INTRODUCTION
Liver resection is the standard treatment for patients with primary or secondary liver malignancies, and offers the only chance of long-term survival[1,2]. With advances in surgical techniques, perioperative management, and anatomical knowledge of the liver, major hepatectomy usually does not carry a high operative mortality in patients with normal hepatic function, or in those with large tumors, unless accompanied by technical failure. However, the morbidity and mortality after extensive hepatectomy, or major hepatectomy in patients with obstructive jaundice, hepatic dysfunction, or tumors increase due to postoperative liver failure caused by excessive loss of functional residual liver mass[3,4]. It has been reported that there is a strong correlation between the expected remnant liver volume and postoperative liver failure in patients who undergo liver resection[5]. Surgical resection of liver tumors requires a sufficient surgical margin that can lead to substantial loss of residual mass. However, it is essential to secure sufficient functional liver mass to prevent postoperative liver failure.
In 1920, Rous and Larimore showed that selective portal occlusion can produce atrophy of the occluded lobe and compensatory hypertrophy of the contralateral lobe in rabbits[6]. In the clinical setting, Makuuchi et al[7] first proposed portal vein embolization as a preoperative treatment to avoid postoperative liver failure due to insufficient remnant liver mass. Portal vein embolization is now widely accepted as a useful procedure to extend eligibility of patients with liver cancer for liver resection. However, one study has shown that some patients can become ineligible for scheduled surgery due to tumor progression after portal vein embolization[8], whereas another study has indicated that portal vein embolization neither prevents nor accelerates tumor growth[9]. Thus, the effect of portal vein embolization on tumor growth prior to resection is not well understood.
In the present study, we used a murine model of portal vein ligation (PVL) to determine the effects of ligation on the mechanisms of liver growth and regeneration. In addition, we evaluated how these mechanisms influence the growth of colorectal carcinoma tumors in ligated and contralateral lobes after PVL.
MATERIALS AND METHODS
Animal model
Male C57BL/6J and BALB/c mice (Jackson Laboratory, Bar Harbor, ME, USA) weighing 20-26 g were used in all experiments. This project was approved by the University of Cincinnati Animal Care and Use Committee and was in compliance with the National Institutes of Health guidelines. The C57BL/6J mice were randomly separated into a PVL group, partial hepatectomy group, and sham operation group. All mice were anesthetized with sodium pentobarbital (60 mg/kg, ip) and a midline laparotomy was performed. For PVL, the branch of the portal vein that fed the left and median hepatic lobes, which corresponded to 70% of the whole liver, was dissected under an operative microscope and ligated with an 8-0 PROLENE suture (Ethicon, Inc., Somerville, NJ, USA). Partial hepatectomy was performed according to the method of Higgins and Anderson[10], with slight modification. 7-0 PRONOVA sutures (Ethicon) were secured around the base of the left and median hepatic lobes, and the lobes were resected. Mice were sacrificed at the indicated time points after operation, and blood and liver samples were taken for analysis. The liver lobes to body weight ratio was determined.
Blood and tissue analysis
Blood was obtained by cardiac puncture for analysis of serum alanine aminotransferase (ALT) as an index of hepatocellular injury. Measurements of serum ALT were made using a diagnosis kit by bioassay (Wiener Laboratories, Rosario, Argentina). Liver tissues were fixed in 10% neutral-buffered formalin, processed, and embedded in paraffin for light microscopy. Sections were stained with hematoxylin and eosin (HE) for histological examination. Liver content of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β, hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor β1 (TGFβ1) was assessed by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA). Liver samples were weighed and immediately placed in 10 volumes (wt/vol) of a protease inhibitor cocktail that contained 10 nmol/L EDTA, 2mmol/L phenylmethylsulfonyl fluoride (PMSF), 0.1 mg/mL soybean trypsin inhibitor, 1.0 mg/mL bovine serum albumin, and 0.002% sodium azide in isotonic PBS, pH 7.0. Tissues were disrupted with a tissue homogenizer, and lysates were incubated at 4°C for 2 h. Samples were clarified by two rounds of centrifugation at 12 500 g for 10 min at 4°C.
Liver neutrophil accumulation
Liver myeloperoxidase (MPO) content was assessed by methods described elsewhere[11]. Liver tissue (100 mg) was homogenized in 2 mL buffer A (3.4 mmol/L KH2HPO4, 16 mmol/L Na2HPO4, pH 7.4). After being centrifuged for 20 min at 10 000 g, the pellet was resuspended in 10 volumes of buffer B (43.2 mmol/L KH2HPO4, 6.5 mmol/L Na2HPO4, 10 mmol/L EDTA, 0.5% hexadecyltrimethylammonium, pH 6.0) and sonicated for 10 s. After being heated for 2 h at 60°C, the supernatant was reacted with 3,3’,3,5’-tetramethylbenzidine, and the optical density was read at 655 nm.
Proliferating cell nuclear antigen staining
Immunohistochemical staining for proliferating cell nuclear antigen (PCNA) was performed on paraffin-embedded liver tissue with anti-PCNA antibody using DakoCytomation ARK kit (Dako, Copenhagen, Denmark). A three-step peroxidase method was performed according to the manufacturer’s instructions. PC-10 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at a dilution of 1:50, for 15 min at room temperature. The sections were counterstained with hematoxylin. Evaluation of PC-10 immunostaining was performed based on the percentage of positive nuclei of 400-600 hepatocytes from the 4-6 highest positive fields at high power (400 ×), and was expressed as PCNA labeling index.
Western blotting
Liver samples were homogenized in lysis buffer (10 mmol/L HEPES, pH 7.9, 150 mmol/L NaCl, 1 mmol/L EDTA, 0.6% NP-40, 0.5 mmol/L PMSF, 1 μg/mL leupeptin, 1 μg/mL aprotinin, 10 μg/mL soybean trypsin inhibitor, and 1 μg/mL pepstatin). Samples were then sonicated and incubated for 30 min on ice. Cellular debris was removed by centrifugation at 10 000 r/min. Protein concentrations of each sample were determined. Samples that contained equal amounts of protein in equal volumes of sample buffer were separated in a denaturing 10% polyacrylamide gel and transferred to a 0.1 μm pore nitrocellulose membrane. Nonspecific binding sites were blocked with Tris-buffered saline (TBS; 40 mmol/L Tris, pH 7.6, 300 mmol/L NaCl) that contained 5% non-fat dry milk for 1 h at room temperature. Membranes were then incubated with antibodies to cyclin D1 (Santa Cruz Biotechnology), signal transducer and activator of transcription 3 (STAT3) (Cell Signaling Technology, Boston, MA, USA), and phosphorylated STAT3 (Cell Signaling Technology) in TBS with 0.1% Tween 20. Membranes were washed and incubated with secondary antibodies conjugated to horseradish peroxidase. Immunoreactive proteins were detected by enhanced chemiluminescence.
