Published online Aug 14, 2010. doi: 10.3748/wjg.v16.i30.3731
Revised: March 25, 2010
Accepted: April 2, 2010
Published online: August 14, 2010
The adenosine monophosphate-activated protein kinase (AMPK) and p70 ribosomal S6 kinase-1 pathway may serve as a key signaling flow that regulates energy metabolism; thus, this pathway becomes an attractive target for the treatment of liver diseases that result from metabolic derangements. In addition, AMPK emerges as a kinase that controls the redox-state and mitochondrial function, whose activity may be modulated by antioxidants. A close link exists between fuel metabolism and mitochondrial biogenesis. The relationship between fuel metabolism and cell survival strongly implies the existence of a shared signaling network, by which hepatocytes respond to challenges of external stimuli. The AMPK pathway may belong to this network. A series of drugs and therapeutic candidates enable hepatocytes to protect mitochondria from radical stress and increase cell viability, which may be associated with the activation of AMPK, liver kinase B1, and other molecules or components. Consequently, the components downstream of AMPK may contribute to stabilizing mitochondrial membrane potential for hepatocyte survival. In this review, we discuss the role of the AMPK pathway in hepatic energy metabolism and hepatocyte viability. This information may help identify ways to prevent and/or treat hepatic diseases caused by the metabolic syndrome. Moreover, clinical drugs and experimental therapeutic candidates that directly or indirectly modulate the AMPK pathway in distinct manners are discussed here with particular emphasis on their effects on fuel metabolism and mitochondrial function.
- Citation: Yang YM, Han CY, Kim YJ, Kim SG. AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J Gastroenterol 2010; 16(30): 3731-3742
- URL: https://www.wjgnet.com/1007-9327/full/v16/i30/3731.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i30.3731
Metabolic regulation of carbohydrate, lipid and protein, and synthesis of proteins and lipids are the principal functions of the liver, as well as xenobiotic detoxification. The function and survival of organisms are dependent on the dynamic control of energy metabolism. The regulation of fuel metabolic processes can be mediated by hormones and other endogenous ligands in response to changes in energy status. Diverse signaling pathways contribute to the regulation of energy metabolism, which is associated with the activation of cell surface and nuclear receptors in hepatocytes. Thus, the modulation of specific pathways can provide therapeutic strategies for hepatic diseases that result from metabolic derangements[1].
In a variety of hepatic diseases, abnormal fat accumulation in the liver is often a prerequisite metabolic event for further pathogenesis[2]. Lipotoxicity can lead to the generation of oxidative stress and inflammation, ultimately causing apoptosis[3]. Programmed cell death is elicited by cell surface death receptors, the caspase cascade, deranged mitochondrial metabolism, and energy deficiency. Mitochondria, cytoplasmic organelles in eukaryotic cells, play a key role in energy utilization such as oxidative phosphorylation; dysfunction of mitochondria is closely related with apoptosis[4].
The relationship between fuel metabolism and cell survival strongly implies the existence of a shared signaling network, which is responsible for the regulation of both phenomena. Emerging evidence indicates that the adenosine monophosphate (AMP)-activated protein kinase (AMPK) and p70 ribosomal S6 kinase-1 (S6K1) pathway serves as a key pathway that regulates fuel energy metabolism. In addition, it has been suggested that AMPK controls the redox-state and mitochondrial function. In this review, we focus on the role of the AMPK pathway in hepatic fuel metabolism in conjunction with cell survival. Moreover, clinical drugs and experimental therapeutic candidates that activate the AMPK-S6K1 pathway in distinct manners are discussed here with particular reference to their roles in mitochondrial function and energy metabolism.
The liver plays a central role in fuel metabolism, and thus regulates dynamic catabolic and anabolic processes to maintain energy homeostasis of organisms. Breakdown products of carbohydrate and lipid (i.e. glucose and fatty acids) are common energy sources which are converted to adenosine-triphosphate (ATP) in mitochondria. In addition, mitochondria have many other metabolic functions, such as regulation of membrane potential, cellular metabolism, calcium signaling (including calcium-induced apoptosis), and apoptosis. During the process of catabolism, the mitochondrion serves as the main source of energy for the cell because it converts nutrients into energy via cellular respiration[5]. Most of the oxygen delivered to cells or organs is consumed by mitochondria for ATP generation. When the energy is excessive in the cell, mitochondrial energy production is inhibited so that glucose and free fatty acids can be stored as glycogen and fat through anabolic processes.
AMPK: AMPK is a heterotrimer complex that consists of a catalytic subunit (α1/2) and two regulatory subunits (β1/2 and γ1/2/3), and functions as a serine/threonine protein kinase[6]; AMPK activation is mediated by phosphorylation of threonine-172 in the catalytic domain of the α subunit[7]. The activity of AMPK can be regulated by upstream kinases, which include liver kinase B1 (LKB1)[8], Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) β[9], and transforming growth factor β-activated kinase-1[10]. Both LKB1 and CaMKK increase the AMPK activity through direct phosphorylation of threonine-172 in the α subunit. In addition, LKB1 is constitutively active as a major upstream kinase. The upstream signaling molecules of LKB1 may include protein kinase C (PKC)-ζ[11], protein kinase A[12], and p90 kDa ribosomal S6 kinase[13]. The fact that the calcium/calmodulin complex regulates CaMKK suggests that AMPK may be involved in Ca2+ modulation in cells.
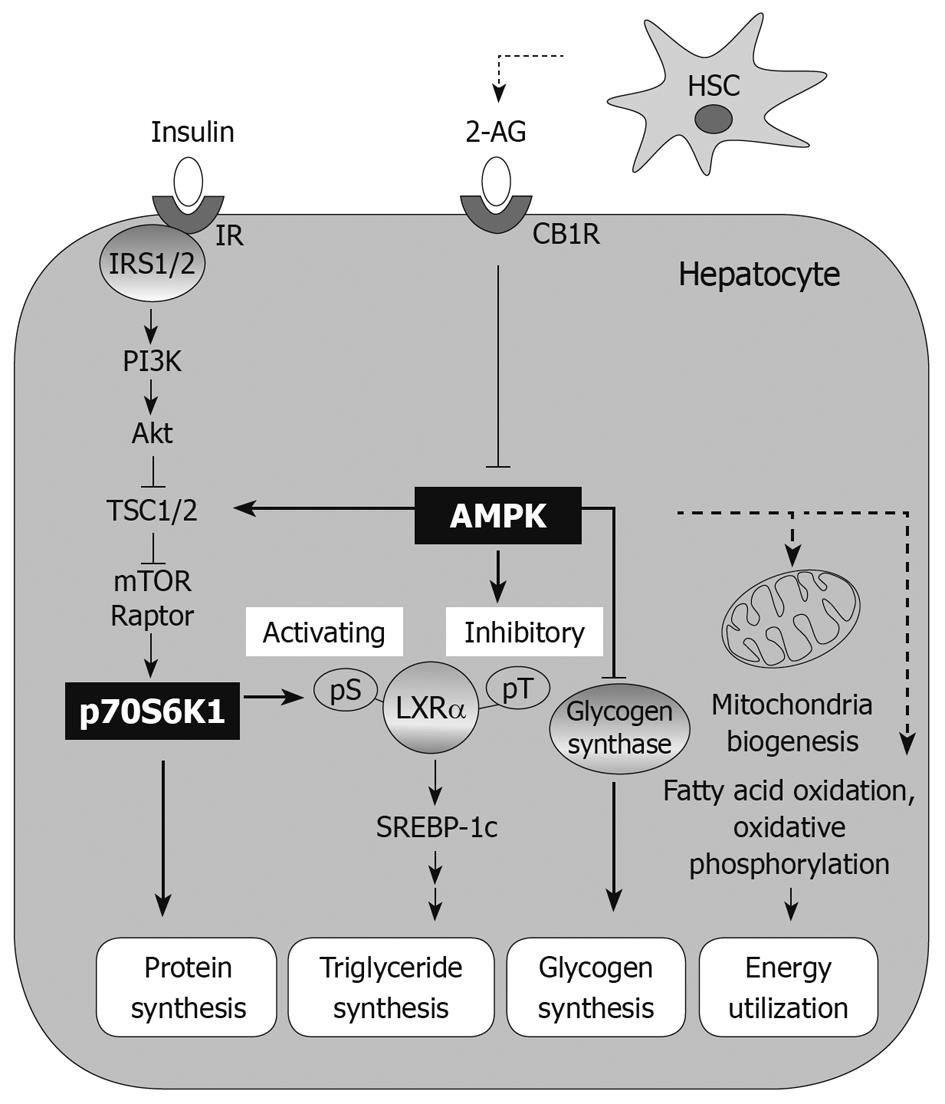
AMPK regulates energy homeostasis in various organs through response to hormones and nutrient signals. AMPK physiologically responds to the change in the AMP:ATP ratio, and thus serves as an intracellular sensor for energy homeostasis[7]. In addition to ATP production with switching off from anabolic processes in tissues, the activation of AMPK affects whole body fuel utilization and induces fatty acid oxidation and glucose uptake in skeletal muscle and heart, but inhibits lipogenesis and adipocyte differentiation[6-7]. In the liver, AMPK inhibits gluconeogenesis and synthesis of glycogen, fatty acid and cholesterol. Since AMPK plays a key role in metabolic regulation, it is recognized as an important target for metabolic disorders such as obesity, diabetes, and metabolic liver diseases.
