Published online Jun 28, 2010. doi: 10.3748/wjg.v16.i24.3002
Revised: April 18, 2010
Accepted: April 25, 2010
Published online: June 28, 2010
AIM: To investigate the effect of sulforaphane (SFN) on regulation of NF-E2-related factor-2 (Nrf2)-antioxidant response element (ARE) pathway in liver injury induced by intestinal ischemia/reperfusion (I/R).
METHODS: Rats were divided randomly into four experimental groups: control, SFN control, intestinal I/R and SFN pretreatment groups (n = 8 in each group). The intestinal I/R model was established by clamping the superior mesenteric artery for 1 h and 2 h reperfusion. In the SFN pretreatment group, surgery was performed as in the intestinal I/R group, with intraperitoneal administration of 3 mg/kg SFN 1 h before the operation. Intestine and liver histology was investigated. Serum levels of aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured. Liver tissue superoxide dismutase (SOD), myeloperoxidase (MPO), glutathione (GSH) and glutathione peroxidase (GSH-Px) activity were assayed. The liver transcription factor Nrf2 and heme oxygenase-1 (HO-1) were determined by immunohistochemical analysis and Western blotting analysis.
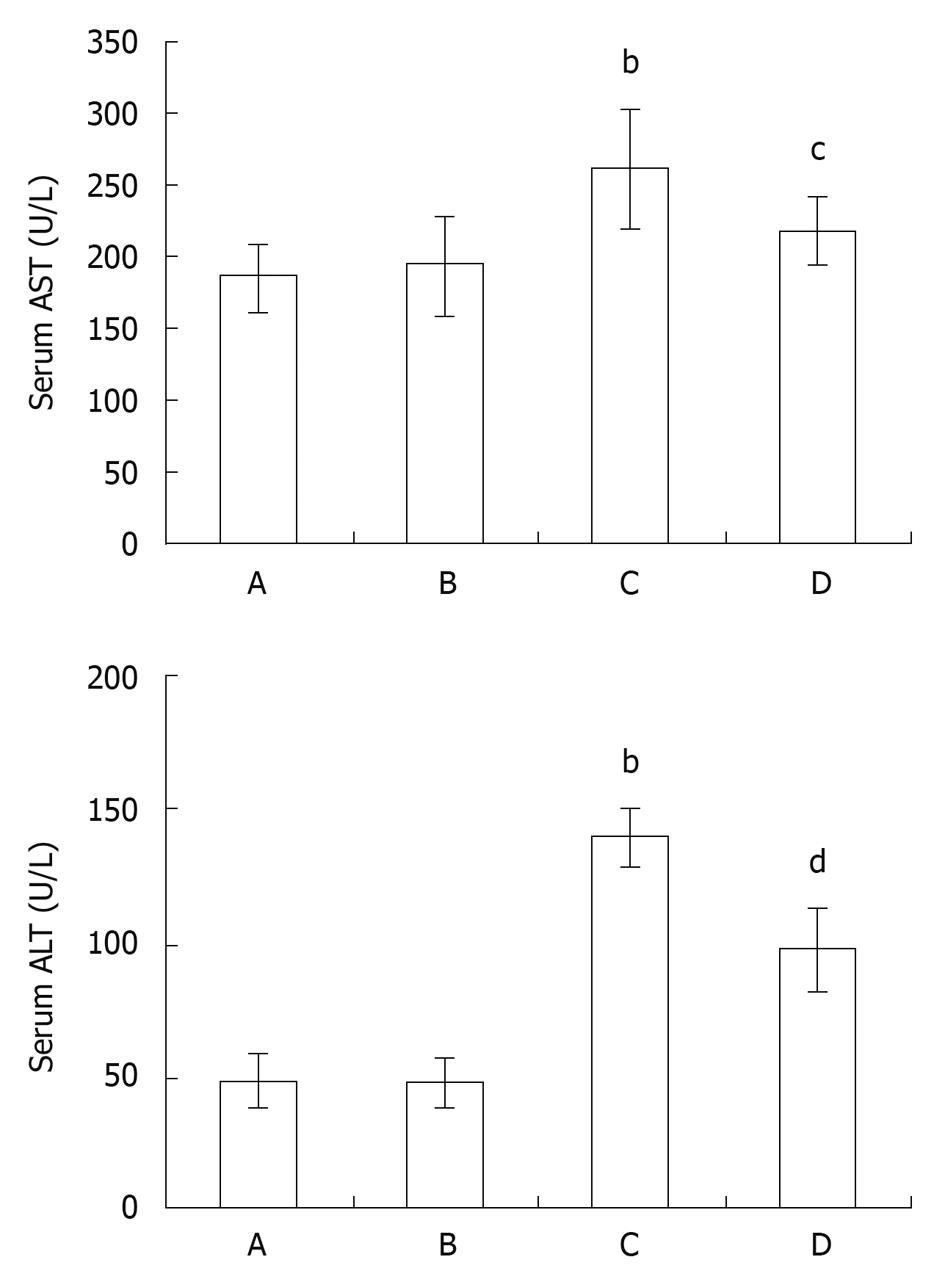
RESULTS: Intestinal I/R induced intestinal and liver injury, characterized by histological changes as well as a significant increase in serum AST and ALT levels (AST: 260.13 ± 40.17 U/L vs 186.00 ± 24.21 U/L, P < 0.01; ALT: 139.63 ± 11.35 U/L vs 48.38 ± 10.73 U/L, P < 0.01), all of which were reduced by pretreatment with SFN, respectively (AST: 260.13 ± 40.17 U/L vs 216.63 ± 22.65 U/L, P < 0.05; ALT: 139.63 ± 11.35 U/L vs 97.63 ± 15.56 U/L, P < 0.01). The activity of SOD in the liver tissue decreased after intestinal I/R (P < 0.01), which was enhanced by SFN pretreatment (P < 0.05). In addition, compared with the control group, SFN markedly reduced liver tissue MPO activity (P < 0.05) and elevated liver tissue GSH and GSH-Px activity (P < 0.05, P < 0.05), which was in parallel with the increased level of liver Nrf2 and HO-1 expression.
