Published online Jul 7, 2010. doi: 10.3748/wjg.v16.i25.3187
Revised: March 30, 2010
Accepted: April 6, 2010
Published online: July 7, 2010
AIM: To evaluate the relationship between thiopurine S-methyltransferase (TPMT) polymorphisms and thiopurine-induced adverse drug reactions (ADRs) in inflammatory bowel disease (IBD).
METHODS: Eligible articles that compared the frequency of TPMT polymorphisms among thiopurine-tolerant and -intolerant adult IBD patients were included. Statistical analysis was performed with Review Manager 5.0. Sub-analysis/sensitivity analysis was also performed.
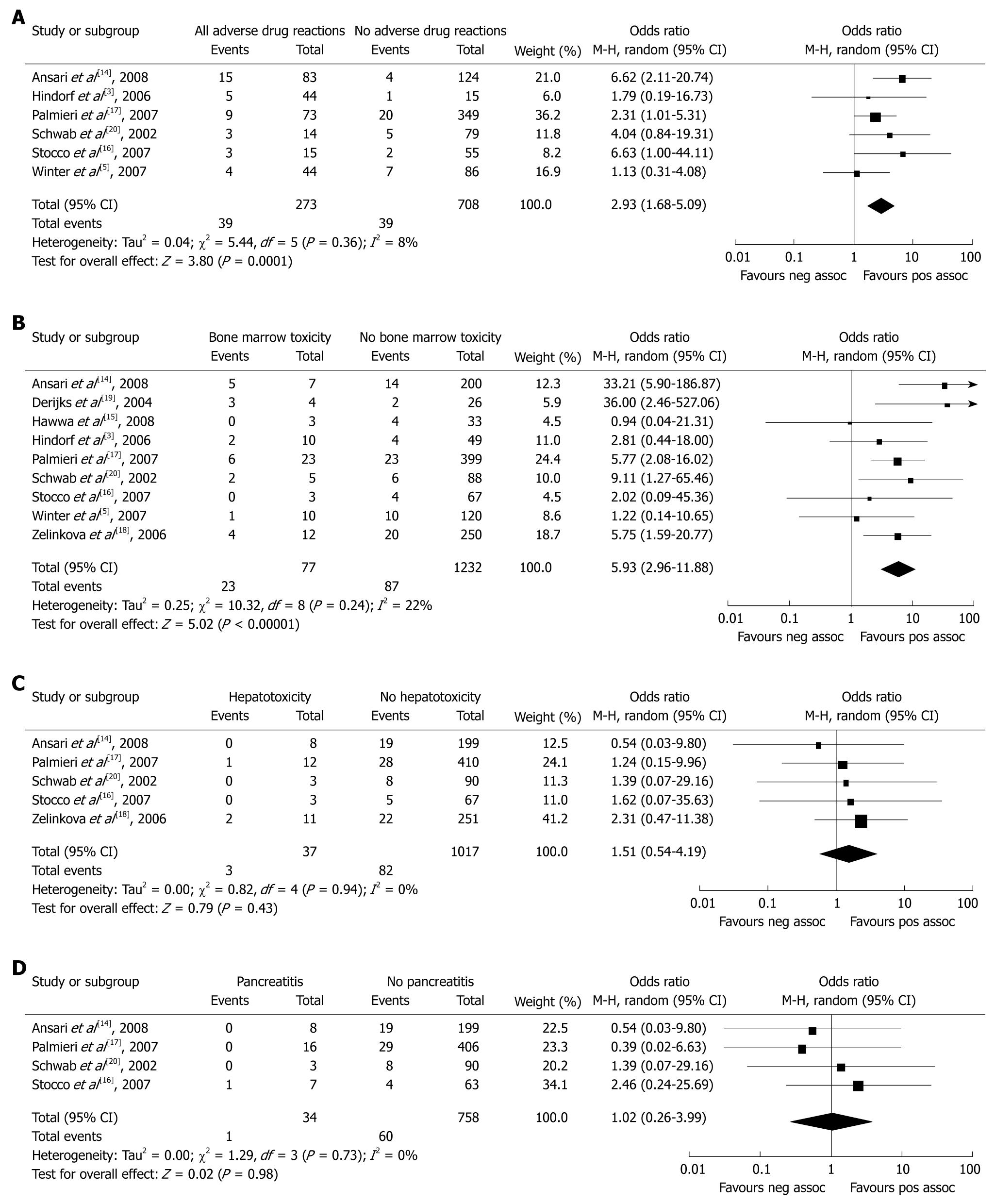
RESULTS: Nine studies that investigated a total of 1309 participants met our inclusion criteria. The incidence of TPMT gene mutation was increased 2.93-fold (95% CI: 1.68-5.09, P = 0.0001) and 5.93-fold (95% CI: 2.96-11.88, P < 0.00001), respectively, in IBD patients with thiopurine-induced overall ADRs and bone marrow toxicity (BMT), compared with controls. The OR for TPMT gene mutation in IBD patients with thiopurine-induced hepatotoxicity and pancreatitis was 1.51 (95% CI: 0.54-4.19, P = 0.43) and 1.02 (95% CI: 0.26-3.99, P = 0.98) vs controls, respectively.
CONCLUSION: This meta-analysis suggests that the TPMT polymorphisms are associated with thiopurine-induced overall ADRs and BMT, but not with hepatotoxicity and pancreatitis.
- Citation: Dong XW, Zheng Q, Zhu MM, Tong JL, Ran ZH. Thiopurine S-methyltransferase polymorphisms and thiopurine toxicity in treatment of inflammatory bowel disease. World J Gastroenterol 2010; 16(25): 3187-3195
- URL: https://www.wjgnet.com/1007-9327/full/v16/i25/3187.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i25.3187
Inflammatory bowel disease (IBD) is a chronic, relapsing and remitting disease of the gastrointestinal tract. The major types of IBD are Crohn’s disease (CD) and ulcerative colitis (UC). The thiopurine drugs azathioprine (AZA) and 6-mercaptopurine (6-MP) are widely used to treat patients with active, steroid-refractory, and steroid-dependent IBD, and have been proven to be effective for inducing and maintaining remission of CD[1,2]. Unfortunately, the dose of thiopurine often has to be reduced or the therapy has to be discontinued in 9%-28% of patients because of adverse drug reactions (ADRs)[3]. Gastrointestinal disturbances, bone marrow toxicity (BMT), hepatotoxicity and pancreatitis are among the most frequent reasons to prevent their use in some patients.
