl) treated group served as a normal control. Efficacy was assessed at 24 h by determination of serum levels of amylase, lipase and pro-inflammatory cytokines [tumor necrosis factor (TNF)-α and interleukin (IL)-6], pancreatic myeloperoxidase (MPO) activity, lipid peroxidation [thiobarbituric acid reactive substances (TBARS)], nitrate/nitrite levels, and the wet weight/body weight ratio. Tissue histology and the immunoreactivity for TNF-α and inducible nitric oxide synthetase (iNOS) were performed.
RESULTS: α,β-amyrin and methylprednisolone treatments significantly (P < 0.05) attenuated the L-arginine-induced increases in pancreatic wet weight/body weight ratio, and decreased the serum levels of amylase and lipase, and TNF-α and IL-6, as compared to the vehicle control. Also, pancreatic levels of MPO activity, TBARS, and nitrate/nitrite were significantly lower. Histological findings and TNF-α and iNOS immunostaining further confirmed the amelioration of pancreatic injury by α,β-amyrin.
CONCLUSION: α,β-amyrin has the potential to combat acute pancreatitis by acting as an anti-inflammatory and antioxidant agent.
- Citation: Melo CM, Carvalho KMMB, Neves JCS, Morais TC, Rao VS, Santos FA, Brito GAC, Chaves MH. α,β-amyrin, a natural triterpenoid ameliorates L-arginine-induced acute pancreatitis in rats. World J Gastroenterol 2010; 16(34): 4272-4280
- URL: https://www.wjgnet.com/1007-9327/full/v16/i34/4272.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i34.4272
Acute pancreatitis (AP) is a life-threatening inflammatory disorder with a significant impact on patient health. Although its pathogenesis is not fully understood, microcirculatory disturbances, leukocyte activation, and oxidative stress are the main events in AP that is characterized by activation of digestive proteases, a widespread inflammatory cell infiltration, leukocyte activation and the release of various kinds of inflammatory mediators and reactive oxygen and nitrogen species[1-4]. Repeated attacks of acute pancreatitis have the potential to evolve into chronic disease that is characterized by fibrosis and loss of pancreatic function[5]. There are no specific therapies for acute pancreatitis. Medical management is aimed at the control of symptoms with anti-inflammatory agents, steroids, and analgesics. As a result of the limitations of conventional therapy, many ethnobotanical agents are being pursued as alternative sources to develop novel and safe therapeutic agents to treat pancreatitis[6-11].
The resin (oily amorphous exudate) obtained from the trunk wood of Protium heptaphyllum (P. heptaphyllum) (Aubl.) March (Burseraceae) that grows abundantly in Brazil and South America is a reputed folk medicinal agent because of its analgesic and anti-inflammatory properties[12,13]. Chemical investigations have revealed the presence of α,β-amyrin, a pentacyclic triterpene as the major component of resin, and pharmacological studies have revealed its anti-inflammatory, antipruritic, gastroprotective and hepatoprotective effects[14-17]. Also, a few other studies have shown its efficacy in suppressing the acute visceral and orofacial nociception and bladder inflammation[17,18]. Recently, Vitor et al[19] have demonstrated that α,β-amyrin exerts a marked and rapid suppression of inflammatory cytokines and cyclooxygenase-2 levels in a murine model of trinitro-benzene-sulfonic acid (TNBS)-induced colitis. Since these studies have established the anti-inflammatory, antinociceptive, and antioxidant properties of α,β-amyrin at non-toxic doses, which range from 10 to 100 mg/kg, the present study evaluated its potential to ameliorate pancreatic injury in a rat model of acute pancreatitis induced by L-arginine, wherein inflammation and oxidative stress play a pathogenic role.
The resinous exudate from the trunk wood of P. heptaphyllum (March.) was collected from the municipal areas of Timon, Maranhão state of Brazil, after its identification by botanist Professor Roseli Farias de Melo Barros. A voucher sample (#18247) has been deposited at the Herbarium Graziela Barroso of the Federal University of Piauí, Teresina, Brazil. The extraction and isolation of α,β-amyrin from the crude resin of P. heptaphyllum (March.) was carried out as described earlier[20] and its structural identity was confirmed by 1H- and 13C-NMR spectral analysis, based on the method developed by Gallegos et al[21] and in comparison to literature data[22]. The ratio of α- and β-amyrin in this mixture was 63:37.
Forty-eight male Wistar rats obtained from the Central Animal House of Federal University of Ceara, Fortaleza were maintained at a constant room temperature (23 ± 2°C) with light-dark cycles of 12/12 h and free access to water and standard laboratory chow. The rats were randomly divided into six groups of eight in each and experiments were performed after 12 h of fasting. Their body weights ranged between 180 and 200 g at the time of experimentation.
Experimental protocols were approved by the Institutional Committee on Care and Use of Animals for experimentation (No. 84/08) in accordance with the guidelines of the National Institutes of Health, Bethesda, MD, USA.
L-arginine, hexadecyltrimethylammonium bromide (HETAB), o-dianisidine dihydrochloride, thiobarbituric acid and methylprednisolone were from Sigma Chemical Co. (St Louis, MO, USA). All other chemicals and reagents were of the highest commercial grade available.
Acute pancreatitis was induced in five groups of rats (n = 8/group) by two intraperitoneal (ip) injections of L-arginine (2.5 g/kg, 1 h apart)[23]. One hour following the last injection of L-arginine, the rats were treated orally as follows: group 1 received the vehicle (3% Tween 80) of α,β-amyrin (vehicle control); groups 2, 3 and 4 were treated with α,β-amyrin (10, 30 and 100 mg/kg, respectively); and group 5 acted as positive control and received methylprednisolone (30 mg/kg), all in a volume of 10 mL/kg.
A sixth group (n = 8) of rats that received saline (0.9%, NaCl, ip) in place of L-arginine served as a normal control. Twenty-four hours after the last injection of L-arginine or saline, a midline laparotomy was performed in rats under ketamine anesthesia and blood samples were collected from the inferior vena cava, the rats were then exsanguinated, the whole pancreas was quickly removed and stored at -70°C until use. The pancreatic weight/body weight ratio was evaluated as an estimate of the degree of pancreatic edema (mg/g)[24].