S6K1: S6K1 is a mitogen-activated serine/threonine protein kinase that is associated with growth and cell cycle progression. In translational processes, S6K1 phosphorylates the S6 protein of the 40S ribosomal subunit. Phosphoinositide-3 kinase (PI3K)-the mammalian target of rapamycin (mTOR) regulates S6K1 as a distinct pathway from the Ras/mitogen-activated protein kinase cascade[14]. S6K1 signaling suppresses catabolic events such as lipolysis in adipose tissue and fatty acid oxidation in muscle, both of which stimulate ATP generation[15]. Since S6K1 is sensitive to nutrients including amino acids, nutrients negatively regulate insulin signaling by phosphorylating insulin receptor substrate-1 (IRS1) through S6K1 activation. Thus, S6K1 may also affect the regulation of nutrient and hormone signaling pathways under normal and pathological conditions (e.g. obesity, diabetes, and cancer). Moreover, S6K1 may play a role in the balance between survival and death in tissues including the liver. It is noteworthy that AMPK activation leads to inhibition of the mTOR/S6K1 pathway through tuberous sclerosis protein 2 (TSC2) phosphorylation[16]. The regulation of S6K1 by AMPK is now recognized as an important regulatory step, by which cells maintain energy metabolism.
Nonalcoholic fatty liver disease (NAFLD) is defined as a common liver disease ranging from steatosis to nonalcoholic steatohepatitis, and cirrhosis[2]. Moreover, NAFLD is considered as a main hepatic component of metabolic syndrome[17]. The characteristics of metabolic syndrome are obesity, insulin resistance, and cardiovascular disorders. In obese people mostly with insulin resistance, excessive fat is deposited in the liver and the raised hepatic lipid amount is closely associated with pathogenic processes of the syndrome[18,19].
Hepatic steatosis by liver X receptor-α-sterol regulatory element, binding protein-1c: A variety of conditions such as excess delivery of fatty acids, decreased oxidation of hepatic fatty acid and/or impaired synthesis or secretion of very low-density lipoprotein increase the sources of hepatic lipids, leading to fatty liver disease. The amount of accumulated fat is also increased by lipogenesis; emerging evidence supports the importance of de novo lipogenesis in abnormal hepatic fat accumulation in NAFLD patients[20,21]. Lipogenesis is transcriptionally regulated by the membrane-bound sterol regulatory element, binding protein-1c (SREBP-1c), which belongs to the basic helix-loop-helix-leucine zipper family. In the nucleus, SREBP-1c activates transcription of genes involved in lipogenesis, as supported by the finding that the overexpression of SREBP-1c in transgenic mice promotes the development of fatty liver. In animal models of insulin-resistant diabetes and obesity, the increased synthesis of fatty acids contributes to the development of hepatic steatosis.
Liver X receptor-α (LXRα), a transcriptional nuclear receptor, is a key regulator of lipogenic genes encoding for the enzymes that promote hepatic fat accumulation (e.g. fatty acid synthase, FAS; acetyl-CoA carboxylase, ACC; and stearoyl-CoA desaturase-1, SCD-1)[22,23]. Ligand activation of LXRα promotes induction of the lipogenic genes through SREBP-1c, causing increases in fatty acid synthesis and progression to steatosis, hypertriglyceridemia, and steatohepatitis[22]. Thus, SREBP-1c is an important target gene of LXRα. Since the LXRα-SREBP-1c pathway activates lipogenesis in the liver, it is an attractive target for the treatment of hepatic steatosis and hepatitis. In clinical situations, the expression of SREBP-1c and lipogenic genes including ACC and FAS is enhanced in NAFLD patients[24,25]. In addition, increases in LXRα target gene expression (e.g. ACC and FAS) were observed in the patients with fatty liver, which was accompanied by SREBP-1c activation, but not activation of carbohydrate responsive element-binding protein[26].
The AMPK-S6K1 pathway is involved in the regulation of LXRα-SREBP-1c and thus in LXRα-induced lipogenesis; chemical activation of AMPK in conjunction with its inhibition of S6K1 leads to the intervention of hepatic steatosis (Figure 1)[27]. As an example, AMPK activation by oltipraz treatment inhibits S6K1 activity, which inhibits the activity of LXRα[27] and prevents the ability of activated LXRα to bind the LXR binding site upstream of the genes including SREBP-1c and CYP7A1. Therefore, the consequent repression of SREBP-1c expression contributes to decreased synthesis of fat in the liver[27].
Repeated alcohol consumption decreases the production of adiponectin secreted from adipocytes[28]. Adiponectin increases hepatic fatty acid oxidation through AMPK activation[29]. Therefore, it is tempting to speculate that AMPK activity is repressed as hepatic function deteriorates in alcoholic patients. Similarly, AMPK activity was decreased in animals which consumed alcohol for 4 wk[30]. As a compensatory response, alcohol consumption increased lipogenesis in the liver, which may also result from the reduced rate of fatty acid oxidation. Thus, pharmacological activation of AMPK may be of help in treating hepatic steatosis. Peroxisome proliferator-activated receptors (PPARs) play a role in sensing nutrient levels and regulating lipid and glucose metabolism[31]. Thiazolidinediones (TZDs) and fibrates that activate PPARγ and PPARα, respectively, are prescribed for patients with diabetes and/or dyslipidemia. In those taking PPARγ agonists, insulin-mediated adipose tissue uptake and storage of free fatty acids are augmented with the inhibition of hepatic fatty acid synthesis, which may result in part from indirect activation of AMPK[32,33].