Blood tests have been performed regularly to monitor the blood count and liver function to detect BMT and hepatotoxicity at an early enough stage to discontinue therapy and avoid life-threatening toxicity. However, the ADRs might develop suddenly and unpredictably during the interval between two tests. Thus, screening the patients before thiopurine treatment for genetic susceptibilities to predict the risk of toxicity has aroused considerable interest in several clinical centers.
Thiopurine S-methyltransferase (TPMT) is a very important enzyme for the metabolism of thiopurine compounds. Lack of its activity has been associated with the incidence of BMT induced by thiopurine therapy. Genetic polymorphisms that account for reduced (heterozygote) or absent (homozygote) TPMT activity have been confirmed. About 10% of the patients have intermediate activity due to heterozygosity of the TPMT, and 1 in 300 patients inherit TPMT deficiency as an homozygote[4]. TPMT*1 is the wild type, TPMT*3A is the most prevalent mutant allele (85%) in Caucasians[4] and TPMT*3C is the most common reported mutant alleles in African and South-East Asian populations[5]. Although several studies have shown that low TPMT activity is associated with BMT, the efficacy of the strategy of screening the TPMT gene mutation in all patients prior to initiating treatment with thiopurine drugs has not yet been definitively confirmed.
The aim of this meta-analysis was to evaluate whether there is a relationship between TPMT polymorphisms and incidence of ADRs in IBD patients.
The databases PubMed (1966 to July 2009), Embase (1980 to July 2009), Cochrane Controlled Trials Register (Issue 1, 2009), Science Citation Index (1945 to July 2009) and Chinese Biomedical Database (1981 to July 2009) were used for systematic literature searches. We employed both MeSH and free-language terms for “TPMT”, “thiopurine S-methyltransferase” AND “inflammatory bowel disease”, “ulcerative colitis”, OR “Crohn’s disease” combined with each of the following: “thiopurine”, “azathioprine”, “imuran”, “6-mercaptopurine” as search terms. A comprehensive search of reference lists of all review articles and original studies retrieved by this method was performed to identify additional studies. Furthermore, we also searched abstracts of major gastroenterological meetings, such as the Digestive Disease Week of the American Gastroenterological Association, the World Congress of Gastroenterology and British Society of Gastroenterology. Authors of some identified trials were asked whether they knew of additional studies, including unpublished ones.
The selection criteria were as follows: cross-sectional cohort, prospective cohort and case-control studies were included in the meta-analysis. Studies in abstract form or meeting reports, without publication of the full paper, were also included in the meta-analysis. Only IBD patients aged over 18 years were included.
Studies were included that compared TPMT polymorphism frequencies among thiopurine-tolerant and -intolerant adult IBD patients. Two of the most prevalent mutant types of the TPMT gene were studied, namely, TPMT*3A and TPMT*3C polymorphisms. Studies were included if they provided information on at least one outcome parameter as follows: proper OR of overall ADRs, BMT, hepatotoxicity or pancreatitis.
Furthermore, articles published in English and Chinese were included. Studies in other languages were excluded unless a translation was available.
Standardized data abstraction sheets were prepared. Data were extracted for author and year, location of trials, trial design, diseases, number of enrolled subjects, and dose of thiopurine, meanwhile, key outcome data, such as TPMT polymorphisms and thiopurine induced overall ADRs, BMT, hepatotoxicity and pancreatitis were abstracted from the selected studies. All papers were examined independently for eligibility by two reviewers (Dong XW and Zheng Q). Disagreements were resolved by consulting a third reviewer (Ran ZH). When the results of a particular study were reported in more than one publication, only the most recent and complete data were included in the meta-analysis. Finally, the manuscripts were studied for their comparability by Zhu MM and Tong JL.
Data were entered into the Cochrane Collaboration RevMan 5.0 (Copenhagen, 2008). OR with 95% CI was calculated for the TPMT*3A and TPMT*3C polymorphisms vs overall ADRs, BMT, hepatotoxicity and pancreatitis. Because of too small a number of patients, heterozygous and homozygous patients were combined as “polymorphism-positive”. An OR of < 1 favored the control group, P < 0.05 and 95% CI that did not include the value 1 were considered to be statistically significant. The included studies displayed heterogeneity regarding study design, definition of ADRs, and follow-up periods. Therefore, it would be inappropriate to combine the data for further analysis with the fixed-effects model, whereas the random effects model was used for calculations. Finally, we used funnel plot asymmetry to detect any publication bias in the meta-analysis, and Egger’s regression test to measure funnel plot asymmetry.