For serum assays, blood samples were centrifuged at 3000 ×g at 4°C for 10 min. The serum amylase and lipase were determined by routine colorimetric methods using the commercial kits for amylase (Labtest Diagnostica SA, Lagoa Santa, Brazil) and lipase (Bioclin-Quibasa, Belo Horizonte, Minas Gerais, Brazil) and expressed as U/dL and U/L, respectively. Serum tumor necrosis factor (TNF)-α and interleukin (IL)-6 were measured using an ELISA kit according to the manufacturer’s instructions (Quantikine®; R&D Systems, Minneapolis, MN, USA). The cytokine levels were calculated from the standard curve and expressed as pg/mL.
The degree of neutrophil infiltration was quantified by the measurement of pancreatic myeloperoxidase (MPO) activity[25]. Pancreatic tissue (50 mg) was minced and homogenized in 0.5 mL of 50 mmol/L phosphate buffer solution (PBS) (pH 6) that contained 0.5% HETAB. The homogenate was subjected to three cycles of freezing (-30°C) and thawing (37°C) and brief periods (15 s) of sonication, after which, they were centrifuged at 12 000 ×g for 15 min at 4°C. The supernatant (0.1 mL) was mixed with 2.9 mL of 50 mmol/L PBS, pH 6, which contained 0.167 mg/mL o-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. The change in absorbance at 470 nm was then measured for 5 min using a Beckman spectrophotometer (Beckman DU 640B; CA, USA).
Thiobarbituric acid-reactive substances (TBARS) level in the pancreatic tissue was determined as an indicator of lipid peroxidation according to a previously described method[26]. Briefly, 500 μL of 10% tissue homogenate in 0.15 mol/L KCl was mixed with 200 μL 8.1% SDS, and then incubated at room temperature for 5 min. The reaction mixture was heated at 95°C for 1 h after the addition of 1.5 mL 20% acetic acid (pH 3.5) and 1.5 mL 0.8% thiobarbituric acid. After the mixture had cooled, 1.0 mL distilled water and 5.0 mL butanol/pyridine (15:1) solution were added under agitation using a vortex. This solution was centrifuged at 1000 ×g for 15 min, and the resultant colored layer was measured at 532 nm using a Beckman DU 640B spectrophotometer.
Total nitrate/nitrite levels were determined as a measure of nitric oxide with the use of Griess reagent[27]. The pancreatic tissue was homogenized in 50 mmol/L potassium phosphate buffer (pH 7.8) and centrifuged at 11 000 ×g for 15 min at 4°C. One hundred microliters of the supernatant was mixed with 100 μL Griess reagent [0.1% N-(1-naphthyl) ethylenediamide dihydrochloride, 1% sulfanilamide in 5% phosphoric acid], and after 10 min, the absorbance was measured at 540 nm using a Beckman DU 640B spectrophotometer. The standard curve was obtained by using sodium nitrite. The results were calculated from a standard curve by using sodium nitrite and expressed as micromoles of nitrate/nitrite.
Samples of pancreatic tissue were fixed in 10% buffered formalin solution, embedded in paraffin by standard methods, cut into 5-μm sections, stained with hematoxylin-eosin, and then assessed under light microscopy and examined blind by a morphologist for grading the histological alterations. Pancreatic edema, leukocyte infiltration, hemorrhage, acinar vacuolization and necrosis were described with scores ranging from 0 to 3 as described previously[28].
Immunohistochemical analysis of the expression of TNF-α and inducible nitric oxide synthase (iNOS) was performed. Sections of pancreas (4 μm) were transferred to a gelatin-coated slide. The tissue sections were deparaffinized, and endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide (30 min). Nonspecific protein binding was blocked by incubating the tissue sections with goat serum (0.5% in PBS for 45 min). The slides were then incubated overnight with primary rabbit anti-TNF-α or rabbit anti-iNOS (Sigma), diluted 1:400 in PBS plus bovine serum albumin. For TNF-α, the slides were incubated with avidin-biotin-horseradish peroxidase conjugate (Vectastain® ABC kit; Vector Laboratories, Burlingame, CA, USA) for 30 min, and TNF-α was visualized with the chromogen 3,3’ diaminobenzidine and counterstained with Harry’s hematoxylin. For iNOS, the slides were incubated with alkaline-phosphatase-conjugated secondary antibody (EnVisionTM/AP, K1396; Dako, Carpinteria, CA, USA). The reaction was developed by applying on the slides a solution containing levamisole and Fast Red Substrate (EnVisionTM/AP, K1396; Dako).
Statistical analysis was performed by analysis of variance followed by Kruskal-Wallis or Student Newman Keul’s as post-hoc tests using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA). The non-parametric data are expressed as median (with low and high ranges), and parametric data, expressed as mean ± SE. Differences were considered to be statistically significant when P was < 0.05.