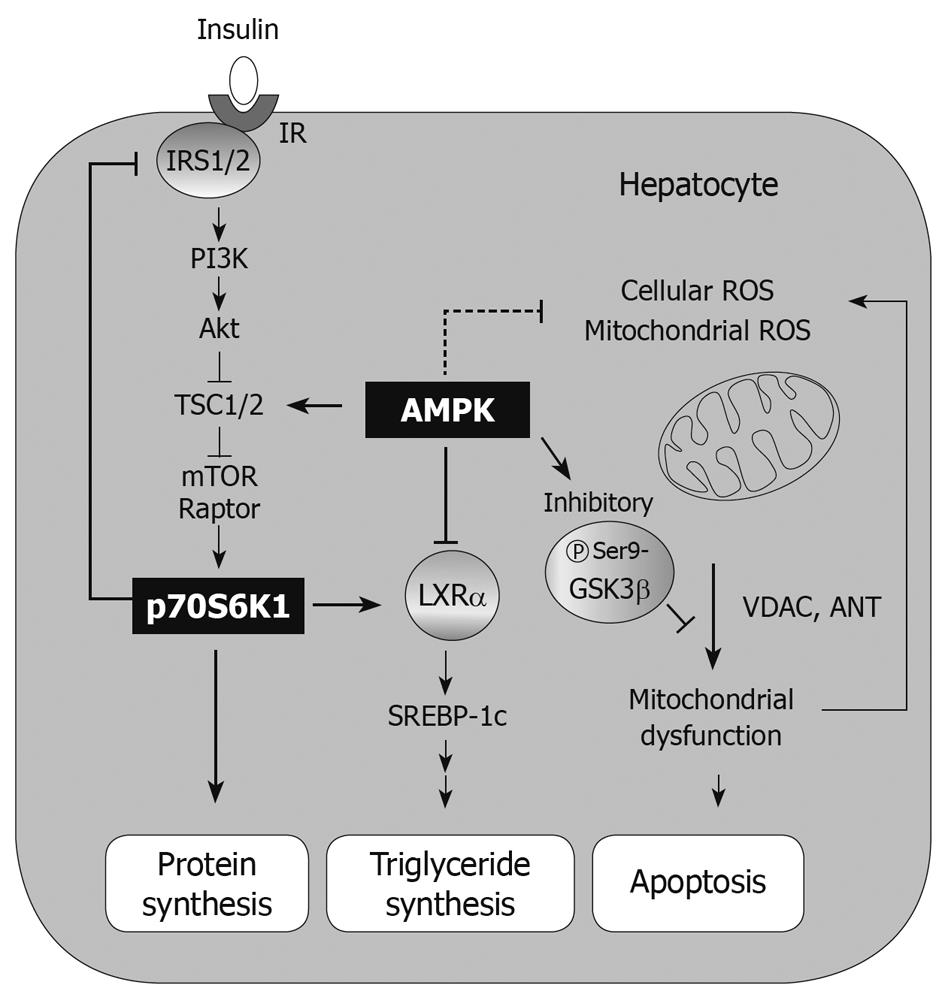
Hepatic insulin resistance: Insulin signaling is important in maintaining homeostasis of glucose, lipid, and protein metabolism, and thus induces anabolism in tissues. In addition, it has effects on normal growth and development. Insulin receptor and its associated protein IRS1 relay signal transmission to the PI3K-Akt pathway, which consequently increases mTOR-S6K activity. Activation of the mTOR-S6K1 pathway by insulin may lead to fat accumulation in adipose tissue, hypertrophy of skeletal muscle, growth of pancreatic β cells, and protein synthesis[15]. Therefore, the control of insulin signaling is tightly regulated by a negative feedback mechanism. In fact, the downstream components of the insulin receptor give inhibitory autoregulatory signals to upstream molecules along the insulin-signaling pathway or through unrelated pathways that cause insulin resistance. In particular, phosphorylation of IRS proteins on serine residues is a key step in the processes of physiological and pathological conditions. So, the kinases that phosphorylate IRS1/2 have been extensively studied.
Hepatic steatosis alone, or to a greater degree in combination with endotoxin challenge, makes the liver susceptible to oxidative damage and thus facilitates the pathologic process of hepatitis. The cytokines produced by accumulated fat with or without endotoxin cause insulin resistance. In particular, tumor necrosis factor α (TNFα) and interleukin-6 (IL-6) lead to insulin resistance through multiple mechanisms. These include c-Jun N-terminal kinase 1 (JNK1)-mediated serine phosphorylation of IRS-1, IκB kinase-dependent nuclear factor-κB activation, and suppressors of cytokine signaling-3 (SOCS-3) induction[34-36]. Since TNFα increases insulin resistance in peripheral organs, inhibition of TNFα activity and/or its decreased expression would be of help to overcome insulin resistance. However, IL-6 displays pleiotropic functions in a tissue-specific and time-dependent manner. IL-6 confers insulin resistance in hepatocytes through activation of SOCS protein through the Jak/Stat pathway to inhibit tyrosine phosphorylation of IRS1[36], while IL-6 increases insulin sensitivity by stimulating basal glucose transport in 3T3-L1 adipocytes[37], smooth muscle[38] and chondrocytes[39]. Acute treatment with IL-6 increases insulin sensitivity due to AMPK activation[40], while chronically elevated IL-6 leads to impaired insulin signaling and cellular insulin resistance via activating SOCS-3[36] and reducing the expression of the adiponectin, GLUT4, IRS1 mRNA, IRS-1 protein and its tyrosine phosphorylation[41,42].
Glucose is overproduced in the liver of patients with type 2 diabetes[43]. Because AMPK serves as an energy-saving mechanism, its activation decreases hepatic gluconeogenesis. The experimental results using gene knockouts, pharmacological means, or adenoviral activation of AMPK support the role of AMPK in the regulation of glucose production in the liver. Consistently, hepatic glucose production increased to show hyperglycemia and glucose intolerance in liver-specific AMPKα2 deficient mice. Hence, it is highly likely that the hepatic AMPKα2 isoform is critical for repressing hepatic glucose production and maintaining fasting blood glucose levels in the physiological range[44]. Consistently, AMPK activation by adenovirus expressing a constitutively active form of AMPKα2 as well as by 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR, a direct AMPK activator) or metformin reduced glucose output[45-47].
Activation of S6K1 exerts a negative feedback action on insulin signaling. As an example, TNFα secreted by non-parenchymal cells activates S6K1 in pathologic states. The important role of S6K1 on insulin resistance was proven by a study using S6K1-null mice[48,49]. A key role for mTOR-S6K1 regulation of insulin resistance was also supported by the finding that rapamycin blocked IRS1 phosphorylation[50,51], confirming the importance of S6K1 activity in inducing insulin resistance. Hence, insulin resistance induced by abnormal conditions such as hyperinsulinemia, obesity and excess nutrient availability is accompanied by an increase in S6K1 activity[48,52]. The result of a study using a knockout model proved the critical role of S6K1 and its physiological feedback importance to IRS1/2 and PI3K for insulin resistance. In an experimental model, the inhibitory effect of high-fat diet consumption on the insulin receptor-PI3K pathway is also mediated by S6K1. In our laboratory, it was found that the inhibitory modulation of S6K1 activity by beneficial candidates reversed insulin resistance and hyperglycemia[50]. In particular, oltipraz treatment inhibited S6K1 through AMPK activation. Consistently, a dominant negative mutant of AMPK abrogated S6K1 phosphorylation[50]. AMPK activation by other drugs like metformin and rosiglitazone also contribute to insulin sensitivity enhancement[47,53]. Similarly, other agents that inhibit insulin resistance also antagonize S6K1 activation downstream of AMPK[50]. So, these agents have the effects of improving insulin sensitivity through a mechanism involving AMPK-mediated S6K1 inhibition in hepatocytes[50].
JNK1 is activated by various stress signals such as cytokines or oxidative stress, and thus the activity of JNK1 increases under prediabetic or diabetic conditions. This important kinase is also implicated in the phosphorylation of IRS1/2[54-56], interfering with insulin action. The JNK pathway is stimulated by oxidative stress conditions, increased flux of free fatty acids and TNFα production, which contributes to developing insulin resistance. The importance of JNK activation is supported by the finding that a deficiency of JNK1 prevented insulin resistance in an experimental model[54]. Moreover, JNK mediates dysfunction of insulin secretion from β cells[57]. Hence, inhibition of JNK by chemical means may help improve insulin resistance and ameliorate hepatic energy metabolism[54,58]. For example, isoliquiritigenin from various natural herbs including licorice has a JNK-inhibitory effect. Thus, isoliquiritigenin is capable of repressing lipogenesis in the liver and protecting hepatocytes from oxidative injury inflicted by fat accumulation through a novel JNK-dependent pathway that acts as an upstream component of LXRα (unpublished data).