Electrophoretic mobility shift assay
Nuclear extracts of liver tissue were prepared by the method of Deryckere and Gannon[12], and analyzed by electrophoretic mobility shift assay. Double-stranded consensus oligonucleotides to nuclear factor (NF)-κB and activator protein (AP)-1 (Promega, Madison, WI, USA) were end-labeled with γ[32P]-ATP (3000 Ci/mmol at 10 mCi/mL; Perkin Elmer, Waltham, MA, USA). Binding reactions (total volume 15 μL) that contained equal amounts of nuclear protein extract (20 μg) and 35 fmol (approximate 50 000 cpm, Cherenkov counting) of oligonucleotide were incubated at room temperature for 30 min. Binding reaction products were separated on a 4% polyacrylamide gel and analyzed by autoradiography.
Liver tumor model
The CT26 cell line is from an undifferentiated colon adenocarcinoma induced by N-nitroso-N-methylurethane injection in BALB/c mice. The CT26.WT cell line was obtained from American Type Culture Collection (ATCC; Rockville, MD, USA). CT26.WT cells were maintained in RPMI-1640 medium (ATCC) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin. Cells were incubated at 37°C in a humidified atmosphere that contained 5% CO2 in air. The cells were harvested from subconfluent cultures by 0.05% trypsinization and washed twice in PBS on the day of implantation. For the portal vein injection model, a midline incision was made and the portal vein was exposed by removing the intestine. A suspension of 2 × 105 CT26.WT cells was injected into the portal vein using a 31 G needle. After injection, a small piece of Gelfoam (Pharmacia Co., Kalamazoo, MI, USA) was pressed over the injection site for 2-3 min to obtain hemostasis. One week after tumor cell implantation, mice were subjected to portal vein ligation or sham surgery. One week after the operation, all mice were sacrificed and blood and liver samples were collected. The tumor growth was evaluated on HE slides and tumor area was determined by morphometry. Morphometric analysis was performed by image analysis software in five representative fields at low power (10 ×).
Statistical analysis
All data are expressed as mean ± SE. Data were analyzed with one-way analysis of variance with subsequent Student-Newman-Keuls test. Differences were considered significant when P < 0.05.
RESULTS
Liver growth and regeneration after PVL vs partial hepatectomy
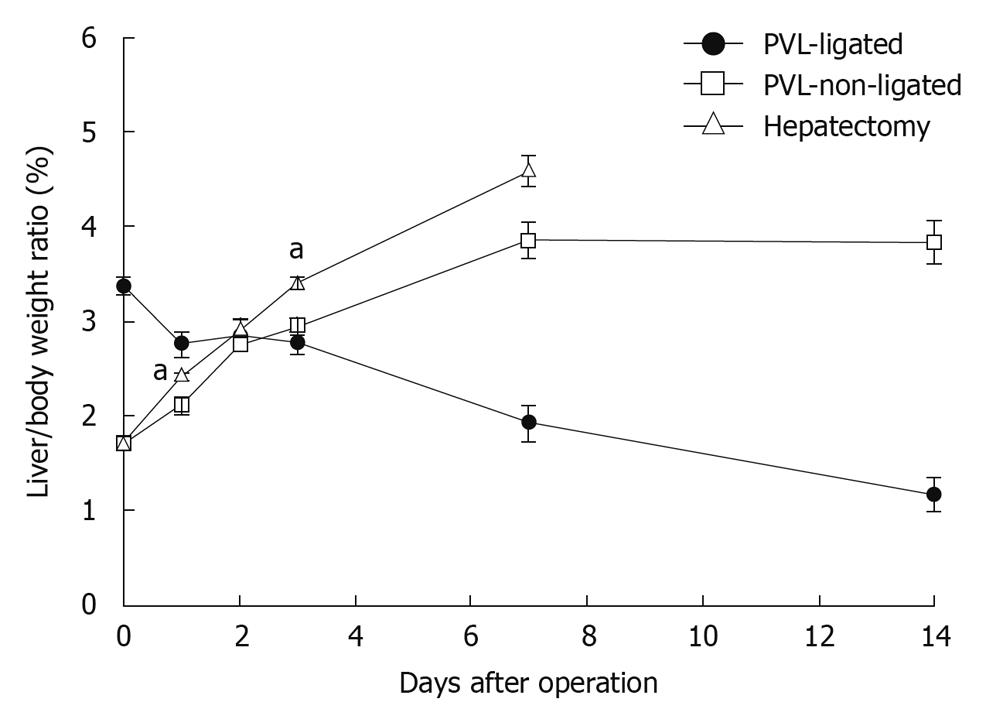
To evaluate liver growth and regeneration after PVL or partial hepatectomy, we measured liver/body weight ratios. After partial hepatectomy, liver regenerated at the expected rate and returned to normal liver mass within 7 d (Figure 1). After PVL, non-ligated lobes grew at a rate similar to liver after partial hepatectomy, but reached a plateau of mass below that after partial hepatectomy (Figure 1). The ligated lobes atrophied at a constant rate and after 14 d, the mass of the ligated lobes was approximately one third of the starting mass (Figure 1).
Figure 1 Changes in liver lobe to body weight ratio after portal vein ligation or partial hepatectomy.
To evaluate liver regeneration after portal vein ligation (PVL) or partial hepatectomy, liver lobe to body weight ratio was determined. Data are mean ± SE with n = 4-6 per group. aP < 0.05 vs PVL-non-ligated.