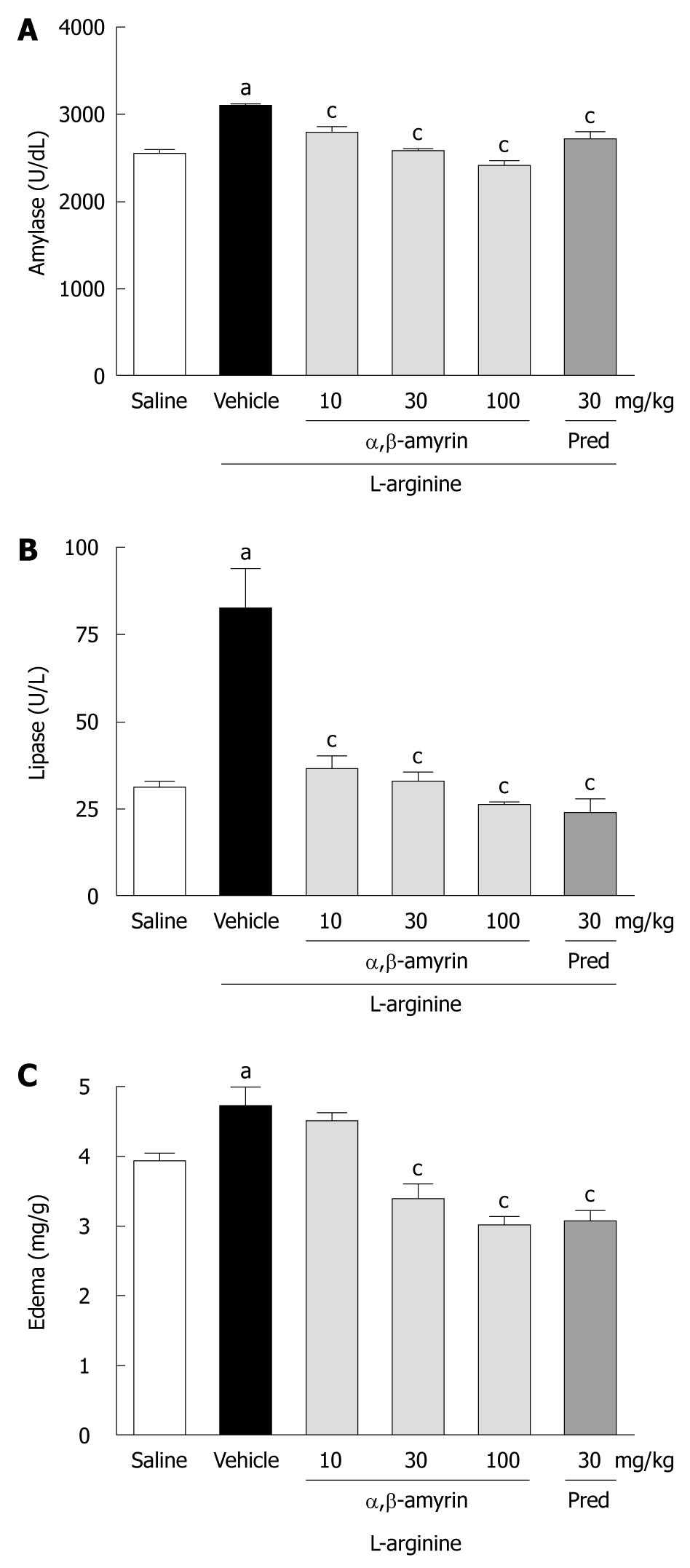
Figure 1 shows the levels of serum amylase and lipase activities and the pancreatic edema in rats under different treatments. When compared to the saline-treated group, the levels of serum amylase and lipase were significantly (P < 0.05) higher in the L-arginine-induced acute pancreatitis group. However, L-arginine caused a much higher increase in lipase as compared to amylase. Besides, L-arginine markedly increased the pancreatic wet weight/body weight ratio, an index of pancreatic edema. While treatment with α,β-amyrin (10, 30 and 100 mg/kg) significantly (P < 0.05) lowered the L-arginine-induced elevation of serum amylase and lipase (Figure 1A and B) at all doses, a significant decrease in pancreatic edema was observed only at 30 and 100 mg/kg (Figure 1C). Methylprednisolone (30 mg/kg), the reference anti-inflammatory drug included in the study, manifested similar reductions in serum amylase and lipase activities, as well as in pancreatic edema (Figure 1).
Figure 2 depicts the serum levels of pro-inflammatory cytokines TNF-α and IL-6 in rats under different treatments. Both TNF-α and IL-6 were significantly elevated in the L-arginine-induced pancreatitis control group when compared to the saline group. Treatment with α,β-amyrin (10, 30 and 100 mg/kg) effectively decreased the enhanced levels of TNF-α at all doses, however, IL-6 inhibition was statistically significant only at 30 and 100 mg/kg of α,β-amyrin.
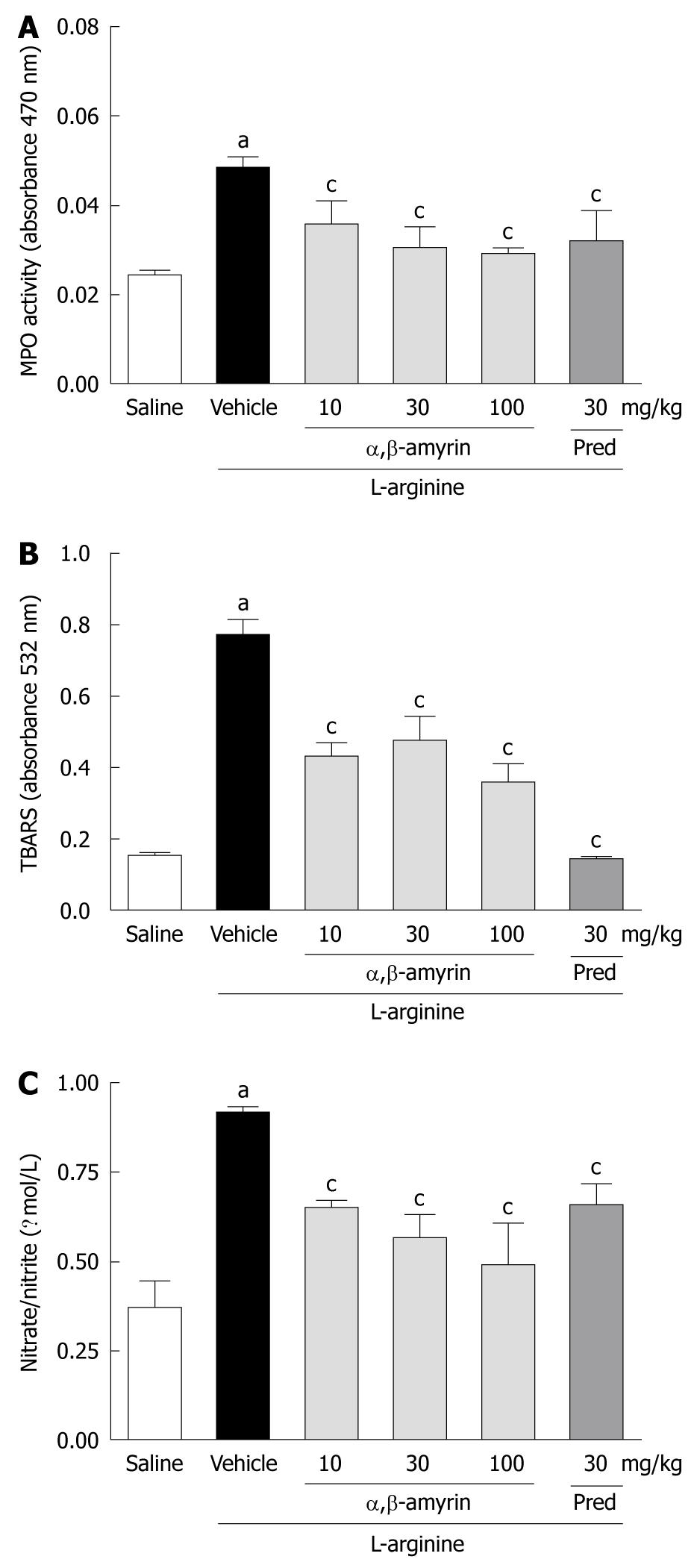
In the L-arginine-induced acute pancreatitis group, pancreatic MPO activity, TBARS, and nitrate/nitrite levels were significantly elevated when compared to the saline-treated group (Figure 3A-C). Treatment with α,β-amyrin (10, 30 and 100 mg/kg) and methylprednisolone significantly (P < 0.05) reduced the L-arginine-evoked increase in pancreatic MPO activity, TBARS and nitrate/nitrite levels.