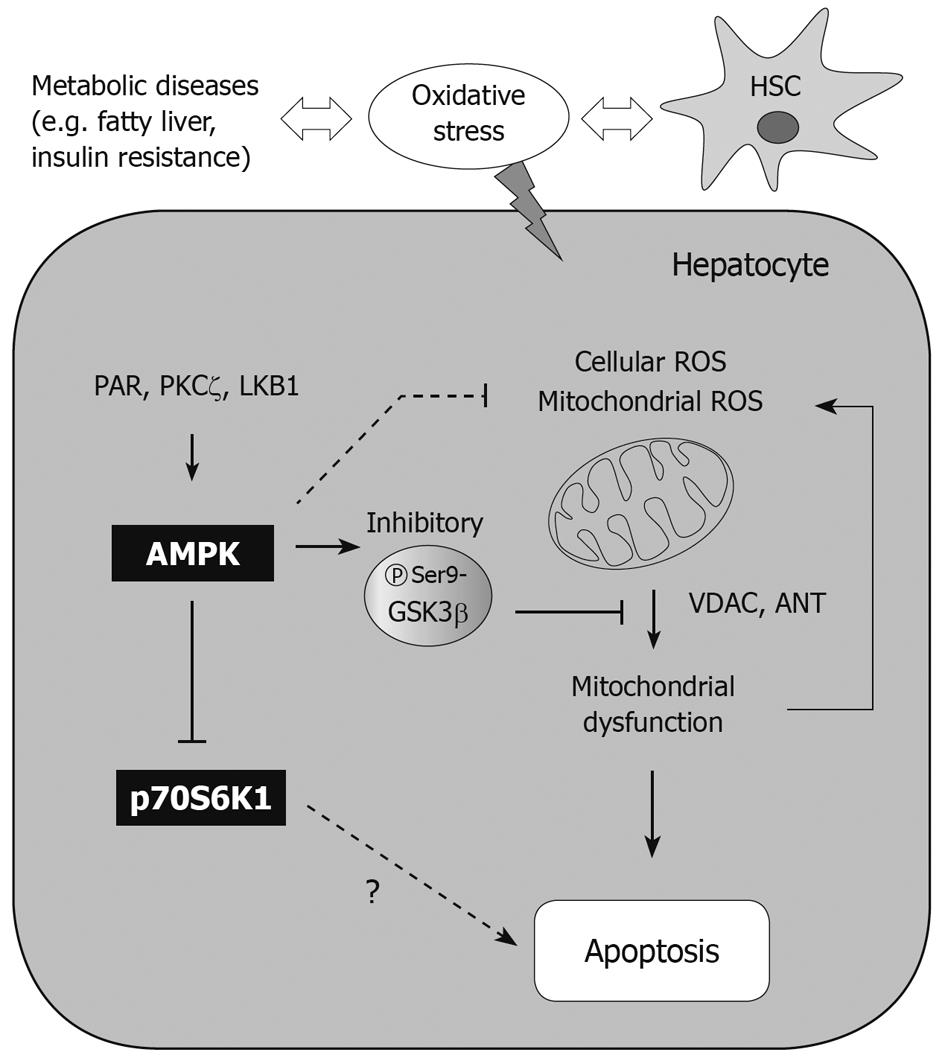
An energy flux is a crucial factor for cell viability. To keep the energy supply constant, eukaryotic cells use AMPK as a mechanism to monitor ATP production and expenditure. As a consequence of its sensitivity to AMP levels, AMPK is activated by treatment with drugs including metformin and TZDs as well as by conditions of metabolic stress that repress ATP production (e.g. hypoxia or glucose deprivation). Thus, AMPK activation causes upregulation of ATP-producing catabolic pathways. However, AMPK inhibits ATP-consuming pathways including synthesis of fatty acids, cholesterol, glycogen, and proteins[59]. Although AMPK signaling is intricately tied to energy metabolism and homeostasis, it is also critical for various physiological processes including inflammation, and proliferation[60,61]. It is noteworthy that the AMPK-associated pathway may suppress apoptosis induced by glucocorticoids[62], hyperglycemia[63], hepatic ischemia-reperfusion[64] and oxidative stress[65-69]. AMPK activation has a beneficial effect on cell viability via protection of mitochondria from apoptosis: phosphorylation of glycogen synthase kinase 3β (GSK3β)[66], and phosphorylation of Bad, which leads to inhibition of cytochrome c release and attenuation of caspase-3 activity[70]. AMPK is also implicated in other pathophysiological responses in various cell types: a decrease in endoplasmic reticulum (ER) stress[71], DNA damage repair[72,73], autophagy[74,75], and the antioxidant defense system[65-69]. This review focuses on the role of AMPK in hepatocyte viability.
Regulation of cellular balance between biosynthesis and turnover is crucial for the maintenance of metabolic homeostasis. Autophagy is an evolutionally conserved pathway for self-digesting of cytoplasmic components and organelles by lysosomal degradation[76]. Autophagy contributes to cell survival via removal of long-lived proteins and damaged organelles, thus this event plays a role in adaptive protection upon starved conditions[77]. In addition, a recent study showed that autophagy regulates lipid metabolism by inducing lipid utilization in hepatocytes, implicating a possible link with metabolic diseases[78]. The autophagic processes are regulated by several signal transduction mechanisms. Among them, AMPK activation induces autophagy in response to diverse stress conditions including energy depletion, ER stress, and hypoxia. The action of AMPK is mediated by the inhibition of mTOR-dependent signaling, which is a central inhibitory pathway of autophagy[79]. The AMPK-induced autophagy exerts a cytoprotective effect, which can be regulated by upstream kinases such as LKB1[74,75]. However, the role of S6K1 inhibition by AMPK in the modulation of autophagy is unclear. Despite these primarily defensive effects, autophagy mediates cell death under certain conditions[77], thus further study would help understand the role of AMPK in autophagy-associated cell viability.
Insulin resistance has been associated with a reduction in mitochondrial oxidative phosphorylation and ATP production, and thus downregulates the expression of genes encoding for oxidative metabolism[80-82]. Thus, mitochondrial dysfunction is frequently observed in the metabolic syndrome[82]. Under mitochondrial dysfunction caused by several endogenous or exogenous stimulants, it is difficult to maintain redox-homeostasis. In this situation, changes in mitochondrial membrane permeability (MMP) cause the release of proapoptotic mediators that can damage DNA and lead to apoptosis[83,84]. Oxidative stress inhibits endoplasmic reticulum calcium pumps, releasing calcium into the cytoplasm from endoplasmic reticulum. The cytoplasmic calcium is taken up by mitochondria, which makes the mitochondrial permeability transition pore (mPTP)[85,86]. In basal conditions, the mPTP is closed but opens in response to stress, allowing passage of small molecules. Opening of the mPTP causes MMP transition and cytochrome c release, inducing apoptosis. A number of studies have shown that chemical inhibitors of the mPTP have the ability to prevent the release of cytochrome c and protect cells from death[87]. Excess reactive oxygen species may enhance the opening of the mPTP, and cause mitochondrial depolarization and cytochrome c leakage[88,89]; the release of cytochrome c from mitochondria to cytoplasm activates procaspase-9 and Apaf-1, and stimulates apoptosome formation and caspase-3 activation so that it induces cell death[90].
AMPK: AMPK responds to external stress as a modulator of cell viability or death. In many cases, AMPK activation exerts a cytoprotective effect[62-64,66]. Chemical activation of AMPK protected cells from arachidonic acid-induced apoptosis and restored MMP. In this model, cell viability depended on mitochondrial function; treatment of the AMPK activator (e.g. oltipraz and resveratrol) protected cells from mitochondrial injury. Thus, the direct or indirect AMPK activators have the ability to protect cells from mitochondrial oxidative stress. This mitochondrial protective effect could be reversed by either compound C treatment or overexpression of the dominant negative mutant of AMPKα. In our laboratory, the AMPK-dependent antioxidant and cytoprotective effects had been tested with AICAR. Cellular H2O2 production increased by arachidonic acid treatment impairs mitochondrial function, and promotes apoptosis. Thus, arachidonic acid propagates apoptotic signals due to oxidative stress alone or in combination with an increase in mitochondrial Ca2+ uptake[91]. In this model, AICAR exhibited a cytoprotective effect against injury caused by arachidonic acid so that it abolished reactive oxygen species production in the cell. The data showing that compound C treatment induced MMP transition indicate that AMPK is necessary for MMP regulation. AMPK increases its activity through TSC2 phosphorylation, which leads to translational suppression and cell size reduction under the situation of energy deprivation. Moreover, the phosphorylation of TSC2 protects cells from apoptosis induced by energy deprivation[16], suggesting that the downstream components of AMPK may be responsible for MMP regulation.