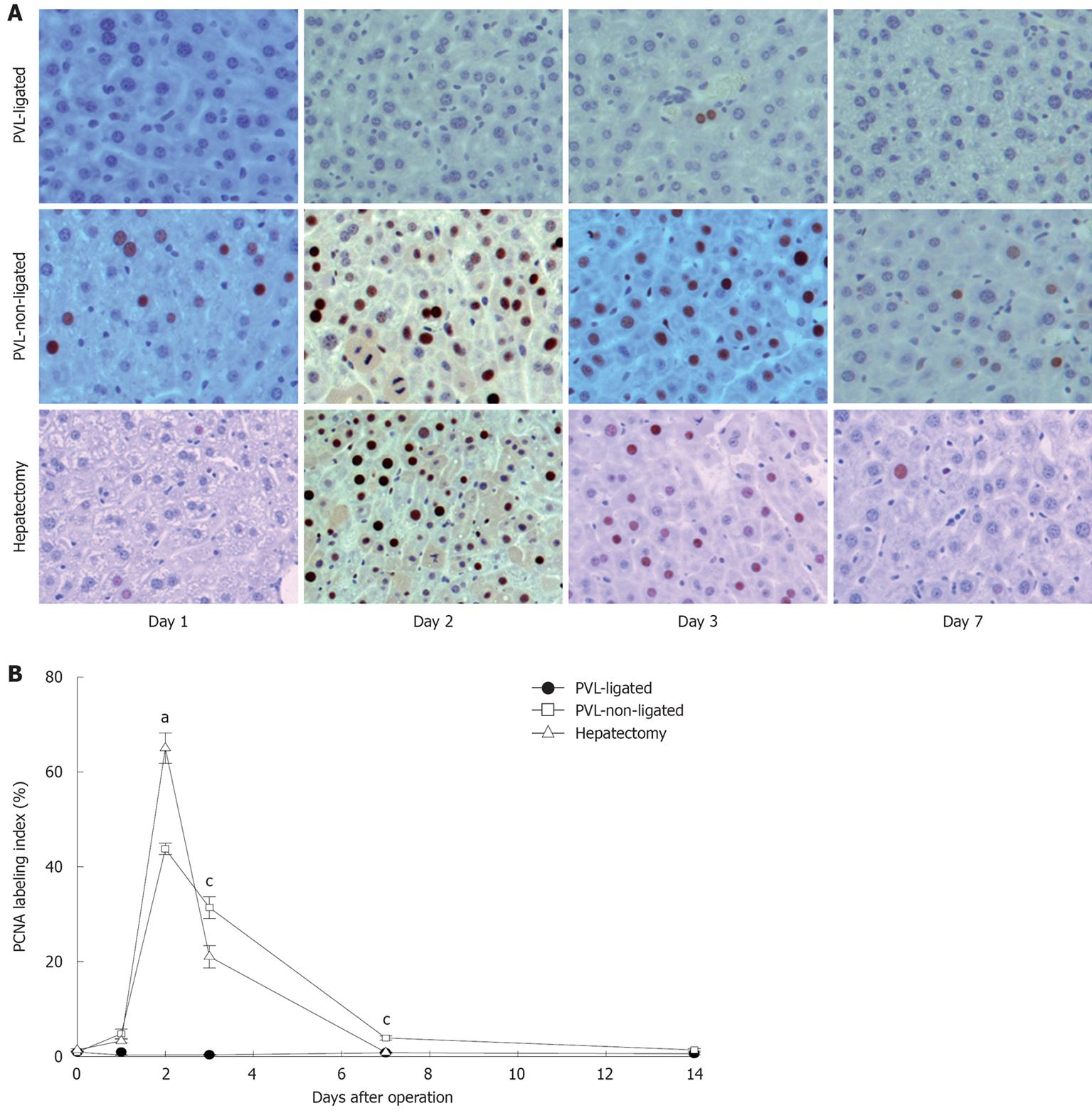
In accordance with the changes in liver growth and regeneration, similar patterns were found when we examined hepatocyte proliferation by staining for PCNA. PCNA-positive hepatocytes increased in a similar fashion after partial hepatectomy and in non-ligated lobes after PVL (Figure 2). However, there were subtle differences noted. While the number of PCNA-positive hepatocytes was maximal in both partial hepatectomy and non-ligated lobes after PVL at 2 d after surgery, there were significantly more PCNA-positive hepatocytes in the partial hepatectomy group (Figure 2). Furthermore, the number of PCNA-positive hepatocytes dropped dramatically by day 3 after partial hepatectomy, whereas after PVL, the number of PCNA-positive hepatocytes in non-ligated lobes had a more gradual decrease and was significantly higher compared to that after partial hepatectomy (Figure 2). Despite these minor differences, our data confirm previous studies that the mechanisms of liver growth and regeneration are similar between that occurring in non-ligated lobes after PVL and that occurring after partial hepatectomy[13].
Figure 2 Hepatocyte proliferation after portal vein ligation.
A: Hepatocyte proliferation was determined by immunohistochemical staining for proliferating cell nuclear antigen (PCNA). Original magnification was 200 ×; B: Quantitative analysis of PCNA labeling. PCNA labeling index was expressed as percentage of positive nuclei of 400-600 hepatocytes from the 4-6 highest positive fields at high power (400 ×). Data are mean ± SE with n = 4-6 per group. aP < 0.05 vs PVL-non-ligated; cP < 0.05 vs partial hepatectomy.