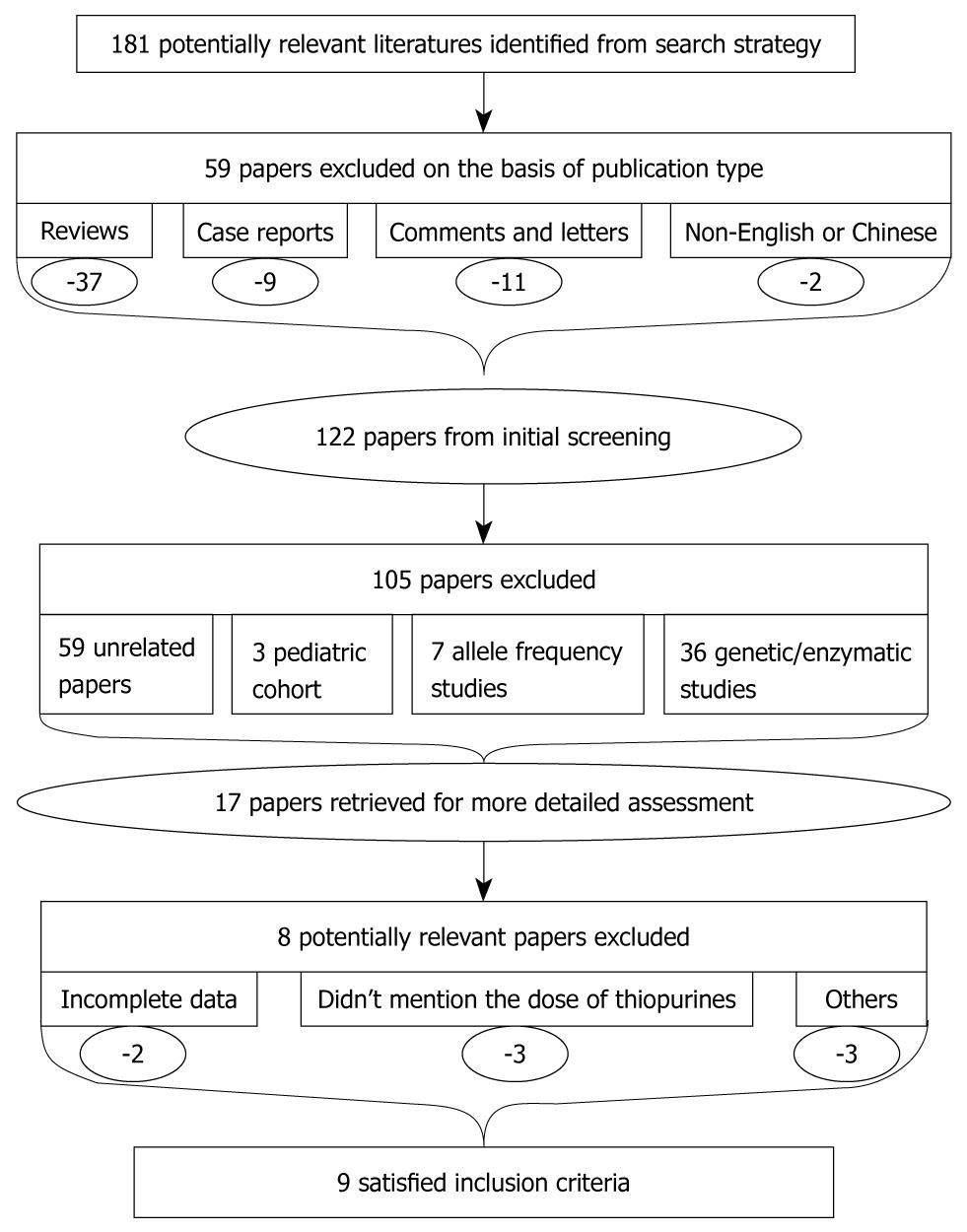
We reviewed 181 citations and abstracts obtained from our computerized literature searches. Fifty-nine papers were excluded on the basis of publication type and another 105 were excluded after examining the title and abstract. Among these 17 potentially appropriate studies, we found that all of them had studied the association between TPMT polymorphisms and thiopurine-induced ADRs. Abstracts and full texts of the remaining 17 papers were retrieved for further assessment. Of these potential eligible articles, we excluded another eight[6-13], which are listed in Table 1. Two studies were not able to extract the data from the published results, and we also failed to contact the authors of this manuscript, therefore, these two studies were excluded. Three studies were excluded for not mentioning the prescription dose of thiopurine. One study was excluded as it did not set a control group, such that a proper OR could not be calculated. One study was excluded since it included non-IBD patients. In one study, not all of the patients had determination of TPMT genotype. Finally, nine studies[3,5,14-20] met the inclusion criteria; all of which were published in the last 7 years. Three were prospective cohort studies, with the remaining six being cross-sectional. The flowchart of reviews showed the detailed process of selection (Figure 1). The characteristics of the nine trials included in the meta-analysis are summarized in Table 2. The outcomes of the meta-analysis are shown in Figure 2.
| Author | Location | Study design | Participants | TPMT genotypes was determined | Dose of thiopurine | Reason for exclusion |
| Cao et al[6], 2009 | China | Cross-sectional | 43 treated IBD patients | TPMT*2, TPMT*3A | AZA 1.35 mg/kg per day | Incomplete data |
| TPMT*3B, TPMT*3C | ||||||
| Takatsu et al[7], 2009 | Japan | Cross-sectional | 147 treated IBD patients | TPMT*2, TPMT*3B | AZA 25 mg/d | Incomplete data |
| TPMT*3C, TPMT*8 | or 75 mg/d | |||||
| or 100 mg/d | ||||||
| Uchiyama et al[8], 2009 | Japan | Cross-sectional | 16 treated IBD patients with ADRs | TPMT*2, *3A, *3B | AZA < 50 mg/d | Incomparable control group |
| TPMT*3C, *3D, *4 | or 6-MP < 30 mg/d | No definition of ADRs | ||||
| TPMT*5, *6, *7, *8 | Pediatric patients included | |||||
| Ban et al[9], 2008 | Japan | Case-control | 70 treated IBD patients | TPMT*2, TPMT*3A | Not mentioned | Dose of thiopurine not mentioned |
| 41 healthy controls | TPMT*3B, TPMT*3C | |||||
| Gearry et al[10], 2003 | New Zealand | Case-control | 56 treated IBD patients with ADRs | TPMT*2, TPMT*3A | Not mentioned | Dose of thiopurine not mentioned |
| 50 treated IBD patients without ADRs | TPMT*3B, TPMT*3C | |||||
| Regueiro et al[11], 2002 | USA | Cross-sectional | 71 treated CD patients | TPMT*3A, TPMT*3B | AZA 2.35 mg/kg per day | 38 patients had determination of TPMT by phenotype |
| TPMT*3C | or 1.28 mg/kg per day | |||||
| Evans et al[12], 2001 | USA | Cross-sectional | 23 treated patients with ADRs | TPMT*2, TPMT*3A | AZA 32 mg/m2 per week | Non-IBD patients |
| TPMT*3B, TPMT*3C | or 175 mg/m2 per week | |||||
| or 280 mg/m2 per week | ||||||
| Naughton et al[13], 1999 | UK | Cross-sectional | 15 treated IBD patients | TPMT*2, TPMT*3A | Not mentioned | Dose of thiopurine not mentioned |
| TPMT*3B, TPMT*3C |
| Author | Location | Study design | Participants | TPMT genotypes was determined | Dose of thiopurine | ADRs and definitions |
| Ansari et al[14], 2008 | UK | Prospective cohort | 215 treated IBD patients | TPMT*3A, TPMT*3B | AZA 2 mg/kg per day | All ADRs |
| TPMT*3C | BMT: WBC < 3.5 × 109 or Ne < 1.5 × 109 | |||||
| H: ALT > 2 × ULN | ||||||
| P: AP and amylase > 4 × ULN and supportive radiological findings | ||||||
| Hawwa et al[15], 2008 | UK | Cross-sectional | 36 treated IBD patients | TPMT*3A, TPMT*3B | AZA 1.49 mg/kg per day | BMT: WBC < 3.0 × 109 or Ne < 1.5 × 109 or PLT < 150 × 109 |
| TPMT*3C | ||||||
| Winter et al[5], 2007 | UK | Cross-sectional | 130 treated IBD patients | TPMT*2, TPMT*3A | AZA 1.6 mg/kg per day | All ADRs |