In a recent study, resveratrol, a polyphenolic component found in grapes and red wine, was shown to protect mitochondria from oxidative stress in an AMPK-dependent manner. AMPK activation by resveratrol depended on LKB1, but not CaMKK. Thus, LKB1 activation protects cells from apoptosis under the condition of energy stress[92]. The importance of LKB1 for AMPK-dependent cytoprotection is also supported by the result of the sauchinone study: sauchinone exerted a protective effect against MMP transition via LKB1 activation[69]. The upstream components that activate LKB1 include SIRT1[93], nitric oxide synthase[94], and protein kinase A[12]. In addition, we identified the formation of poly (ADP-ribose) (PAR) as the upstream event, by which resveratrol activates LKB1[66]. PAR polymerase (PARP) represents a nuclear enzyme that plays a role in DNA damage repair through PAR formation. In an energetic process, PAR causes rapid depletion of NAD+, decreases ATP production, and thus leads to cell death[75]. In contrast, PARP prevents cell death through LKB1-AMPK-mediated autophagy activation[75], which may be associated with LKB1. Sometimes, AMPK activation may cause apoptosis; sustained AMPK activation (> 10 h) triggered hepatocyte death through JNK and caspase-3 activation. In this process, p53, Bax and Fas ligand are upregulated or activated by activated JNK[95]. Hence, AMPK-dependent cell survival may rely on cell type, environmental conditions and on the duration of this kinase activation[95].
Mn-superoxide dismutase (Mn-SOD) as a mitochondrial enzyme converts the superoxide anion to hydrogen peroxide, and plays a role in cytoprotection[96]. Pro-oxidants like paraquat and dinitrophenol induce Mn-SOD in the liver[97,98]. Treatment with metformin or AICAR, an AMPK activator, increases the expression of MnSOD mRNA, suggesting that Mn-SOD induction may be coupled to the AMPK-associated pathway.
GSK3β: GSK3β is a constitutively activated serine/threonine kinase in normal state. This enzyme is well known as a regulator of glycogen metabolism, gene expression, and cell cycle progression[99]. GSK3β is inactivated by serine 9 phosphorylation[100], enabling cells to suppress mPTP opening[101] and prevent apoptosis of hepatocytes[66]. Hence, this kinase may contribute to cell viability against external stress (e.g. ischemia/reperfusion injury). It has also been recognized that inhibitory phosphorylation of GSK3β prevents phosphorylation of voltage-activated anion channel, and promotes binding of GSK3β with adenine nucleotide translocase. In our study, GSK3β inhibition protected mitochondria from mPTP opening and contributed to cell survival against severe oxidative stress[66], as also supported by other reports[102,103]. This contention is supported by the finding that treatment by a direct AMPK activator (i.e. AICAR) leads to GSK3β inhibition (Figure 2), as mediated with Raf1/ERK/p90 kDa ribosomal S6 kinase[104]. Some other cytoprotective compounds also act as AMPK activators, which include resveratrol and isoliquiritigenin, and cause GSK3β inhibition[66]. Thus, GSK3β phosphorylation may lie downstream of AMPK.
PKC: In certain situations, necrosis may also be programmed through specific pathways. Hepatocytes undergo necrosis several hours after H2O2 treatment in association with PKC activation and/or AMPK inhibition, as evidenced by a decrease in cell death by PKC inhibitor treatment. Interestingly, PKC inhibition results in AMPK upregulation, suggesting that these two pathways are inversely coupled to each other[105]. Apparently, these pathways are linked to a cytoprotective effect, as shown by decreased H2O2-induced necrosis after treatment with PKC inhibitor or AMPK activator. Consistently, compound C treatment (an AMPK inhibitor) abrogated the ability of PKC inhibitor to protect cells, suggesting that PKC inhibitors have a cytoprotective effect through AMPK upregulation.
S6K1: In S6K1-/- hepatocytes, caspase-8 and Bid (a pro-apoptotic protein) were both down-regulated relative to control. A deficiency of S6K1 was not sensitive to the cascades of death receptor activation, as shown by no caspase-8 activation or FLIPL degradation in hepatocytes challenged by TNF-α or anti-Fas antibody treatment. The finding that Bid cleavage, cytochrome c release, caspase-3 activation, and DNA laddering were all attenuated by a deficiency of S6K1 raises the importance of S6K1 in the apoptotic process. Consistently, the lack of S6K1 did not diminish the BclxL/Bim ratio in cells deprived of serum, and thus prevented cytochrome c release and DNA fragmentation[106]. In an animal model, S6K1 deficiency enabled hepatocytes to survive against concanavalin A-induced apoptosis[106]. Inhibition of S6K1 may activate survival pathways through PI3K/Akt and ERK pathways. However, hepatocytes deficient in S6K1 underwent apoptosis on serum withdrawal when combined with PI3K or ERK inhibitor treatment[106]. In this sense, S6K1 inhibition along with Akt and ERK inhibitors, would enhance the efficacy of cancer chemotherapy for hepatocarcinoma[106]. In our oxidative stress model, rapamycin, an inhibitor of mTOR-S6K1 activity that causes dissociation of raptor from mTOR by binding FK506 binding protein 12, had no effect on apoptosis elicited by arachidonic acid + iron, suggesting that the inhibition of S6K1 alone may not be sufficient to promote cell viability. Overall, the inhibition of S6K1 may contribute to protecting hepatocytes from liver failure, and if so, it might result from improvement in insulin signaling.
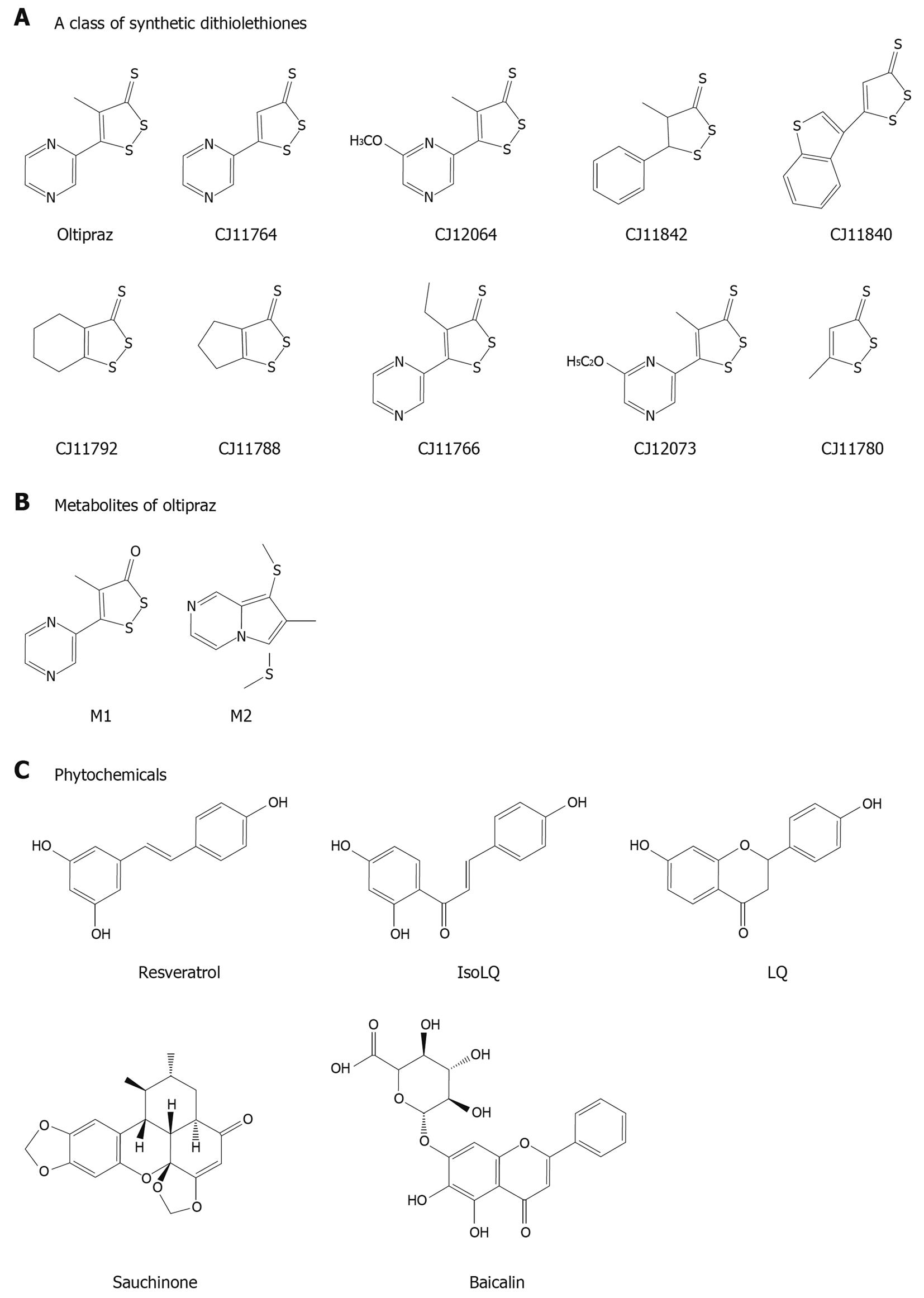
A series of beneficial compounds with the abilities of AMPK activation are listed in Table 1 and Figure 3, which may have liver-protective effects against external stimuli. Thus, the compounds that have modulating activities on metabolism may also have cytoprotective effects (Figure 4). In these actions, LKB1-dependent AMPK activation may be one of the key molecular pathways for cell survival. A number of studies have shown how AMPK responds to an increase in AMP as an energy sensing enzyme. In this way, it integrates diverse signal inputs, controls a number of metabolic enzymes in various cell types, and adapts cellular processes to the energy status. Since AMPK activation may not always be on the side of cell survival, the specific AMPK pathways responsible for cell viability still remain to be elucidated.