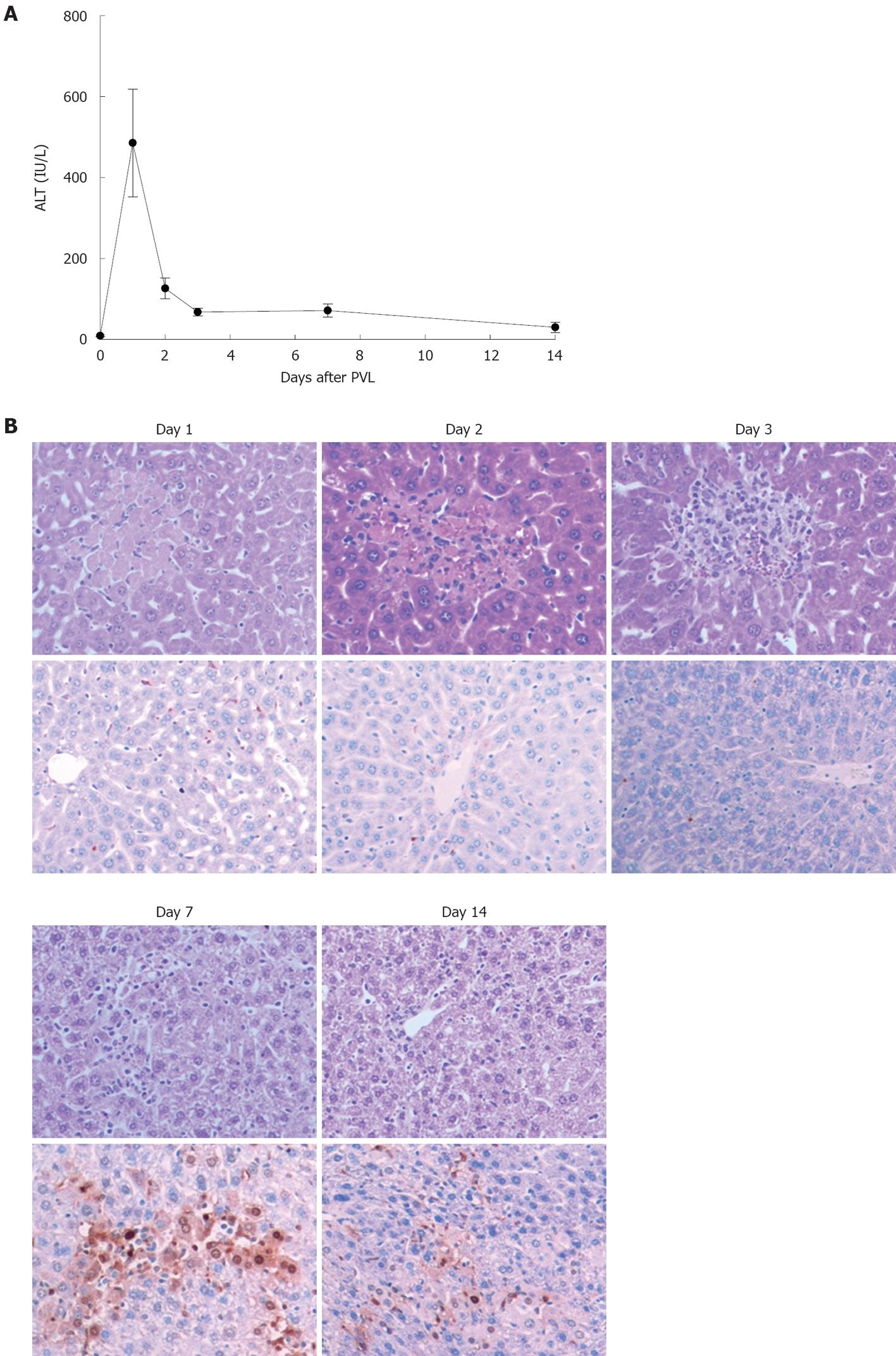
We next examined the mode of cell death after PVL. Serum levels of ALT were assessed as a measure of hepatocyte necrosis and TUNEL staining was performed to determine the amount of hepatocyte apoptosis. Serum levels of ALT peaked 1 d after PVL, but remained elevated for 14 d (Figure 3A). Corresponding with the ALT data, ligated lobes showed areas of necrosis within 1 d after PVL (Figure 3B, upper panels). These regions persisted for up to 7 d after PVL and were undetectable by day 14. In contrast, significant hepatocyte apoptosis was detected in ligated lobes, beginning at 7 d after PVL and persisting until 14 d after PVL (Figure 3B, lower panels). In non-ligated lobes, no evidence of hepatocyte necrosis or apoptosis was observed (data not shown).
Figure 3 Effects of portal vein ligation on liver necrosis and apoptosis.
A: Liver injury was measured by serum levels of alanine aminotransferase (ALT). Data are mean ± SE with n = 4-6 per group; B: Representative pictures of HE staining (upper) and TUNEL staining (bottom). TUNEL staining was performed to determine the amount of hepatocyte apoptosis. Original magnification was 200 ×. PVL: Portal vein ligation.
Lobar differences in cytokine and growth factor expression after PVL
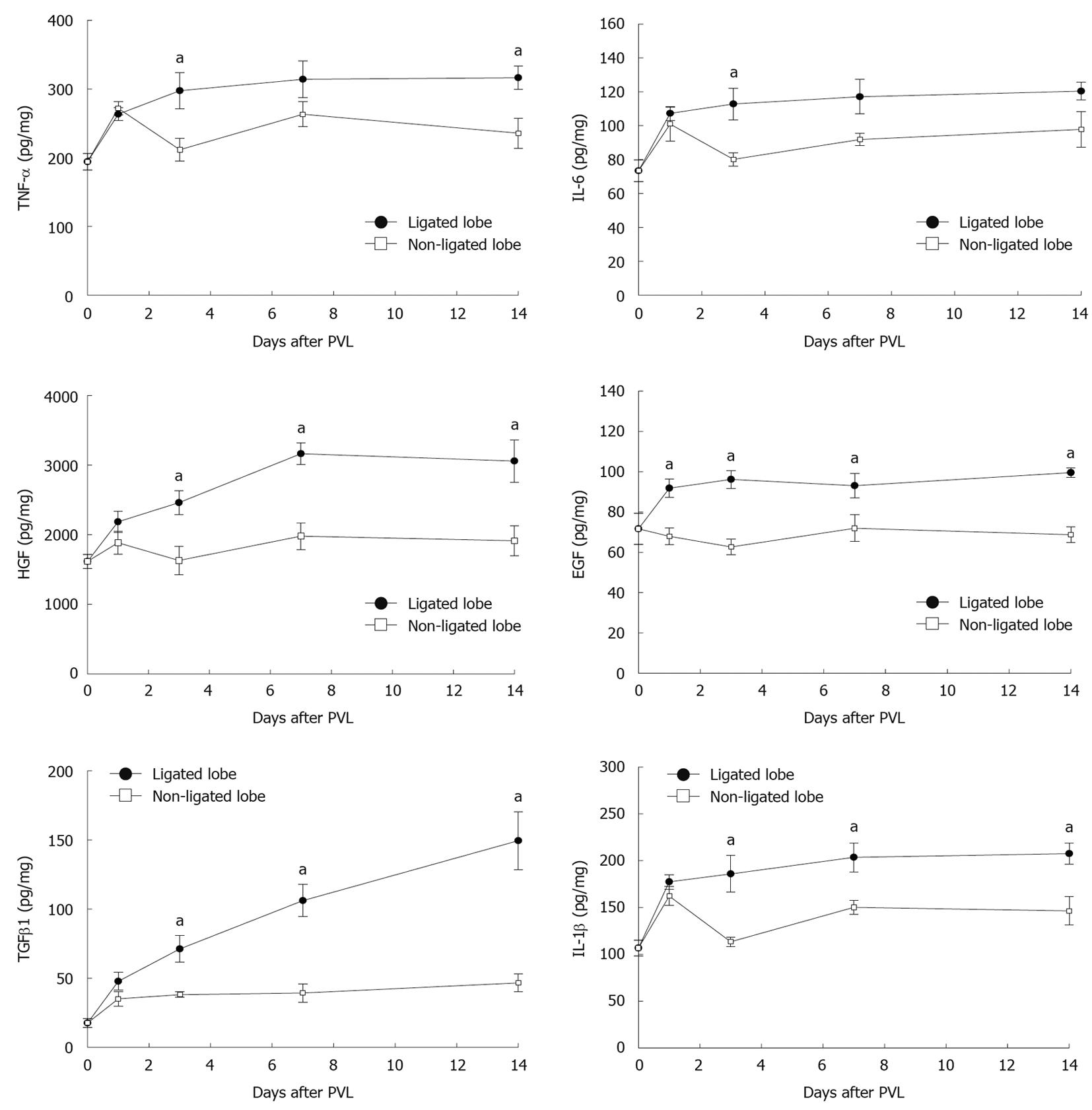
A variety of cytokines and growth factors are known to modulate liver growth and regeneration. To evaluate whether expression of relevant cytokines and growth factors is related to the growth of non-ligated lobes and/or the atrophy of ligated lobes, we measured the protein levels of HGF, EGF, TNF-α, IL-6, TGFβ1, and IL-1β in liver tissues. HGF and EGF are direct mitogens for hepatocytes and are crucial inducers of liver regeneration[14-16]. Expression of HGF and EGF were increased in both ligated and non-ligated lobes after PVL (Figure 4). However, expression of these mediators was much higher in ligated lobes compared to non-ligated lobes. TNF-α and IL-6 have been implicated as important contributors to liver growth and