| TPMT*3C | BMT: WBC < 3 × 109 | |||||
| H: No definition | ||||||
| P: No definition | ||||||
| Stocco et al[16], 2007 | Italy | Cross-sectional | 70 treated IBD patients | TPMT*2, TPMT*3A | AZA: 2 mg/kg per day | All ADRs |
| TPMT*3B, TPMT*3C | BMT: WBC < 3 × 109 or PLT < 100 × 109 | |||||
| H: ALT, AST or ALP > 2 × ULN | ||||||
| P: AP and amylase > 2 × ULN | ||||||
| Palmieri et al[17], 2007 | Italy | Cross-sectional | 422 treated IBD patients | TPMT*3A, TPMT*3B | AZA 2-2.5 mg/kg per day | All ADRs |
| TPMT*3C | or 6-MP 1-1.25 mg/kg per day | BMT: WBC < 3 × 109 or PLT < 100 × 109 | ||||
| H: ALT > 2 × ULN | ||||||
| P: Amylase, lipase > 2 × ULN and AP | ||||||
| Zelinkova et al[18], 2006 | Netherlands | Cross-sectional | 262 treated IBD patients | TPMT*2, TPMT*3A | AZA 2-2.5 mg/kg per day1 | BMT: WBC < 3 × 109 or PLT < 100 × 109 |
| TPMT*3B, TPMT*3C | H: ALT > 2 × ULN | |||||
| Hindorf et al[3], 2006 | Sweden | Prospective cohort | 60 treated IBD patients | TPMT*2, *3A, *3B | AZA 2.5 mg/kg per day | All ADRs |
| TPMT*3C, *3D, *4 | or 6-MP 1.25 mg/kg per day | BMT: WBC < 3 × 109 or PLT < 100 × 109 | ||||
| TPMT*5, *6, *7, *8 | or Ne < 1.5 × 109 | |||||
| TPMT*10, *14, *15 | P: No definition | |||||
| Derijks et al[19], 2004 | Netherlands | Prospective cohort | 30 treated IBD patients | TPMT*2, TPMT*3A | 6-MP 0.71 mg/kg per day | BMT: WBC < 4 × 109 or PLT < 100 × 109 |
| TPMT*3B, TPMT*3C | ||||||
| Schwab et al[20], 2002 | Germany | Cross-sectional | 93 treated IBD patients | TPMT*2, TPMT*3A | AZA 1.5 mg/kg per day | All ADRs |
| TPMT*3B, *3C, *3D | or 1.9 mg/kg per day | BMT: WBC < 3 × 109 or PLT < 100 × 109 | ||||
| H: ALT or AST > 2 × ULN | ||||||
| P: Amylase, lipase > 2 × ULN and AP |
Six[3,5,14,17,18,20] of the nine studies reported on the incidence of overall ADRs in 273 IBD patients exposed to thiopurine drugs vs 708 IBD patients without ADRs. ADRs included BMT, hepatotoxicity, pancreatitis, gastrointestinal disturbances and adverse reactions during treatment that required reduction of thiopurine dose or discontinuation of therapy. Three[3,5,20] of the studies demonstrated that the AZA/6-MP-induced ADRs were independent of the TPMT polymorphisms; the remaining three showed that TPMT polymorphisms strongly predicted ADRs. The TPMT polymorphisms were significantly associated with ADRs in the overall calculated OR (OR: 2.93, 95% CI: 1.68-5.09, P = 0.0001). When excluding hepatotoxicity and pancreatitis from the overall ADRs group, it still indicated that IBD patients exposed to thiopurine drugs, with ADRs, were more likely to have TPMT polymorphisms than the controls (OR: 4.37, 95% CI: 1.69-11.29, P = 0.002).
All of the nine studies[3,5,14-20] reported on the incidence of BMT in 1309 IBD patients treated with thiopurine. Twenty-three of 77 IBD patients with BMT and 87 of 1232 IBD patients without BMT had the TPMT gene mutation. Researchers from the United Kingdom[14] defined BMT as a white blood cells count of < 3.5 × 109/L or a neutrophil count of < 1.5 × 109/L. In the study of Winter et al[5], BMT was defined as a white blood cells count of < 3.0 × 109/L. Researchers from Sweden[3] have defined BMT as a white blood cells count of < 3.0 × 109/L, a neutrophil count of < 1.5 × 109/L, or a platelet count of < 100 × 109/L. Hawwa et al[15] have defined BMT as a white blood cells count of < 3.0 × 109/L, a neutrophil count of < 1.5 × 109/L, or a platelet count of < 150 × 109/L. Dutch researchers[19] have defined BMT as a white blood cells count of < 4.0 × 109/L or a platelet count of < 100 × 109/L. BMT was defined in the remaining four studies[16-18,20] as a white blood cells count of < 3.0 × 109/L or a platelet count of < 100 × 109/L. Of these selected studies, five reported that heterozygous TPMT genotype strongly predicted BMT, and the remaining four failed to demonstrate an association between TPMT gene mutation and risk of BMT. The pooled OR demonstrated that TPMT polymorphisms were significantly associated with BMT (OR: 5.93, 95% CI: 2.96-11.88, P < 0.00001).