| Chemicals | AMPK | S6K1 | NAFLD | Hepatic insulin resistance | Cyto-protection in the liver | Effective conc. | Ref. |
| A class of synthetic dithiolethiones | |||||||
| Oltipraz | ↑ | ↓ | + | + | + | 30 μmol/L, 30 mg/kg | [27,50,67,107] |
| CJ11764 | ↑ | ↓ | + | + | + | 30 μmol/L | [27,50,67] |
| CJ12064 | ↑ | ↓ | + | + | + | 30 μmol/L | [27,50,67,107] |
| CJ11842 | ↑ | ↓ | + | + | + | 30 μmol/L | [27,50,67,107] |
| CJ11840 | ↑ | ↓ | + | + | + | 30 μmol/L | [27,50,67] |
| CJ11792 | ↑ | ↓ | + | + | + | 30 μmol/L | [27,50,67,107] |
| CJ11788 | ↑ | ↓ | + | + | + | 30 μmol/L | [27,50,67,107] |
| CJ11766 | ↑ | ↓ | ND | + | + | 30 μmol/L | [67,107] |
| CJ12073 | ↑ | - | + | + | + | 30 μmol/L | [27,67,107] |
| CJ11780 | ↑ | ND | + | ND | ND | 30 μmol/L | [27] |
| Metabolites of oltipraz | |||||||
| M1 | ↑ | ↓ | ND | + | + | 30 μmol/L | [50,68] |
| M2 | ↑ | ND | ND | ND | + | 30 μmol/L | [68] |
| Phytochemicals | |||||||
| Resveratrol | ↑ | ND | + | + | + | 30 μmol/L | [66,108,109] |
| Isoliquiritigenin (Glycyrrhizae radix) | ↑ | - | + | ND | + | 20 μmol/L, 30 mg/kg | [65], UD |
| Liquiritigenin (Glycyrrhizae radix) | ↑ | - | + | ND | + | 100 μmol/L, 30 mg/kg | [65], UD |
| Sauchinone (Saururus chinensis) | ↑ | - | + | ND | + | 30 μmol/L, 30 mg/kg | [69,110] |
| Baicalin (Scutellaria baicalensis) | ↑ | ND | + | ND | ND | 10 μmol/L, 80 mg/kg | [111] |
Metformin is a major drug used in the treatment of type 2 diabetes. AMPK activation by metformin suppresses hepatic glucose production and lowers blood glucose levels[47,112]. In addition, metformin has been shown to reverse fatty liver disease in humans[113,114]. TZDs belong to another important class of antidiabetic drugs that augment systemic insulin sensitivity. In diabetic patients, pioglitazone decreases hepatic fat content and increases splanchnic glucose uptake presumably through AMPK[115]. In addition, these medications may prevent simple hepatic steatosis from progressing to steatohepatitis. Although the molecular mechanism of AMPK activation by TZDs is unclear, AMPK activation is attributed to their ability to increase plasma adiponectin levels[53].
Hepatic ischemia-reperfusion injury, usually in association with liver transplantation and hepatic resection, is an important clinical issue. Ischemic preconditioning may be beneficial to patients with hepatic resections in which long periods of ischemia are necessary. Ischemic preconditioning prevents ATP degradation and intracellular accumulation of AMP induced by subsequent ischemia[116]. Increases in AMP levels during ischemia activate AMPK, while AMPK inhibition abolishes the effect of preconditioning, indicating that AMPK plays a role in this effect[64]. So, hepatic preconditioning may allow the liver to preserve energy metabolism during sustained ischemia[116]. Since AMPK activation by preconditioning may represent a new strategy to reduce the ischemia-reperfusion injury, modified preservation solutions containing AMPK activators may be of use, which should be evaluated in clinical settings.
As the mitochondrion plays a diverse role in essential cellular functions including energy production and homeostasis, redox cell signaling, and apoptosis, the chemical activators of AMPK protect hepatic mitochondria against toxic stress. The inhibition of S6K1 downstream of AMPK may also have a distinct role in liver biology. Thus, the AMPK pathway is associated with various pathological conditions, including metabolic syndrome and numerous apoptotic conditions. Because of the shared regulatory functions of AMPK in metabolism and cell viability, it becomes an advantageous target. In this review, we have proposed the concept that AMPK-associated signaling bridges the gap between fuel metabolism and hepatocyte viability, which may be of help in identifying valuable potential targets to prevent and/or treat derangement of metabolism and cell death in the liver.