regeneration[14-16]. Expression of TNF-α and IL-6 increased similarly at 1 d after PVL in ligated and non-ligated lobes (Figure 4). By day 3, expression of TNF-α and IL-6 was significantly higher in ligated lobes compared to non-ligated lobes. TGFβ1 and IL-1β are known as suppressors of cell proliferation and might be involved in termination of liver regeneration[15,17]. We found that expression of TGFβ1 and IL-1β was increased 1 d after PVL in both ligated and non-ligated lobes (Figure 4). However, by day 3, expression of TGFβ1 had reached a plateau and IL-1β decreased in non-ligated lobes, whereas their expression had increased further in ligated lobes.
Figure 4 Effect of portal vein ligation on liver cytokines, growth factors, and chemokines.
To evaluate whether expression of relevant cytokines and growth factors are related to the growth of non-ligated lobes and/or the atrophy of ligated lobes, liver levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor β1 (TGFβ1), and IL-1β were analyzed by enzyme-linked immunosorbent assay (ELISA). Liver lysates were processed for ELISA. Data are mean ± SE with n = 4-15 per group. aP < 0.05 vs portal vein ligation (PVL)-non-ligated.
Divergent signaling mechanisms in ligated and non-ligated lobes after PVL
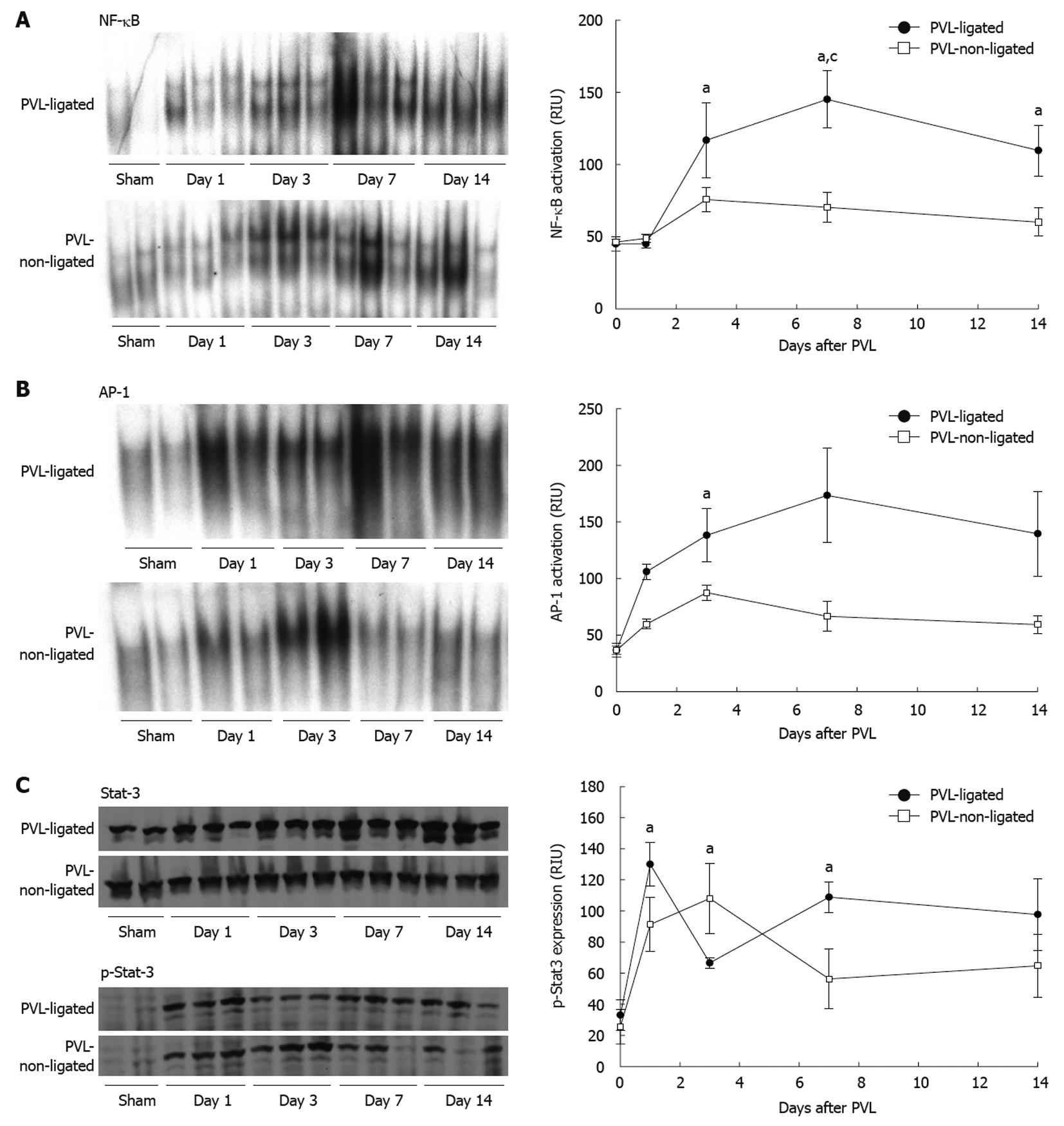
NF-κB, AP-1 and STAT3 are known to be important mediators of liver growth and regeneration[18,19], therefore, we assessed the activation of these transcription factors in ligated and non-ligated lobes after PVL. NF-κB activation increased in both ligated and non-ligated lobes by day 3 after PVL; however, it was much greater in ligated lobes (Figure 5A). In non-ligated lobes, NF-κB activation remained elevated, albeit modestly, throughout the 14-d experimental period (Figure 5A). In contrast, activation of NF-κB in ligated lobes increased further, and remained significantly higher than in non-ligated lobes (Figure 5A). Supershift assays of NF-κB from each lobe indicated that the composition was composed primarily of p50/p65 heterodimers (data not shown).