Six studies[5,14,16-18,20] have reported on the incidence of hepatotoxicity in IBD patients treated with thiopurines. Three[14,17-18] of these have defined hepatotoxicity as alanine transaminase (ALT) levels of at least twice the upper limit of normal (ULN). A German research group[20] has defined hepatotoxicity as either ALT levels or aspartate aminotransferase (AST) levels of at least twice the ULN. Stocco et al[16] have defined hepatotoxicity as ALT, AST or alkaline phosphatase (ALP) levels of at least twice the ULN. No definition of hepatotoxicity was provided in the study of Winter et al[5], therefore, this study was excluded in this sub-analysis. Only three of the 37 IBD patients with hepatotoxicity were TPMT heterozygotes/homozygotes, while 82 of 1017 IBD patients without hepatotoxicity were TPMT heterozygotes/homozygotes. All of the remaining five have shown that there was no association between AZA-related hepatotoxicity and TPMT polymorphisms. The pooled OR indicated that no significant difference in TPMT polymorphisms was seen between IBD patients with thiopurine-induced hepatotoxicity and controls (OR: 1.51, 95% CI: 0.54-4.19, P = 0.43).
Six studies[3,5,14,17,18,20] have reported on the incidence of pancreatitis in IBD patients treated with thiopurine and detected TPMT polymorphisms. In the study of Ansari et al[14], pancreatitis was defined as serum amylase of more than four times the ULN, associated with severe abdominal pain, and supportive radiological findings. Winter et al[5] and Hindorf et al[3] have not defined the criteria of pancreatitis, thus, we also excluded these two studies from the sub-analysis. The remaining studies[17,18,20] have defined pancreatitis as either serum amylase or serum lipase of more than two times the ULN, associated with severe abdominal pain. Only one of 34 IBD patients with pancreatitis and 60 of 758 without pancreatitis had the TPMT gene mutation. All of the four studies have demonstrated that there was no association between pancreatitis and TPMT polymorphisms. Pooled data have demonstrated that there was no significant difference in TPMT polymorphisms between IBD patients with thiopurine-induced pancreatitis and controls (OR: 1.02, 95% CI: 0.26-3.99, P = 0.98).
We performed subgroup analysis in which we excluded the studies with different definitions of BMT to determine the effect on the test of heterogeneity and the overall pooled estimates. Because the results were nearly identical, only results based on the four studies[16-18,20] that have defined BMT as a white blood cells count of < 3.0 × 109/L or a platelet count of < 100 × 109/L were reported. The pooled OR of the four studies[16-18,20] was 5.05 (95% CI: 2.58-9.88, P < 0.01). This result still showed a significant difference in TPMT polymorphisms between IBD patients with and without BMT. Similarly, we also performed subgroup analysis in which we individually excluded the studies with different definitions of hepatotoxicity and pancreatitis, to determine the effect on the test of heterogeneity and the overall pooled estimates. Because the results were nearly identical, they were not reported.
Studies that prescribed AZA ≤ 2 mg/kg per day or 6-MP ≤ 1 mg/kg per day.
When the studies[5,14,16,19,20] that prescribed AZA > 2 mg/kg per day or 6-MP > 1 mg/kg per day were excluded, results remained consistent with original results. A significant difference in the incidence of TPMT polymorphisms was observed in the overall ADRs group (OR: 7.82, 95% CI: 2.18-28.12, P < 0.01) and BMT group (OR: 6.73, 95% CI: 3.52-12.85, P < 0.01) compared with the controls. However, no significant difference was found in the hepatotoxicity group (OR: 1.04, 95% CI: 0.18-5.90, P = 0.96) and pancreatitis group (OR: 1.36, 95% CI: 0.28-6.49, P = 0.70) compared with the controls.
When the excluded studies[9,10,13] that provided information on at least one outcome parameter such as proper OR of overall ADRs, BMT, hepatotoxicity or pancreatitis were taken into account, there was still a significant difference in TPMT polymorphisms between IBD patients with or without ADRs. It also showed an increase in the risk of the incidence of TPMT polymorphisms in the overall ADRs group (OR: 2.76, 95% CI: 1.69-4.50, P < 0.0001) and BMT group (OR: 6.73, 95% CI: 3.52-12.58, P < 0.00001) compared with the controls. No significant difference in the incidence of TPMT polymorphisms was found in the hepatotoxicity group (OR: 1.27, 95% CI: 0.53-3.07, P = 0.59) and pancreatitis group (OR: 1.32, 95% CI: 0.46-3.76, P = 0.60) compared with the controls.
Studies reporting on ≥ 100 patients
Analysis of the studies[5,14,17,18] that reported on ≥ 100 IBD patients treated with thiopurine, and detected TPMT polymorphisms showed that the results were consistent with the overall results and the analysis of the original studies. A significant difference in the incidence of TPMT polymorphisms was observed in the overall ADRs group (OR: 2.64, 95% CI: 1.06-6.53, P = 0.04) and BMT group (OR: 6.53, 95% CI: 2.35-18.19, P = 0.0003) compared with the controls. No significant difference in the incidence of TPMT polymorphisms was found in the hepatotoxicity group (OR: 1.51, 95% CI: 0.47-4.82, P = 0.49) and pancreatitis group (OR: 0.46, 95% CI: 0.06-3.47, P = 0.45) compared with the controls.
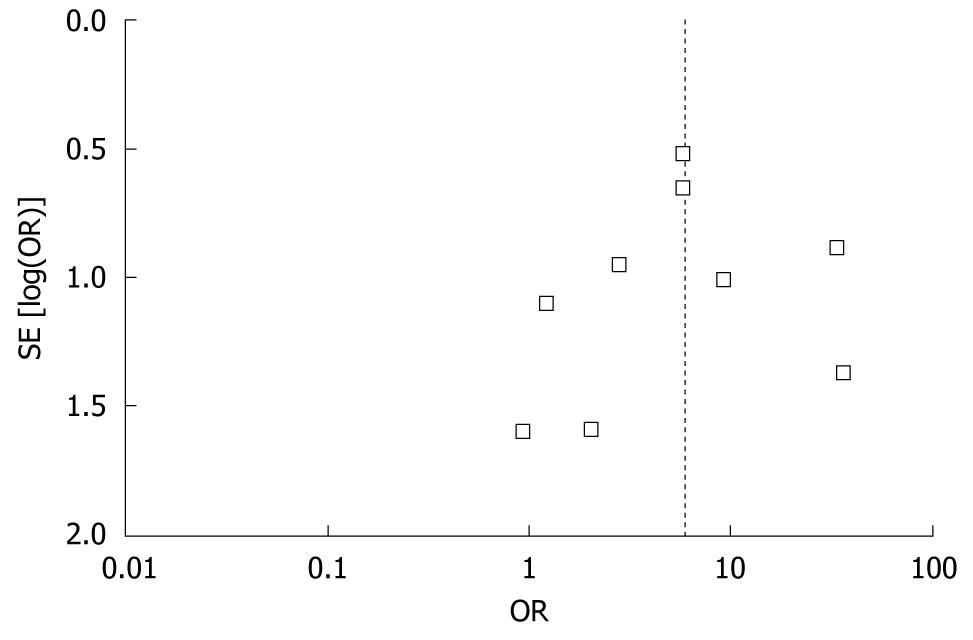
Figure 3 shows a funnel plot of the studies used in this meta-analysis that have reported on the incidence of TPMT polymorphisms in BMT. We found that the funnel plot was slightly asymmetrical in distribution, but Egger’s regression test suggested no significant asymmetry of the funnel plot (P = 0.623), which indicated no evidence of substantial publication bias.