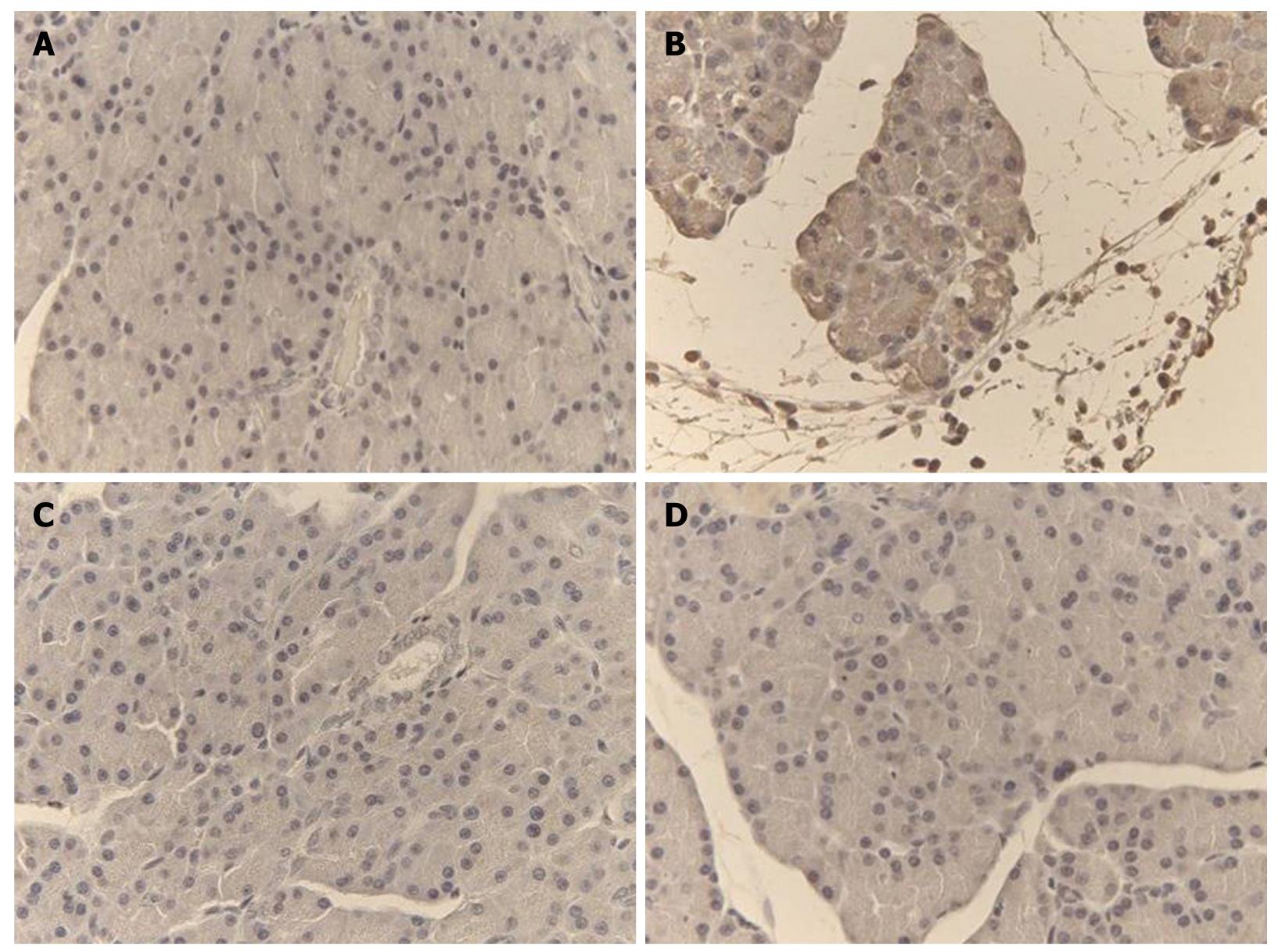
Representative TNF-α and iNOS immunostaining of the pancreas for different treatments are shown in Figures 4 and 5. In saline-treated control rats, the pattern of TNF-α and iNOS staining was very mild (Figures 4A and 5A). On the other hand, there was a high intensity staining for TNF-α and iNOS in the acinar cells, inflammatory cells and blood vessels of the pancreas in the L-arginine-induced acute pancreatitis group that received only the vehicle (Figures 4B and 5B), however, in rats treated with α,β-amyrin (100 mg/kg) or methylprednisolone (30 mg/kg), immunostaining intensity for TNF-α and iNOS was much less (Figures 4C, D, and 5C, D).