Figure 5 Transcription factor activation after portal vein ligation.
Nuclear factor (NF)-κB (A), activator protein (AP)-1 (B) and signal transducer and activator of transcription 3 (STAT3) (C) were examined in liver extracts. For NF-κB and AP-1, liver nuclear extracts were analyzed by electrophoretic mobility shift assay. For STAT3, liver lysates were assessed by Western blotting. Results were quantitated by image analysis of autoradiograms and chemiluminescence films. Data are mean ± SE with n = 4 per group. A: aP < 0.05 vs sham-operated group; cP < 0.05 vs portal vein ligation (PVL)-non-ligated group; B: aP < 0.05 vs PVL-non-ligated group; C: aP < 0.05 vs sham-operated group.
In contrast to NF-κB, which did not become activated until 3 d after surgery, AP-1 activation occurred rapidly after PVL in both ligated and non-ligated lobes (Figure 5B). In non-ligated lobes, activation of AP-1 was increased modestly throughout the 14-d experiment. Similar to NF-κB, activation of AP-1 was much greater in the ligated lobes compared to non-ligated lobes at every time point (Figure 5B). Supershift assays determined that the composition of AP-1 was similar in each lobe, primarily c-Fos, JunB, and JunD (data not shown).
STAT3 activation, as determined by STAT3 phosphorylation, was rapidly increased in both ligated and non-ligated lobes after PVL (Figure 5C). Interestingly, STAT3 activation decreased in ligated lobes at day 3 and then increased at days 7 and 14. In contrast, STAT3 activation in non-ligated lobes peaked at day 3 and then decreased at days 7 and 14.
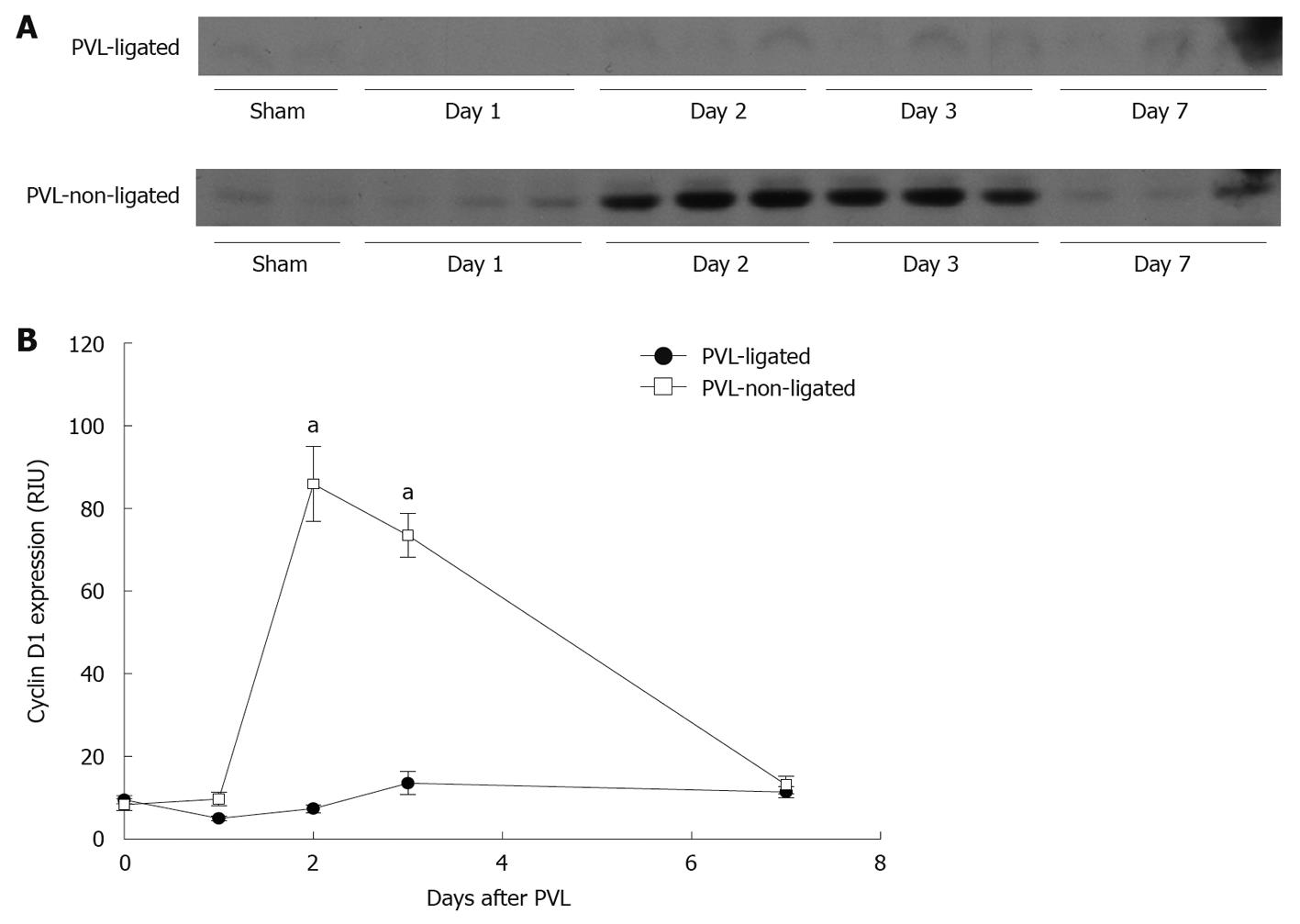
Cyclin D1 is known to play a crucial role in the control of hepatocyte proliferation from G1- to S-phase[16,20,21]. Expression of cyclin D1 in non-ligated lobes was significantly increased after PVL, whereas there was no induction of cyclin D1 expression in ligated lobes (Figure 6).