This review evaluated 17 published studies that have studied the association between TMPT gene polymorphisms and the development of ADRs in thiopurine-treated IBD patients. Nine studies among adult IBD patients were included, while the random effects model was used for the meta-analysis. The results of the present meta-analysis suggested that IBD patients with TPMT polymorphisms are more likely to experience ADRs, in particular BMT, but not hepatotoxicity and pancreatitis. Notably, the result remained significant after the sensitivity analysis.
The association of TPMT polymorphisms with thiopurine toxicity suggested by previous studies[14] is confirmed in this meta-analysis. The study of Ansari et al[14] was the largest prospective study to use full-dose AZA (2 mg/kg) without dose adjustment in IBD patients. They have found that heterozygous TPMT genotype strongly predicted ADRs (79% heterozygous vs 35% wild-type TPMT, P < 0.001), especially for gastric intolerance and BMT. Another study[21] has shown that heterozygous TPMT genotypes were significantly associated with IBD patients experiencing nausea and vomiting. Although several studies[3,5,20] have demonstrated that there was no association between thiopurine-related ADRs and TPMT polymorphisms, the retrospective nature of most of these studies may not have provided a true measure of ADRs. The OR for mutations of the TPMT gene in thiopurine-treated IBD patients suffering from overall ADRs was 2.93.
A higher frequency of TPMT mutation was seen in thiopurine-treated IBD patients with BMT than in those without BMT. This was corroborated in the sensitivity analysis. TPMT polymorphisms results in greater conversion of 6-MP to 6-thioguanine nucleotides (6-TGNs) and methylthioinosine monophosphate (meTIMP) via the hypoxanthine guanine phosphoribosyltransferase pathway. 6-TGNs and meTIMP are thought to be associated with BMT[3,22]. The study by Hindorf et al[3] has suggested that BMT might be dose-related; 67% of the 27 patients with ADRs tolerated long-term treatment with a lower dose (median 1.32 mg/kg per day AZA), and low-dose AZA/6-MP was as effective as the standard dose for remission induction and maintenance of remission in patients with UC and CD[23], but this was not confirmed in the sensitivity analysis of studies that prescribed AZA ≤ 2 mg/kg per day or 6-MP ≤ 1 mg/kg per day. That could be explained by the small sample sizes. Hawwa et al[15] have found that patients with a heterozygous TPMT genotype usually experienced early and severe myelosuppression when they were treated with AZA/6-MP at standard dosage, but all of the included studies showed that BMT occurred after an average exposure time of 1 mo, except the studies by Hawwa et al[15] and Derijks et al[19].
Previous studies[24] have found an association between heterozygotes for the TPMT*3A allele and hepatotoxicity in acute lymphocytic leukemia patients who received a standard dose of 6-MP/6-TG. However, it was not shown in this meta-analysis. The results of the present study showed no increase in the incidence of TPMT polymorphisms in thiopurine-treated IBD patients with hepatotoxicity compared with those without hepatotoxicity. This was confirmed in the sensitivity analysis. TPMT polymorphisms are seldom found in patients exhibiting thiopurine-related hepatotoxicity, and thiopurine-induced hepatotoxicity cannot be generally attributed to it[20]. It has been suggested that elevated concentrations of 6-methylmercaptopurine (6-MMP) are associated with thiopurine-induced hepatotoxicity, and elevated concentrations of 6-MMP could be due to high TPMT activity but not TPMT polymorphisms[25].
No significant difference was found in the incidence of TPMT polymorphisms between the thiopurine-treated IBD patients with and without pancreatitis. The results of the sensitivity analysis also showed no significant difference. Thiopurine-induced pancreatitis is dose-independent and seems to be independent of TPMT polymorphisms. AZA is a drug that can induce pancreatitis, especially when it is used in the treatment of CD, which might be due to an immune-mediated idiosyncratic drug reaction because of a genetic predisposition[26].
Recently, Higgs et al[27] have published a meta-analysis of myelosuppression in patients with intermediate TPMT activity compared with wild-type. They have found that individuals with intermediate TPMT activity or one TPMT variant allele had an increased risk of developing thiopurine-induced BMT, compared with individuals with normal activity. The search was not limited to a specific disease or condition. They included a total of 67 studies, but most of them were retrospective cohorts. The genotype and/or phenotype tests were described in 57 studies. As we know, the overall concordance rate between the genetic and phenotypic tests for TPMT was 71.6%[28]. In addition, the cutoff points of TPMT activity have varied in different groups. The phenotype test might also be influenced by blood transfusion during the previous 3 mo. The most important thing is that prospective studies that have evaluated the cost-effectiveness of the phenotype test used in IBD treatment have been rather scarce.
This meta-analysis has several weaknesses, most of which relate to heterogeneous definitions of BMT, hepatotoxicity and pancreatitis. Therefore, we had to base our analysis on studies that were vulnerable to bias. Second, all of the included studies were performed in populations of European descent. Further studies are needed in other countries because of ethnic differences in TPMT polymorphisms. Third, the low number of ADRs made statistical precision difficult. Finally, it should be noted that not all of the ADRs could be explained only by TPMT genotyping, but also by other reasons, such as concurrent viral infections[29] or co-medication (e.g. mesalamine or sulfasalazine)[30], which can interfere with thiopurine metabolism.