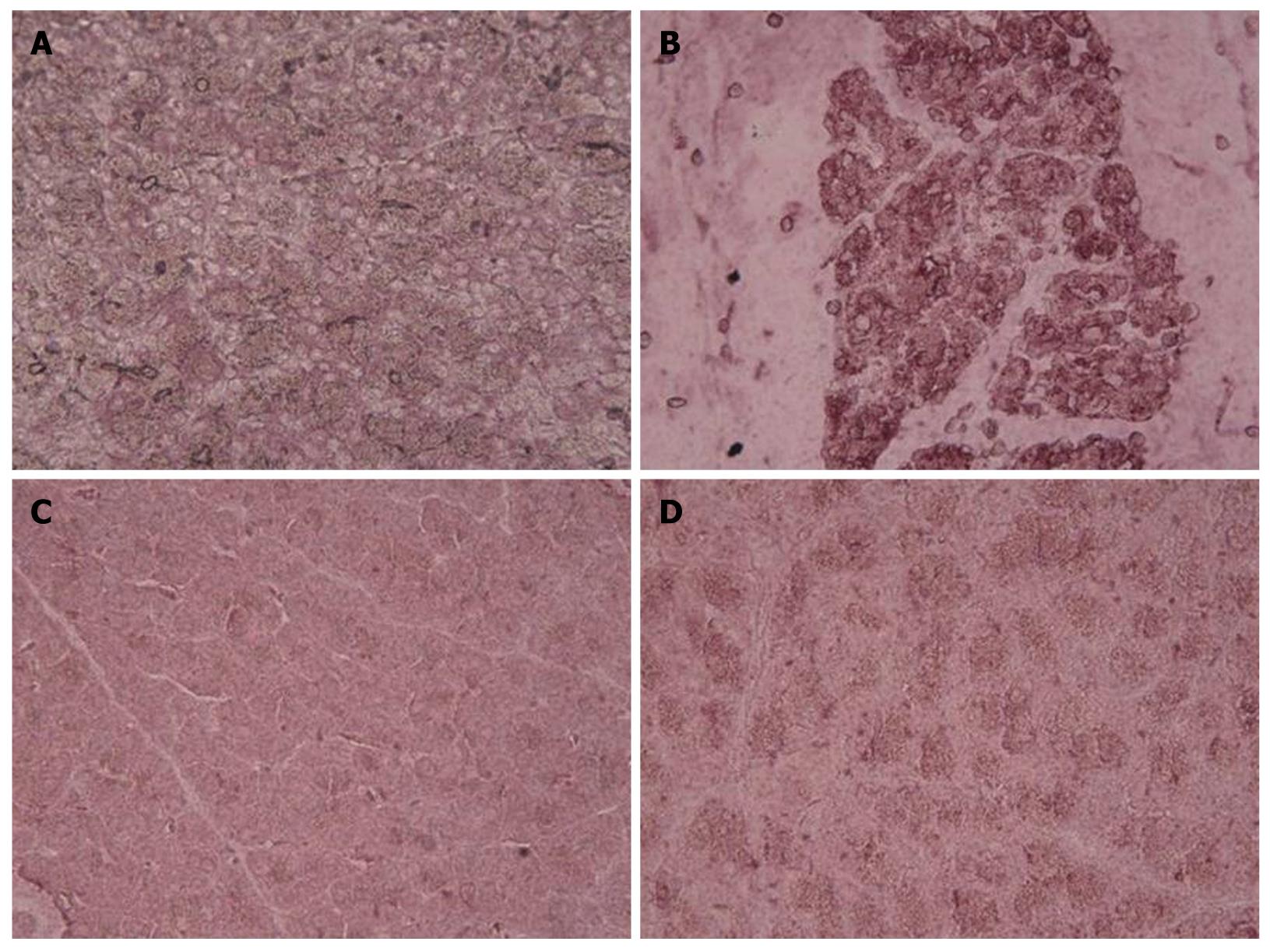
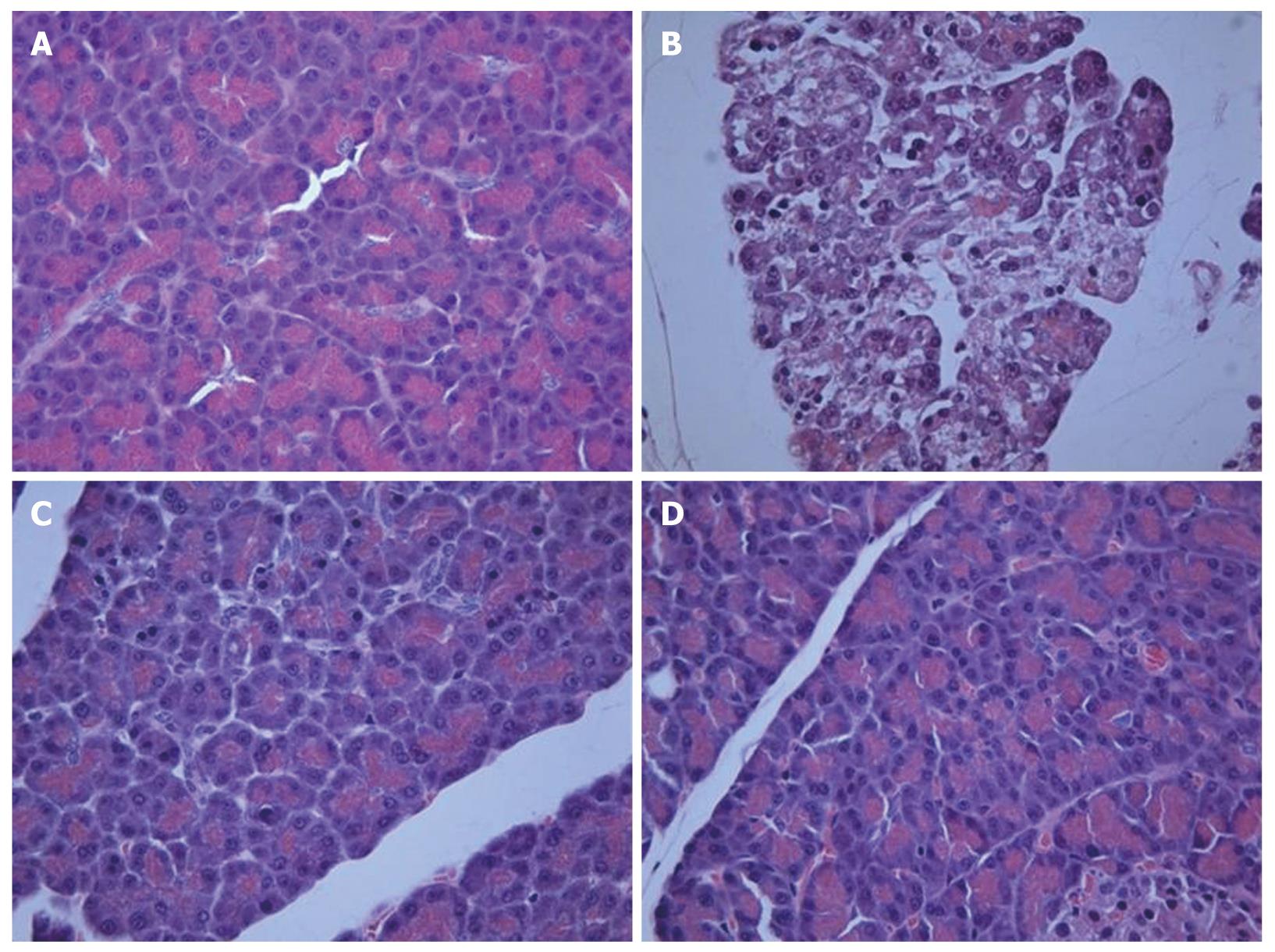
Histological examination of the saline-treated controls showed normal architecture and absence of edema, leukocyte infiltration, acinar vacuolization, hemorrhage and necrosis (Figure 6A and Table 1). In contrast, pancreatic sections from the L-arginine-induced acute pancreatitis group of rats revealed extensive tissue damage that was characterized by significant disruption of normal architecture, with massive edema, acinar cell vacuolization, necrosis, hemorrhage and inflammatory cell infiltration, and thus received significantly higher scores (Figure 6B and Table 1). Treatment with α,β-amyrin (100 mg/kg) and methylprednisolone (30 mg/kg) resolved the inflammation, and most strikingly the edema, and protected the pancreas from histological damage induced by L-arginine. In addition, the total pathological scores were significantly decreased by α,β-amyrin treatment (Figure 6C and D, Table 1).
| Group | Edema (0-3) | Inflammatory infiltration (0-3) | Acinar vacuolization (0-3) | Hemorrhage (0-3) | Necrosis (0-3) |
| Saline control | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
| Vehicle + L-arginine | 3 (2-3)a | 2 (2-3)a | 3 (3-3)a | 2 (2-3)a | 3 (3-3)a |
| α,β-amyrin (100 mg/kg) + L-arginine | 0 (0-0)c | 0 (0-0)c | 0 (0-1)c | 0 (0-0)c | 0 (0-0)c |
| Pred + L-arginine | 0 (0-1)c | 0 (0-0)c | 1 (0-1)c | 0 (0-1)c | 0 (0-0)c |
Our study demonstrated that the natural triterpene α,β-amyrin has the potential to attenuate the severity of L-arginine-induced pancreatitis in rats. In agreement with previous studies[29,30], we found significant increases in serum amylase and lipase levels, neutrophil infiltration, massive edema, necrosis and hemorrhage in this experimental model of pancreatitis. Besides, we noticed an increase in the serum levels of pro-inflammatory cytokines TNF-α and IL-6, and a higher expression of TNF-α and iNOS in pancreatic tissue, consistent with earlier reports that have described similar increases in experimental pancreatitis and in clinical patients as well[23,31,32]. TNF-α plays a pivotal role in severe acute pancreatitis, acting early in the disease course[33], and IL-6 constitutes the principal mediator in the synthesis of acute-phase proteins, in addition to transitioning the acute inflammatory response