Figure 6 Liver cyclin D1 expression after portal vein ligation.
A: Liver lysates were assessed for cyclin D1 protein expression by Western blotting; B: Chemiluminescence films were quantitated by image analysis. Data are mean ± SE with n = 4 per group. aP < 0.05 vs portal vein ligation (PVL)-ligated group.
PVL accelerates tumor growth in ligated, but not in non-ligated lobes
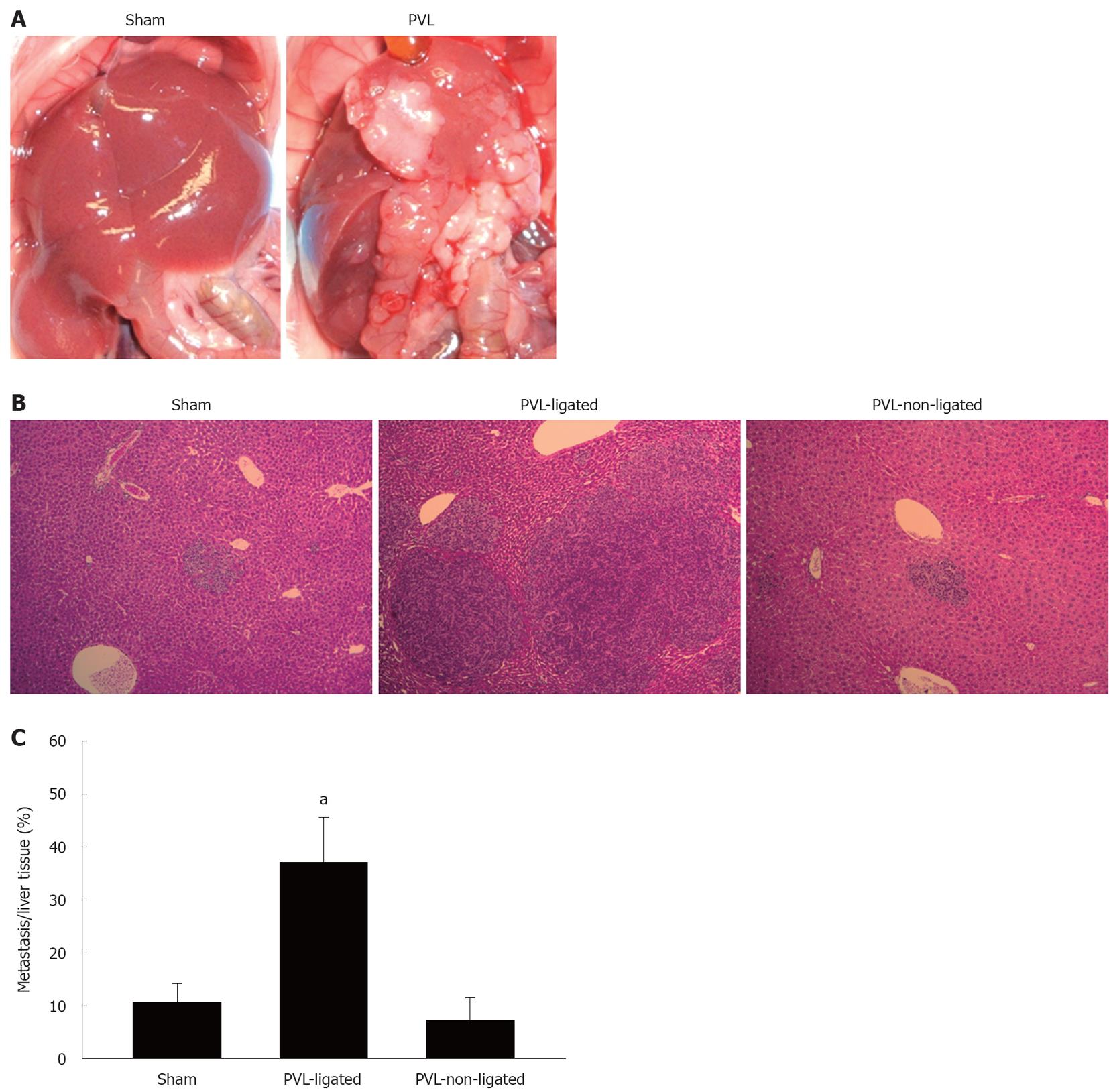
To investigate how the different milieus in ligated vs non-ligated lobes might alter the growth of liver tumors, mice were injected via the portal vein with murine colorectal carcinoma cells 7 d prior to PVL or sham surgery. We used murine colorectal carcinoma cells, CT26.WT, to reproduce the nature of colorectal liver metastases by injecting the cells into the portal vein. In sham-operated mice, there were similar amounts of small tumors in lobes that corresponded to ligated and non-ligated lobes (Figure 7A and B). In mice undergoing PVL, ligated lobes had large tumor nodules that were clearly visible on gross examination as well as histologically (Figure 7A and B). Quantitation of tumor area in liver sections demonstrated a fourfold increase in relative tumor size in ligated lobes vs non-ligated lobes (Figure 7C).
Figure 7 Effect of portal vein ligation on tumor growth.