In conclusion, this meta-analysis provides strong evidence that TPMT polymorphisms are associated with overall ADRs and BMT in patients taking 6-MP or AZA, and thus may be useful in the management of these patients. The present study also clearly demonstrates that thiopurine-related hepatotoxicity and pancreatitis cannot be explained by TPMT polymorphisms. Although there is no consensus that TPMT genotype should be measured before embarking on therapy with thiopurine in IBD patients[31], based on our meta-analysis, we believe that TPMT genotyping is warranted before embarking on therapy with thiopurine in IBD patients. This is also suggested by the US Food and Drug Administration recommendations[32] and by one socioeconomic study of IBD patients[33].
The efficacy of the thiopurines has been well established for the treatment of inflammatory bowel disease (IBD). However, they have severe adverse drug reactions (ADRs) such as gastrointestinal disturbances, bone marrow toxicity (BMT), hepatotoxicity and pancreatitis that prevent their use in some patients. The impact of genetic variation of the thiopurine S-methyltransferase (TPMT) gene on its toxicity has been evaluated in several studies, with varying outcomes.
Recent pharmacogenetic advances have led to the development of novel strategies to optimize and individualize therapy with azathioprine (AZA) and 6-mercaptopurine (6-MP). The strategy of screening the TPMT gene mutation might maximize efficacy while minimizing toxicity.
The results of the previous studies on TPMT polymorphisms and thiopurine toxicity have been inconsistent. Recently, one meta-analysis has found that individuals with both intermediate and absent TPMT activity had an increased risk of developing thiopurine-induced BMT. However, there is still no prospective study that has evaluated the cost-effectiveness and utility of the TPMT phenotyping test before taking thiopurine medication, while TPMT genotyping is recommended by one socioeconomic study in IBD patients. This meta-analysis has shown that IBD patients with TPMT polymorphisms are at risk of increased thiopurine toxicity when taking thiopurine medications.
The meta-analysis provides strong evidence that the TPMT polymorphisms are associated with overall ADRs and BMT in patients taking 6-MP or AZA. Based on the meta-analysis, it is believed that TPMT genotyping is warranted before embarking upon therapy with thiopurine in IBD patients.
TPMT is a cytosolic enzyme that methylates thiopurine compounds. This gene encodes the enzyme that metabolizes thiopurine drugs via S-adenosyl-L-methionine as the S-methyl donor and S-adenosyl-L-homocysteine as a byproduct. Genetic polymorphisms that affect this enzymatic activity are correlated with variations in sensitivity and toxicity to such drugs within individuals.
The authors describe a meta-analysis of associations between TPMT genetic polymorphisms and ADRs, including BMT, hepatotoxicity, and pancreatitis. The authors’ attempt at meta-analysis is interesting, considering current contradictory data about the efficacy of TPMT genotyping in predicting AZA/6-MP toxicity. Moreover, their claims are reasonable.
Peer reviewers: Gabor Veres, MD, Assistant Professor, First Department of Pediatrics, Semmelweis Medical University, Bókay street. 53, Budapest 1083, Hungary; Won Ho Kim, MD, Professor, Department of Internal Medicine, Yonsei Uiversity College of Medicine, 134 Shinchon-dong Seodaemun-ku, Seoul 120-752, South Korea
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
| 1. | Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2009;CD000067. [Cited in This Article: ] |
| 2. | Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2009;CD000545. [Cited in This Article: ] |
| 3. | Hindorf U, Lindqvist M, Peterson C, Söderkvist P, Ström M, Hjortswang H, Pousette A, Almer S. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55:1423-1431. [Cited in This Article: ] |
| 4. | Yates CR, Krynetski EY, Loennechen T, Fessing MY, Tai HL, Pui CH, Relling MV, Evans WE. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med. 1997;126:608-614. [Cited in This Article: ] |
| 5. | Winter JW, Gaffney D, Shapiro D, Spooner RJ, Marinaki AM, Sanderson JD, Mills PR. Assessment of thiopurine methyltransferase enzyme activity is superior to genotype in predicting myelosuppression following azathioprine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2007;25:1069-1077. [Cited in This Article: ] |
| 6. | Cao Q, Zhu Q, Shang Y, Gao M, Si J. Thiopurine methyltransferase gene polymorphisms in Chinese patients with inflammatory bowel disease. Digestion. 2009;79:58-63. [Cited in This Article: ] |
| 7. | Takatsu N, Matsui T, Murakami Y, Ishihara H, Hisabe T, Nagahama T, Maki S, Beppu T, Takaki Y, Hirai F. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2009;24:1258-1264. [Cited in This Article: ] |
| 8. | Uchiyama K, Nakamura M, Kubota T, Yamane T, Fujise K, Tajiri H. Thiopurine S-methyltransferase and inosine triphosphate pyrophosphohydrolase genes in Japanese patients with inflammatory bowel disease in whom adverse drug reactions were induced by azathioprine/6-mercaptopurine treatment. J Gastroenterol. 2009;44:197-203. [Cited in This Article: ] |
| 9. | Ban H, Andoh A, Tanaka A, Tsujikawa T, Sasaki M, Saito Y, Fujiyama Y. Analysis of thiopurine S-methyltransferase genotypes in Japanese patients with inflammatory bowel disease. Intern Med. 2008;47:1645-1648. [Cited in This Article: ] |
| 10. | Gearry RB, Barclay ML, Burt MJ, Collett JA, Chapman BA, Roberts RL, Kennedy MA. Thiopurine S-methyltransferase (TPMT) genotype does not predict adverse drug reactions to thiopurine drugs in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18:395-400. [Cited in This Article: ] |
| 11. | Regueiro M, Mardini H. Determination of thiopurine methyltransferase genotype or phenotype optimizes initial dosing of azathioprine for the treatment of Crohn's disease. J Clin Gastroenterol. 2002;35:240-244. [Cited in This Article: ] |