to a chronic response[34]. These findings suggest that inflammatory cytokines and neutrophil-mediated oxidative stress have a central role in the pathogenesis of acute pancreatitis induced by L-arginine, and it also implies that compounds that combat inflammation and oxidative stress ameliorate acute pancreatitis. Treatment with triterpene α,β-amyrin and glucocorticoid methylprednisolone resulted in a significant decrease of serum amylase and lipase, pancreatic edema, and serum TNF-α and IL-6 levels, as well as the TNF-α and iNOS expression induced by L-arginine. Both drugs effectively improved the pancreatic morphology, and these results clearly point out their anti-inflammatory potential in obliterating the pancreatic inflammation and the associated tissue injury.
The glucocorticoids are useful for the treatment of a wide range of inflammatory and autoimmune conditions[35]. The effect of α,β-amyrin treatment is the same as that of glucocorticoid methylprednisolone that has been previously shown to be effective in the L-arginine model of experimental pancreatitis[36]. Since α,β-amyrin belongs to the group of ursane and oleanane pentacyclic triterpenes that have a chemical structure that resembles glucocorticoids, it might exercise similar anti-inflammatory effects by affecting the transcription of inflammatory mediators[37].
α,β-amyrin therapy significantly reduces the extent of edema and the total pathological score, possibly due to its anti-inflammatory action[17-19]. NO produced via activity of NOS is one of the factors that is involved in the regulation of the rate of perfusion of the pancreatic microvessels[38]. These authors have suggested that excess arginine can induce iNOS activity, which results in high tissue levels of NO that might cause a direct toxic effect on pancreatic acinar cells. The ability of NO to increase the vascular/microcapillary permeability might contribute to the occurrence of pancreatic edema. Total nitrate/nitrite, a marker of endogenous NO, was markedly increased by L-arginine treatment, and was found to be significantly reduced in rats pretreated with α,β-amyrin (100 mg/kg). Furthermore, immunohistochemical staining for iNOS showed that α,β-amyrin could inhibit their expression. It implies that inhibition of NO also participates in the protective effect of triterpenoid.