A: CT26.WT cells were injected into portal the vein. Mice were sacrificed at 14 d after injection following sham operation or portal vein ligation (PVL) performed on day 7 after injection; B: Representative pictures of liver histology after PVL. Small metastatic foci were observed in sham-operated and non-ligated lobes. Large metastatic foci were observed in ligated lobes. Original magnification was 10 ×; C: The ratio of metastases to normal liver was measured by morphometry. Data are mean ± SE with n = 4 per group. aP < 0.05 vs sham-operated group and PVL-non-ligated group.
DISCUSSION
In the current study, we evaluated the effects of PVL on expression of cytokines and growth factors and signaling pathways that are known to contribute to liver growth and regeneration. Although the trigger for growth of contralateral lobes after PVL has not been fully elucidated, hemodynamic changes after PVL have been proposed as an initial event that contributes to this process[22]. Following PVL, arterial blood flow to the ligated lobe roughly doubles, while arterial blood flow to the non-ligated lobes is roughly 60% of normal[22]. Portal flow to the non-ligated lobes, however, more than doubles[22] and this increase in supply helps trigger growth mechanisms in the non-ligated lobes[23]. Hemodynamic changes are more drastic after partial hepatectomy because both arterial and portal flows to the remnant liver are increased. The difference in hemodynamic changes between PVL and partial hepatectomy could affect the degree and/or timing of expression of some proteins involved in regeneration[13,24] and cause a slight delay of regeneration in the non-ligated lobes. However, the gross regenerative responses are similar, as shown by our data.
Some studies have shown that the early growth/regenerative response, including activation of NF-κB, STAT3, IL-6, c-fos, c-myc, and c-jun, are similarly induced in both ligated and non-ligated lobes[18]. Other studies have shown differences in growth factor mRNA expression between ligated and non-ligated lobes[25]. Our data demonstrate that these growth and regenerative mechanisms are greatly increased in both ligated and non-ligated lobes, but are significantly greater in the ligated compared to non-ligated lobes. Cyclin D1 is the sole exception, being induced only in the non-ligated lobes. Cyclin D1 is known to play a crucial role in the control of hepatocyte proliferation from G1- to S-phase[20,21], and appears to be the determinant of proliferation or atrophy in non-ligated and ligated lobes, respectively.
The milieu in the ligated lobe, with greatly increased expression of cytokines and growth factors and increased activation of NF-κB and AP-1, is rather chaotic and not indicative of either a “survival” or “death” mode. TNF-α can function to promote hepatocyte proliferation or death, depending on the co-stimuli present[26-28]. NF-κB activation in hepatocytes is pro-survival and anti-apoptotic[29,30], whereas activation of AP-1 promotes hepatocellular injury and apoptosis[31]. The fate of the ligated lobe might be less dependent upon the changes in these factors, and more on the lack of nutrient and oxygen delivery. As is clear, the end result is atrophy of the ligated lobe through necrotic and apoptotic mechanisms. Despite this atrophy and the pro-hepatocyte death milieu, colorectal carcinoma metastases grew much faster in the ligated lobes compared to the non-ligated lobes after PVL. These findings are consistent with other studies that have shown increased tumor growth in the ligated lobes after PVL[32,33]. However, our study offers more insight into the potential mechanisms that contribute to the increased tumor growth, as our data provide important information about the expression of growth factors and signaling pathways. HGF and EGF have a stimulatory effect on tumor cells[34,35], and therefore, the increased HGF and EGF observed in the ligated lobe after PVL could explain the accelerated tumor growth. TGFβ1 was also increased in the ligated lobe. Although TGFβ1 is generally known as a negative regulator in liver regeneration[15], some recent studies have reported a tumor promoter role for TGFβ1 in hepatocellular carcinoma and liver metastasis[36-38]. It has been shown that TGFβ1 is highly proliferative in CT26 cells, the colorectal carcinoma cell line used in our studies[39]. Furthermore, TGFβ1 is known to contribute to hepatocyte apoptosis, and colorectal carcinoma cells secrete significant amounts of TGFβ1, which might contribute to tumor growth[39,40]. Therefore, it is plausible that increased TGFβ1 expression in the ligated lobes significantly contributed to the accelerated growth of colorectal carcinoma tumors after PVL.
In summary, the present study demonstrated the signaling pathways that were activated in ligated and non-ligated lobes after PVL. Both lobes had increased expression of pro-proliferative cytokines and growth factors, as well as activation of pro-regenerative transcription factors, which help to define the molecular events that contribute to growth of contralateral lobes. Ligated liver lobes had significant increases in proliferative cytokines, growth factors and transcription factors compared to non-ligated lobes. While this response might constitute a survival mode for the hepatic parenchyma, it appears to provide an environment that facilitates tumor growth. PVL is a proven modality for increasing the functional liver remnant and extending the indications for surgery for metastatic liver disease. Future studies are needed to assess the effects of adjuvant or neoadjuvant chemotherapy on the hepatic expression of growth factors and tumor growth rate in this model.
COMMENTS
Background
Liver resection is the standard treatment for patients with primary or secondary liver malignancies, and offers the only chance of long-term survival. Although, the outcome of hepatic resection is improving, postoperative liver failure that results from insufficient functional liver volume after surgery could be lethal. Portal vein embolization is now widely accepted as a useful procedure to increase remnant liver volume and extend eligibility of patients with liver cancer for liver resection.
Research frontiers
Portal vein embolization is well known to induce hypertrophy of contralateral lobes. However, the manner in which portal vein embolization alters growth of the contralateral lobes and atrophy of the embolized lobe(s) is incompletely understood. Moreover, the effect of portal vein embolization on tumor growth is controversial.
Innovations and breakthroughs
In the current study, the authors demonstrated that various cytokines, transcription factors and regulatory factors were significantly upregulated in ligated lobes compared to non-ligated lobes after portal vein ligation. Tumor growth was accelerated in the ligated compared to non-ligated lobes and appeared to be a result of increased growth factor expression.
Applications
The results provide strong evidence of accelerated tumor growth in ligated lobes. This should be taken into account for the treatment strategy when patients undergo portal vein embolization.
Peer review
The experiments were well designed and well conducted. The topic relates to the advantages and/or disadvantages of the surgical procedure of portal vein embolization prior to major liver resections for hepatocellular carcinoma or other liver cancers.