Peer reviewers: Hui-Kang Liu, PhD, Assistant Research Fellow, National Research Institute of Chinese Medicine, 155-1, Li-nung Street Section 2, Taipei 112, Taiwan, China; Vance Matthews, PhD, BS, Cellular and Molecular Metabolism Laboratory, Baker University of Texas Medical Branch, IDI, PO Box 6492, St Kilda Road Central, VIC 8008, Melbourne, Australia
S- Editor Wang JL L- Editor Webster JR E- Editor Lin YP
| 1. | Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311-357. [Cited in This Article: ] |
| 2. | Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14:72-81. [Cited in This Article: ] |
| 3. | Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med. 2008;44:1259-1272. [Cited in This Article: ] |
| 4. | Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799-845. [Cited in This Article: ] |
| 5. | Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359-407. [Cited in This Article: ] |
| 6. | Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328-341. [Cited in This Article: ] |
| 7. | Lage R, Diéguez C, Vidal-Puig A, López M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14:539-549. [Cited in This Article: ] |
| 8. | Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004-2008. [Cited in This Article: ] |
| 9. | Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9-19. [Cited in This Article: ] |
| 10. | Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336-25343. [Cited in This Article: ] |
| 11. | Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952-962. [Cited in This Article: ] |
| 12. | Collins SP, Reoma JL, Gamm DM, Uhler MD. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem J. 2000;345 Pt 3:673-680. [Cited in This Article: ] |
| 13. | Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, Morrice N, Deak M, Alessi DR. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell vrowth. J Biol Chem. 2001;276:19469-19482. [Cited in This Article: ] |
| 14. | Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem. 2005;280:26089-26093. [Cited in This Article: ] |
| 15. | Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393-402. [Cited in This Article: ] |
| 16. | Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577-590. [Cited in This Article: ] |
| 17. | Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev. 2008;29:777-822. [Cited in This Article: ] |
| 18. | Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27-38. [Cited in This Article: ] |
| 19. | Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, Omatsu T, Nakajima T, Sarui H, Shimazaki M. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722-728. [Cited in This Article: ] |
| 20. | Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1139-1142. [Cited in This Article: ] |
| 21. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [Cited in This Article: ] |
| 22. | Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819-2830. [Cited in This Article: ] |
| 23. | Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520-9528. [Cited in This Article: ] |
| 24. | Nakamuta M, Kohjima M, Morizono S, Kotoh K, Yoshimoto T, Miyagi I, Enjoji M. Evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2005;16:631-635. [Cited in This Article: ] |
| 25. | Kohjima M, Enjoji M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. Int J Mol Med. 2007;20:351-358. [Cited in This Article: ] |
| 26. | Higuchi N, Kato M, Shundo Y, Tajiri H, Tanaka M, Yamashita N, Kohjima M, Kotoh K, Nakamuta M, Takayanagi R. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res. 2008;38:1122-1129. [Cited in This Article: ] |
| 27. | Hwahng SH, Ki SH, Bae EJ, Kim HE, Kim SG. Role of adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway in repression of liver X receptor-alpha-dependent lipogenic gene induction and hepatic steatosis by a novel class of dithiolethiones. Hepatology. 2009;49:1913-1925. [Cited in This Article: ] |
| 28. | Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91-100. [Cited in This Article: ] |
| 29. | Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288-1295. [Cited in This Article: ] |
| 30. | Ki SH, Choi JH, Kim CW, Kim SG. Combined metadoxine and garlic oil treatment efficaciously abrogates alcoholic steatosis and CYP2E1 induction in rat liver with restoration of AMPK activity. Chem Biol Interact. 2007;169:80-90. [Cited in This Article: ] |
| 31. | Gervois P, Fruchart JC, Staels B. Drug Insight: mechanisms of action and therapeutic applications for agonists of peroxisome proliferator-activated receptors. Nat Clin Pract Endocrinol Metab. 2007;3:145-156. [Cited in This Article: ] |
| 32. | Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226-25232. [Cited in This Article: ] |
| 33. | Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580-585. [Cited in This Article: ] |
| 34. | Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275:9047-9054. [Cited in This Article: ] |
| 35. | Ogihara T, Asano T, Katagiri H, Sakoda H, Anai M, Shojima N, Ono H, Fujishiro M, Kushiyama A, Fukushima Y. Oxidative stress induces insulin resistance by activating the nuclear factor-kappa B pathway and disrupting normal subcellular distribution of phosphatidylinositol 3-kinase. Diabetologia. 2004;47:794-805. [Cited in This Article: ] |
| 36. | Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003;278:13740-13746. [Cited in This Article: ] |
| 37. | Stouthard JM, Oude Elferink RP, Sauerwein HP. Interleukin-6 enhances glucose transport in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1996;220:241-245. [Cited in This Article: ] |
| 38. | Hardin J, Kroeker K, Chung B, Gall DG. Effect of proinflammatory interleukins on jejunal nutrient transport. Gut. 2000;47:184-191. [Cited in This Article: ] |
| 39. | Shikhman AR, Brinson DC, Valbracht J, Lotz MK. Cytokine regulation of facilitated glucose transport in human articular chondrocytes. J Immunol. 2001;167:7001-7008. [Cited in This Article: ] |
| 40. | Yuen DY, Dwyer RM, Matthews VB, Zhang L, Drew BG, Neill B, Kingwell BA, Clark MG, Rattigan S, Febbraio MA. Interleukin-6 attenuates insulin-mediated increases in endothelial cell signaling but augments skeletal muscle insulin action via differential effects on tumor necrosis factor-alpha expression. Diabetes. 2009;58:1086-1095. [Cited in This Article: ] |
| 41. | Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045-1050. [Cited in This Article: ] |
| 42. | Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777-45784. [Cited in This Article: ] |
| 43. | Viollet B, Mounier R, Leclerc J, Yazigi A, Foretz M, Andreelli F. Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007;33:395-402. [Cited in This Article: ] |
| 44. | Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology. 2006;147:2432-2441. [Cited in This Article: ] |
| 45. | Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331-1339. [Cited in This Article: ] |
| 46. | Bergeron R, Previs SF, Cline GW, Perret P, Russell RR 3rd, Young LH, Shulman GI. Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076-1082. [Cited in This Article: ] |
| 47. | Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167-1174. [Cited in This Article: ] |
| 48. | Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200-205. [Cited in This Article: ] |
| 49. | Tremblay F, Brûlé S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2007;104:14056-14061. [Cited in This Article: ] |
| 50. | Bae EJ, Yang YM, Kim JW, Kim SG. Identification of a novel class of dithiolethiones that prevent hepatic insulin resistance via the adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway. Hepatology. 2007;46:730-739. [Cited in This Article: ] |
| 51. | Takano A, Usui I, Haruta T, Kawahara J, Uno T, Iwata M, Kobayashi M. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol. 2001;21:5050-5062. [Cited in This Article: ] |
| 52. | Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhäusl W, Marette A, Roden M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674-2684. [Cited in This Article: ] |
| 53. | Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654-2660. [Cited in This Article: ] |
| 54. | Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333-336. [Cited in This Article: ] |
| 55. | Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87-96. [Cited in This Article: ] |
| 56. | Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci USA. 2006;103:10741-10746. [Cited in This Article: ] |
| 57. | Hou N, Torii S, Saito N, Hosaka M, Takeuchi T. Reactive oxygen species-mediated pancreatic beta-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology. 2008;149:1654-1665. [Cited in This Article: ] |
| 58. | Nakatani Y, Kaneto H, Kawamori D, Hatazaki M, Miyatsuka T, Matsuoka TA, Kajimoto Y, Matsuhisa M, Yamasaki Y, Hori M. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem. 2004;279:45803-45809. [Cited in This Article: ] |
| 59. | Spasić MR, Callaerts P, Norga KK. AMP-activated protein kinase (AMPK) molecular crossroad for metabolic control and survival of neurons. Neuroscientist. 2009;15:309-316. [Cited in This Article: ] |
| 60. | Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab. 2006;17:205-215. [Cited in This Article: ] |
| 61. | Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys. 2007;47:332-347. [Cited in This Article: ] |