In the present study, possibly, α,β-amyrin inhibited neutrophil infiltration, TNF-α and IL-6 production, and iNOS expression in pancreatic tissue, probably via inhibition of nuclear factor (NF)-κB activity. In this context, recent studies have shown that α,β-amyrin can inhibit NF-κB activation and thereby the production of inflammatory mediators[19]. Oxidative stress plays an important role in the pathophysiology of acute pancreatitis and the beneficial effects of α,β-amyrin might also be associated with the inhibition of NF-κB activity. Excessive reactive oxygen and nitrogen species produced by NOS and isoforms of NADPH oxidase, or as by-products of the mitochondrial electron-transport chain, have been implicated in the pathogenesis of acute pancreatitis[4]. α,β-amyrin potently suppressed neutrophil-mediated MPO and lipoperoxidation, as demonstrated by reduced TBARS formation; events that reflect its antioxidant action. Thus, the present study reveals that α,β-amyrin ameliorates acute pancreatitis by suppressing pro-inflammatory cytokines TNF-α and IL-6, and iNOS expression.
In conclusion, the study provides the first evidence to show that α,β-amyrin attenuates the development of L-arginine-induced acute pancreatitis by reducing the infiltration of neutrophils, generation of inflammatory cytokines and iNOS. This study might provide a basis for future investigations of the therapeutic role of α,β-amyrin in severe necrotizing pancreatitis.
The growing incidence of acute pancreatitis in developing and developed countries has a significant impact on the healthcare system. As a result of the limitations of conventional therapy, many ethnobotanical agents are being pursued as alternative sources to develop novel and safe therapeutic agents for acute pancreatitis. The present experimental study investigated α,β-amyrin, a natural triterpenoid from Protium heptaphyllum as a treatment option for acute pancreatitis.
α,β-amyrin exhibits anti-inflammatory and antioxidant effects. Both inflammation and oxidant stress play a pathogenic role in a rat model of acute pancreatitis induced by L-arginine. α,β-amyrin (100 mg/kg) effectively ameliorates L-arginine-associated pancreatic injury through inhibition of neutrophil infiltration, tumor necrosis factor α and interleukin (IL)-6 production, and inducible nitric oxide synthase expression in pancreatic tissue, probably via inhibition of nuclear factor-κB activity.
This is believed to be the first study that has demonstrated the beneficial effect of α,β-amyrin treatment in a rat model of L-arginine-induced acute pancreatitis. Inhibition of pro-inflammatory cytokine IL-6 by α,β-amyrin might arrest the transition of acute pancreatitis to a chronic state, and thus, progression to the more severe form of pancreatitis that is characterized by fibrosis and loss of pancreatic function.
In this study, acute pancreatic injury caused by L-arginine was ameliorated by α,β-amyrin treatment. This could represent a basis for future investigations on the therapeutic role of α,β-amyrin in severe necrotizing pancreatitis. Clinical studies have suggested that prophylactic administration of anti-inflammatory drugs is useful in preventing pancreatitis in patients undergoing therapeutic endoscopic retrograde cholangiopancreatography (ERCP). α,β-amyrin is an anti-inflammatory and antioxidant agent, therefore, it could also serve as a prophylactic agent in the prevention of ERCP. However, more in-depth experimental studies are warranted to support our observations of its beneficial effects.
α,β-amyrin, is a pentacyclic triterpene that is isolated from the resin of the traditional medicinal plant, Protium heptaphyllum. α,β-amyrin has anti-inflammatory, antinociceptive, gastroprotective and hepatoprotective properties and is non-toxic at the doses employed in this study.
The article describes the beneficial effects of two natural triterpenoids, α and β-amyrin on experimental acute pancreatitis induced by L-arginine in rats. The results presented are very clear, and the authors have been able to demonstrate that these triterpenoids produce anti-inflammatory, antinociceptive, and antioxidant properties at doses that range from 10 to 100 mg/kg, which are non-toxic.
Peer reviewers: Giamila Fantuzzi, PhD, Associate Professor, Department of Kinesiology and Nutrition, University of Illinois at Chicago, 1919 W Taylor Street MC 517, Chicago, IL 60613, United States; José Julián Calvo Andrés, Department of Physiology and Pharmacology, University of Salamanca, Edificio Departamento, Plaza de los Doctores de la Reina, Campus Miguel de Unamuno, 37007 Salamanca, Spain
S- Editor Wang YR L- Editor Kerr C E- Editor Zheng XM
| 1. | Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132-144. [Cited in This Article: ] |
| 2. | Al Mofleh IA. Severe acute pancreatitis: pathogenetic aspects and prognostic factors. World J Gastroenterol. 2008;14:675-684. [Cited in This Article: ] |
| 3. | Regnér S, Manjer J, Appelros S, Hjalmarsson C, Sadic J, Borgström A. Protease activation, pancreatic leakage, and inflammation in acute pancreatitis: differences between mild and severe cases and changes over the first three days. Pancreatology. 2008;8:600-607. [Cited in This Article: ] |
| 4. | Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal. 2009;11:135-165. [Cited in This Article: ] |
| 5. | Vonlaufen A, Wilson JS, Apte MV. Molecular mechanisms of pancreatitis: current opinion. J Gastroenterol Hepatol. 2008;23:1339-1348. [Cited in This Article: ] |
| 6. | Hahm KB, Kim JH, You BM, Kim YS, Cho SW, Yim H, Ahn BO, Kim WB. Induction of apoptosis with an extract of Artemisia asiatica attenuates the severity of cerulein-induced pancreatitis in rats. Pancreas. 1998;17:153-157. [Cited in This Article: ] |
| 7. | Chen XR, Lu JB. The latest research advance in the pharmacological action of Salvia miltiorriza. Chin J Hosp Pharma. 2001;21:44. [Cited in This Article: ] |