| 12. | Evans WE, Hon YY, Bomgaars L, Coutre S, Holdsworth M, Janco R, Kalwinsky D, Keller F, Khatib Z, Margolin J. Preponderance of thiopurine S-methyltransferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J Clin Oncol. 2001;19:2293-2301. [Cited in This Article: ] |
| 13. | Naughton MA, Battaglia E, O'Brien S, Walport MJ, Botto M. Identification of thiopurine methyltransferase (TPMT) polymorphisms cannot predict myelosuppression in systemic lupus erythematosus patients taking azathioprine. Rheumatology (Oxford). 1999;38:640-644. [Cited in This Article: ] |
| 14. | Ansari A, Arenas M, Greenfield SM, Morris D, Lindsay J, Gilshenan K, Smith M, Lewis C, Marinaki A, Duley J. Prospective evaluation of the pharmacogenetics of azathioprine in the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:973-983. [Cited in This Article: ] |
| 15. | Hawwa AF, Millership JS, Collier PS, Vandenbroeck K, McCarthy A, Dempsey S, Cairns C, Collins J, Rodgers C, McElnay JC. Pharmacogenomic studies of the anticancer and immunosuppressive thiopurines mercaptopurine and azathioprine. Br J Clin Pharmacol. 2008;66:517-528. [Cited in This Article: ] |
| 16. | Stocco G, Martelossi S, Barabino A, Decorti G, Bartoli F, Montico M, Gotti A, Ventura A. Glutathione-S-transferase genotypes and the adverse effects of azathioprine in young patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:57-64. [Cited in This Article: ] |
| 17. | Palmieri O, Latiano A, Bossa F, Vecchi M, D'Incà R, Guagnozzi D, Tonelli F, Cucchiara S, Valvano MR, Latiano T. Sequential evaluation of thiopurine methyltransferase, inosine triphosphate pyrophosphatase, and HPRT1 genes polymorphisms to explain thiopurines' toxicity and efficacy. Aliment Pharmacol Ther. 2007;26:737-745. [Cited in This Article: ] |
| 18. | Zelinkova Z, Derijks LJ, Stokkers PC, Vogels EW, van Kampen AH, Curvers WL, Cohn D, van Deventer SJ, Hommes DW. Inosine triphosphate pyrophosphatase and thiopurine s-methyltransferase genotypes relationship to azathioprine-induced myelosuppression. Clin Gastroenterol Hepatol. 2006;4:44-49. [Cited in This Article: ] |
| 19. | Derijks LJ, Gilissen LP, Engels LG, Bos LP, Bus PJ, Lohman JJ, Curvers WL, Van Deventer SJ, Hommes DW, Hooymans PM. Pharmacokinetics of 6-mercaptopurine in patients with inflammatory bowel disease: implications for therapy. Ther Drug Monit. 2004;26:311-318. [Cited in This Article: ] |
| 20. | Schwab M, Schäffeler E, Marx C, Fischer C, Lang T, Behrens C, Gregor M, Eichelbaum M, Zanger UM, Kaskas BA. Azathioprine therapy and adverse drug reactions in patients with inflammatory bowel disease: impact of thiopurine S-methyltransferase polymorphism. Pharmacogenetics. 2002;12:429-436. [Cited in This Article: ] |
| 21. | Marinaki AM, Ansari A, Duley JA, Arenas M, Sumi S, Lewis CM, Shobowale-Bakre el-M, Escuredo E, Fairbanks LD, Sanderson JD. Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics. 2004;14:181-187. [Cited in This Article: ] |
| 22. | Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1149-1157. [Cited in This Article: ] |
| 23. | Kim DU, Kim YH, Kim BJ, Chang DK, Son HJ, Rhee PL, Kim JJ, Rhee JC. The efficacy of low dose azathioprine/6-mercaptopurine in patients with inflammatory bowel disease. Hepatogastroenterology. 2009;56:1395-1402. [Cited in This Article: ] |
| 24. | Alves S, Prata MJ, Ferreira F, Amorim A. Thiopurine methyltransferase pharmacogenetics: alternative molecular diagnosis and preliminary data from Northern Portugal. Pharmacogenetics. 1999;9:257-261. [Cited in This Article: ] |
| 25. | Dubinsky MC, Yang H, Hassard PV, Seidman EG, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology. 2002;122:904-915. [Cited in This Article: ] |
| 26. | Weersma RK, Peters FT, Oostenbrug LE, van den Berg AP, van Haastert M, Ploeg RJ, Posthumus MD, Homan van der Heide JJ, Jansen PL, van Dullemen HM. Increased incidence of azathioprine-induced pancreatitis in Crohn's disease compared with other diseases. Aliment Pharmacol Ther. 2004;20:843-850. [Cited in This Article: ] |
| 27. | Higgs JE, Payne K, Roberts C, Newman WG. Are patients with intermediate TPMT activity at increased risk of myelosuppression when taking thiopurine medications? Pharmacogenomics. 2010;11:177-188. [Cited in This Article: ] |
| 28. | Serpe L, Calvo PL, Muntoni E, D'Antico S, Giaccone M, Avagnina A, Baldi M, Barbera C, Curti F, Pera A. Thiopurine S-methyltransferase pharmacogenetics in a large-scale healthy Italian-Caucasian population: differences in enzyme activity. Pharmacogenomics. 2009;10:1753-1765. [Cited in This Article: ] |
| 29. | Colombel JF, Ferrari N, Debuysere H, Marteau P, Gendre JP, Bonaz B, Soulé JC, Modigliani R, Touze Y, Catala P. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn's disease and severe myelosuppression during azathioprine therapy. Gastroenterology. 2000;118:1025-1030. [Cited in This Article: ] |
| 30. | Lowry PW, Franklin CL, Weaver AL, Szumlanski CL, Mays DC, Loftus EV, Tremaine WJ, Lipsky JJ, Weinshilboum RM, Sandborn WJ. Leucopenia resulting from a drug interaction between azathioprine or 6-mercaptopurine and mesalamine, sulphasalazine, or balsalazide. Gut. 2001;49:656-664. [Cited in This Article: ] |
| 31. | Pierik M, Rutgeerts P, Vlietinck R, Vermeire S. Pharmacogenetics in inflammatory bowel disease. World J Gastroenterol. 2006;12:3657-3667. [Cited in This Article: ] |
| 32. | Package insert for azathioprine/6-mercaptopurine. San Diego, CA: Prometheus Laboratories, Inc., 2005. . [Cited in This Article: ] |
| 33. | Winter J, Walker A, Shapiro D, Gaffney D, Spooner RJ, Mills PR. Cost-effectiveness of thiopurine methyltransferase genotype screening in patients about to commence azathioprine therapy for treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:593-599. [Cited in This Article: ] |