| 62. | Stefanelli C, Stanic I, Bonavita F, Flamigni F, Pignatti C, Guarnieri C, Caldarera CM. Inhibition of glucocorticoid-induced apoptosis with 5-aminoimidazole-4-carboxamide ribonucleoside, a cell-permeable activator of AMP-activated protein kinase. Biochem Biophys Res Commun. 1998;243:821-826. [Cited in This Article: ] |
| 63. | Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159-167. [Cited in This Article: ] |
| 64. | Peralta C, Bartrons R, Serafin A, Blázquez C, Guzmán M, Prats N, Xaus C, Cutillas B, Gelpí E, Roselló-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164-1173. [Cited in This Article: ] |
| 65. | Choi SH, Kim YW, Kim SG. AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem Pharmacol. 2010;79:1352-1362. [Cited in This Article: ] |
| 66. | Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884-895. [Cited in This Article: ] |
| 67. | Shin SM, Kim SG. Inhibition of arachidonic acid and iron-induced mitochondrial dysfunction and apoptosis by oltipraz and novel 1,2-dithiole-3-thione congeners. Mol Pharmacol. 2009;75:242-253. [Cited in This Article: ] |
| 68. | Kwon YN, Shin SM, Cho IJ, Kim SG. Oxidized metabolites of oltipraz exert cytoprotective effects against arachidonic acid through AMP-activated protein kinase-dependent cellular antioxidant effect and mitochondrial protection. Drug Metab Dispos. 2009;37:1187-1197. [Cited in This Article: ] |
| 69. | Kim YW, Lee SM, Shin SM, Hwang SJ, Brooks JS, Kang HE, Lee MG, Kim SC, Kim SG. Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury. Free Radic Biol Med. 2009;47:1082-1092. [Cited in This Article: ] |
| 70. | Kewalramani G, Puthanveetil P, Wang F, Kim MS, Deppe S, Abrahani A, Luciani DS, Johnson JD, Rodrigues B. AMP-activated protein kinase confers protection against TNF-{alpha}-induced cardiac cell death. Cardiovasc Res. 2009;84:42-53. [Cited in This Article: ] |
| 71. | Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554-9575. [Cited in This Article: ] |
| 72. | Fu X, Wan S, Lyu YL, Liu LF, Qi H. Etoposide induces ATM-dependent mitochondrial biogenesis through AMPK activation. PLoS One. 2008;3:e2009. [Cited in This Article: ] |
| 73. | Walker JW, Jijon HB, Madsen KL. AMP-activated protein kinase is a positive regulator of poly(ADP-ribose) polymerase. Biochem Biophys Res Commun. 2006;342:336-341. [Cited in This Article: ] |
| 74. | Herrero-Martín G, Høyer-Hansen M, García-García C, Fumarola C, Farkas T, López-Rivas A, Jäättelä M. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677-685. [Cited in This Article: ] |
| 75. | Huang Q, Shen HM. To die or to live: the dual role of poly(ADP-ribose) polymerase-1 in autophagy and necrosis under oxidative stress and DNA damage. Autophagy. 2009;5:273-276. [Cited in This Article: ] |
| 76. | Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861-2873. [Cited in This Article: ] |
| 77. | Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12 Suppl 2:1509-1518. [Cited in This Article: ] |
| 78. | Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131-1135. [Cited in This Article: ] |
| 79. | He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67-93. [Cited in This Article: ] |
| 80. | Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267-273. [Cited in This Article: ] |
| 81. | Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466-8471. [Cited in This Article: ] |
| 82. | Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140-1142. [Cited in This Article: ] |
| 83. | Kantrow SP, Piantadosi CA. Release of cytochrome c from liver mitochondria during permeability transition. Biochem Biophys Res Commun. 1997;232:669-671. [Cited in This Article: ] |
| 84. | Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184:1155-1160. [Cited in This Article: ] |
| 85. | Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552-565. [Cited in This Article: ] |
| 86. | Szabadkai G, Rizzuto R. Participation of endoplasmic reticulum and mitochondrial calcium handling in apoptosis: more than just neighborhood? FEBS Lett. 2004;567:111-115. [Cited in This Article: ] |
| 87. | Bradham CA, Qian T, Streetz K, Trautwein C, Brenner DA, Lemasters JJ. The mitochondrial permeability transition is required for tumor necrosis factor alpha-mediated apoptosis and cytochrome c release. Mol Cell Biol. 1998;18:6353-6364. [Cited in This Article: ] |
| 88. | Piret JP, Arnould T, Fuks B, Chatelain P, Remacle J, Michiels C. Mitochondria permeability transition-dependent tert-butyl hydroperoxide-induced apoptosis in hepatoma HepG2 cells. Biochem Pharmacol. 2004;67:611-620. [Cited in This Article: ] |
| 89. | Zoratti M, Szabò I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139-176. [Cited in This Article: ] |
| 90. | Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479-489. [Cited in This Article: ] |
| 91. | Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135-139. [Cited in This Article: ] |
| 92. | Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329-3335. [Cited in This Article: ] |
| 93. | Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015-20026. [Cited in This Article: ] |
| 94. | Vázquez-Chantada M, Ariz U, Varela-Rey M, Embade N, Martínez-Lopez N, Fernández-Ramos D, Gómez-Santos L, Lamas S, Lu SC, Martínez-Chantar ML. Evidence for LKB1/AMP-activated protein kinase/ endothelial nitric oxide synthase cascade regulated by hepatocyte growth factor, S-adenosylmethionine, and nitric oxide in hepatocyte proliferation. Hepatology. 2009;49:608-617. [Cited in This Article: ] |
| 95. | Meisse D, Van de Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH, Foufelle F, Hue L. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett. 2002;526:38-42. [Cited in This Article: ] |
| 96. | Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97-112. [Cited in This Article: ] |
| 97. | Dryer SE, Dryer RL, Autor AP. Enhancement of mitochondrial, cyanide-resistant superoxide dismutase in the livers of rats treated with 2,4-dinitrophenol. J Biol Chem. 1980;255:1054-1107. [Cited in This Article: ] |
| 98. | Krall J, Bagley AC, Mullenbach GT, Hallewell RA, Lynch RE. Superoxide mediates the toxicity of paraquat for cultured mammalian cells. J Biol Chem. 1988;263:1910-1914. [Cited in This Article: ] |
| 99. | Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3--an overview of an over-achieving protein kinase. Curr Drug Targets. 2006;7:1377-1388. [Cited in This Article: ] |
| 100. | Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391-426. [Cited in This Article: ] |
| 101. | Park SS, Zhao H, Mueller RA, Xu Z. Bradykinin prevents reperfusion injury by targeting mitochondrial permeability transition pore through glycogen synthase kinase 3beta. J Mol Cell Cardiol. 2006;40:708-716. [Cited in This Article: ] |
| 102. | Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res. 2008;103:983-991. [Cited in This Article: ] |
| 103. | Nishihara M, Miura T, Miki T, Tanno M, Yano T, Naitoh K, Ohori K, Hotta H, Terashima Y, Shimamoto K. Modulation of the mitochondrial permeability transition pore complex in GSK-3beta-mediated myocardial protection. J Mol Cell Cardiol. 2007;43:564-570. [Cited in This Article: ] |
| 104. | Wang HM, Mehta S, Bansode R, Huang W, Mehta KD. AICAR positively regulate glycogen synthase activity and LDL receptor expression through Raf-1/MEK/p42/44MAPK/p90RSK/GSK-3 signaling cascade. Biochem Pharmacol. 2008;75:457-467. [Cited in This Article: ] |
| 105. | Saberi B, Shinohara M, Ybanez MD, Hanawa N, Gaarde WA, Kaplowitz N, Han D. Regulation of H(2)O(2)-induced necrosis by PKC and AMP-activated kinase signaling in primary cultured hepatocytes. Am J Physiol Cell Physiol. 2008;295:C50-C63. [Cited in This Article: ] |
| 106. | González-Rodriguez A, Alba J, Zimmerman V, Kozma SC, Valverde AM. S6K1 deficiency protects against apoptosis in hepatocytes. Hepatology. 2009;50:216-229. [Cited in This Article: ] |
| 107. | Bae EJ, Yang YM, Kim SG. Abrogation of hyperosmotic impairment of insulin signaling by a novel class of 1,2-dithiole-3-thiones through the inhibition of S6K1 activation. Mol Pharmacol. 2008;73:1502-1512. [Cited in This Article: ] |
| 108. | Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712-716. [Cited in This Article: ] |
| 109. | Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337-342. [Cited in This Article: ] |
| 110. | Kim YW, Kim YM, Yang YM, Kim TH, Hwang SJ, Lee JR, Kim SC, Kim SG. Inhibition of SREBP-1c-mediated hepatic steatosis and oxidative stress by sauchinone, an AMPK-activating lignan in Saururus chinensis. Free Radic Biol Med. 2010;48:567-578. [Cited in This Article: ] |
| 111. | Guo HX, Liu DH, Ma Y, Liu JF, Wang Y, Du ZY, Wang X, Shen JK, Peng HL. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol Sin. 2009;30:1505-1512. [Cited in This Article: ] |
| 112. | Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642-1646. [Cited in This Article: ] |
| 113. | Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998-1003. [Cited in This Article: ] |
| 114. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893-894. [Cited in This Article: ] |
| 115. | Bajaj M, Suraamornkul S, Pratipanawatr T, Hardies LJ, Pratipanawatr W, Glass L, Cersosimo E, Miyazaki Y, DeFronzo RA. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52:1364-1370. [Cited in This Article: ] |
| 116. | Peralta C, Bartrons R, Riera L, Manzano A, Xaus C, Gelpí E, Roselló-Catafau J. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol Gastrointest Liver Physiol. 2000;279:G163-G171. [Cited in This Article: ] |