| 8. | Zeybek N, Gorgulu S, Yagci G, Serdar M, Simsek A, Kaymakcioglu N, Deveci S, Ozcelik H, Tufan T. The effects of gingko biloba extract (EGb 761) on experimental acute pancreatitis. J Surg Res. 2003;115:286-293. [Cited in This Article: ] |
| 9. | Genovese T, Mazzon E, Di Paola R, Muià C, Crisafulli C, Menegazzi M, Malleo G, Suzuki H, Cuzzocrea S. Hypericum perforatum attenuates the development of cerulein-induced acute pancreatitis in mice. Shock. 2006;25:161-167. [Cited in This Article: ] |
| 10. | Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;133:1056.e1-1056.e25. [Cited in This Article: ] |
| 11. | Jung WS, Chae YS, Kim DY, Seo SW, Park HJ, Bae GS, Kim TH, Oh HJ, Yun KJ, Park RK. Gardenia jasminoides protects against cerulein-induced acute pancreatitis. World J Gastroenterol. 2008;14:6188-6194. [Cited in This Article: ] |
| 12. | Matos FJA. O Formulário fitoterápico do Professor Dias Rocha: informações sobre o emprego da medicina caseira, de plantas do Nordeste, especialmente do Ceará. 2nd ed. Fortaleza: Imprensa Universitária UFC 1997; . [Cited in This Article: ] |
| 13. | Amorozo MCM. Use and diversity of medicinal plants in Santo Antonio do Leverger. Acta Bot Bras. 2002;16:189-203. [Cited in This Article: ] |
| 14. | Oliveira FA, Lima-Junior RC, Cordeiro WM, Vieira-Júnior GM, Chaves MH, Almeida FR, Silva RM, Santos FA, Rao VS. Pentacyclic triterpenoids, alpha,beta-amyrins, suppress the scratching behavior in a mouse model of pruritus. Pharmacol Biochem Behav. 2004;78:719-725. [Cited in This Article: ] |
| 15. | Oliveira FA, Vieira-Júnior GM, Chaves MH, Almeida FR, Santos KA, Martins FS, Silva RM, Santos FA, Rao VS. Gastroprotective effect of the mixture of alpha- and beta-amyrin from Protium heptaphyllum: role of capsaicin-sensitive primary afferent neurons. Planta Med. 2004;70:780-782. [Cited in This Article: ] |
| 16. | Oliveira FA, Chaves MH, Almeida FR, Lima RC Jr, Silva RM, Maia JL, Brito GA, Santos FA, Rao VS. Protective effect of alpha- and beta-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J Ethnopharmacol. 2005;98:103-108. [Cited in This Article: ] |
| 17. | Holanda Pinto SA, Pinto LM, Cunha GM, Chaves MH, Santos FA, Rao VS. Anti-inflammatory effect of alpha, beta-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology. 2008;16:48-52. [Cited in This Article: ] |
| 18. | Lima-Júnior RC, Sousa DI, Brito GA, Cunha GM, Chaves MH, Rao VS, Santos FA. Modulation of acute visceral nociception and bladder inflammation by plant triterpene, alpha, beta-amyrin in a mouse model of cystitis: role of tachykinin NK(1)-receptors, and K(+)(ATP) channels. Inflamm Res. 2007;56:487-494. [Cited in This Article: ] |
| 19. | Vitor CE, Figueiredo CP, Hara DB, Bento AF, Mazzuco TL, Calixto JB. Therapeutic action and underlying mechanisms of a combination of two pentacyclic triterpenes, alpha- and beta-amyrin, in a mouse model of colitis. Br J Pharmacol. 2009;157:1034-1044. [Cited in This Article: ] |
| 20. | Vieira Júnior GM, Souza CML, Chaves MH. The Protium heptaphyllum resin: isolation, structural characterization and evaluation of thermal properties. Quím Nova. 2005;28:183-187. [Cited in This Article: ] |
| 21. | Gallegos RS, Roque NE. Analysis of mixtures of triterpenes by 13C NMR. Quím Nova. 1990;13:278-281. [Cited in This Article: ] |
| 22. | Mahato SB, Sen S. Advances in triterpenoid research, 1990-1994. Phytochemistry. 1997;44:1185-1236. [Cited in This Article: ] |
| 23. | Czakó L, Takács T, Varga IS, Hai DQ, Tiszlavicz L, Hegyi P, Mándi Y, Matkovics B, Lonovics J. The pathogenesis of L-arginine-induced acute necrotizing pancreatitis: inflammatory mediators and endogenous cholecystokinin. J Physiol Paris. 2000;94:43-50. [Cited in This Article: ] |
| 24. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. [Cited in This Article: ] |
| 25. | Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206-209. [Cited in This Article: ] |
| 26. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [Cited in This Article: ] |
| 27. | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131-138. [Cited in This Article: ] |
| 28. | Dembiński A, Warzecha Z, Ceranowicz P, Warzecha AM, Pawlik WW, Dembiński M, Rembiasz K, Sendur P, Kuśnierz-Cabala B, Tomaszewska R. Dual, time-dependent deleterious and protective effect of anandamide on the course of cerulein-induced acute pancreatitis. Role of sensory nerves. Eur J Pharmacol. 2008;591:284-292. [Cited in This Article: ] |
| 29. | Dawra R, Sharif R, Phillips P, Dudeja V, Dhaulakhandi D, Saluja AK. Development of a new mouse model of acute pancreatitis induced by administration of L-arginine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1009-G1018. [Cited in This Article: ] |
| 30. | Chen H, Sun YP, Li Y, Liu WW, Xiang HG, Fan LY, Sun Q, Xu XY, Cai JM, Ruan CP. Hydrogen-rich saline ameliorates the severity of l-arginine-induced acute pancreatitis in rats. Biochem Biophys Res Commun. 2010;393:308-313. [Cited in This Article: ] |
| 31. | de Beaux AC, Ross JA, Maingay JP, Fearon KC, Carter DC. Proinflammatory cytokine release by peripheral blood mononuclear cells from patients with acute pancreatitis. Br J Surg. 1996;83:1071-1075. [Cited in This Article: ] |
| 32. | Gukovskaya AS, Gukovsky I, Zaninovic V, Song M, Sandoval D, Gukovsky S, Pandol SJ. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853-1862. [Cited in This Article: ] |
| 33. | Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role of tumor necrosis factor-alpha in acute pancreatitis: from biological basis to clinical evidence. Shock. 2007;28:130-140. [Cited in This Article: ] |
| 34. | Papachristou GI. Prediction of severe acute pancreatitis: current knowledge and novel insights. World J Gastroenterol. 2008;14:6273-6275. [Cited in This Article: ] |
| 35. | Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711-1723. [Cited in This Article: ] |
| 36. | Paszt A, Eder K, Szabolcs A, Tiszlavicz L, Lázár G, Duda E, Takács T, Lázár G Jr. Effects of glucocorticoid agonist and antagonist on the pathogenesis of L-arginine-induced acute pancreatitis in rat. Pancreas. 2008;36:369-376. [Cited in This Article: ] |