CONCLUSION: SFN pretreatment attenuates liver injury induced by intestinal I/R in rats, attributable to the antioxidant effect through Nrf2-ARE pathway.
- Citation: Zhao HD, Zhang F, Shen G, Li YB, Li YH, Jing HR, Ma LF, Yao JH, Tian XF. Sulforaphane protects liver injury induced by intestinal ischemia reperfusion through Nrf2-ARE pathway. World J Gastroenterol 2010; 16(24): 3002-3010
- URL: https://www.wjgnet.com/1007-9327/full/v16/i24/3002.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i24.3002
Intestinal ischemia/reperfusion (I/R) is considered to be a grave and triggering event in development of local and distant organ dysfunction[1-4], which occurs in many clinical settings including acute mesenteric ischemia, hemorrhagic, traumatic or septic shock, severe burns, resuscitation, small bowel transplantation and thoracoabdominal aortic aneurysm repair[5,6]. The mechanism of liver injury induced by intestinal I/R is complicated, and it has not been fully elucidated although many studies have been done to mimic the pathophysiologic process by both cell culture and animal models in the past a few years[7-10].
Traditionally, decreased basement membrane integrity and barrier function of the intestine, which facilitate bacterial translocation and local production of inflammatory cytokines[11], promotes the systemic inflammatory response syndrome and multiple organ dysfunction syndrome[12,13]. Recent studies showed that reactive oxygen species (ROS) generated during tissue reperfusion, play an important role in intestinal I/R injury[14], which can initiate lipid peroxidation, oxidize proteins to inactive states and cause DNA strand breaks, and initiation of apoptotic and necrotic cascades[15]. Additionally, ROS also has a function as a second messenger to modulate the secretion of pro-inflammation cytokines and chemokines that can destroy the intestinal barrier, which is a critical junction point to amplify the inflammation reaction described above[11-13]. The administration of antioxidants has been shown to exert beneficial effects in the prevention of ischemia-reperfusion injury[16].
Sulforaphane (SFN) is a natural product derived from isothiocyanate which is present in cruciferous vegetables such as broccoli that has been used as a chemopreventive compound[17]. The cytoprotective effect of this compound is mediated by transcription factor NF-E2-related factor-2 (Nrf2), which binds to the antioxidant response element (ARE) in the promoter region of a number of genes, encoding for antioxidative and phase 2 enzymes, including heme oxygenase-1 (HO-1), NAD(P)H: quinoine oxidoreductase 1, glutathione reductase, and glutathione peroxidase (GSH-Px)[18-20]. Phase 2 enzymes play a major role in the detoxification of ROS during ischemia/reperfusion[21,22]. Nrf2 is held in the cytoplasm by a cytoskeletal-associated specific inhibitory protein (Kelch-like ECH associated protein 1, Keap1) under circumstances of normal cellular quiescent state. Upon stimulation of oxidative stress, cysteine residues within the hinge region of Keap1 can be modified and cause a conformational change in KEAP1 with the loss of Nrf2 binding, then Nrf2 translocated into the nucleus, where it heterodimerizes with members of the maf protein family, and coordinates up-regulation of cytoprotective genes[20]. SFN can disrupt the Nrf2-Keap1 complex, and permit Nrf2 to translocate into the nucleus to activate the ARE-driven genes[23,24]. Studies suggest that Nrf2 activation by SFN results in effective protection from cancers[25] and renal I/R[26] by upregulating ARE-related detoxification enzymes. These studies have focused on the chemoprevention by SFN, and to our knowledge, no one has evaluated SFN to determine if it can protect liver injury induced by intestinal I/R.
In this study, we investigated the effect of SFN on liver injury following intestinal I/R and explored the mechanism of its protective action through Nrf2-ARE pathway.
Male Sprague-Dawley rats weighing 190-220 g (from the Animal center of Dalian Medical University, Dalian, China) were used in this study, which was approved by the Institutional Ethics Committee. All rats were provided with standard laboratory chow and water and were housed in accordance with institutional animal care policies.
The rats were divided into four experimental groups randomly: control (A), SFN control (B), intestinal I/R (C) and SFN pretreatment group (D) (n = 8 in each group). The rats in the control group underwent surgical preparation including isolation of the superior mesenteric artery (SMA) without occlusion; the rats in the SFN control group underwent surgery as in the control group with intraperitoneal administration of 3 mg/kg SFN 1 h before the operation (SFN was purchased from Sigma Chemical Company and was dissolved in 10% dimethylsulfoxide before administration); the rats in intestinal I/R group were subjected to 1 h intestinal ischemia and 2 h reperfusion after the SMA was isolated and occluded[27]; the rats in the SFN pretreatment group underwent surgery as in the intestinal I/R group with intraperitoneal administration of 3 mg/kg SFN 1 h before the operation.
The dose of SFN administration was determined according to the previous studies with modification from preliminary experiments. The rats in the control and intestinal I/R groups were treated with an equal volume of 10% dimethylsulfoxide. Two hours after reperfusion, blood, intestine and liver tissue samples were obtained for further analysis.
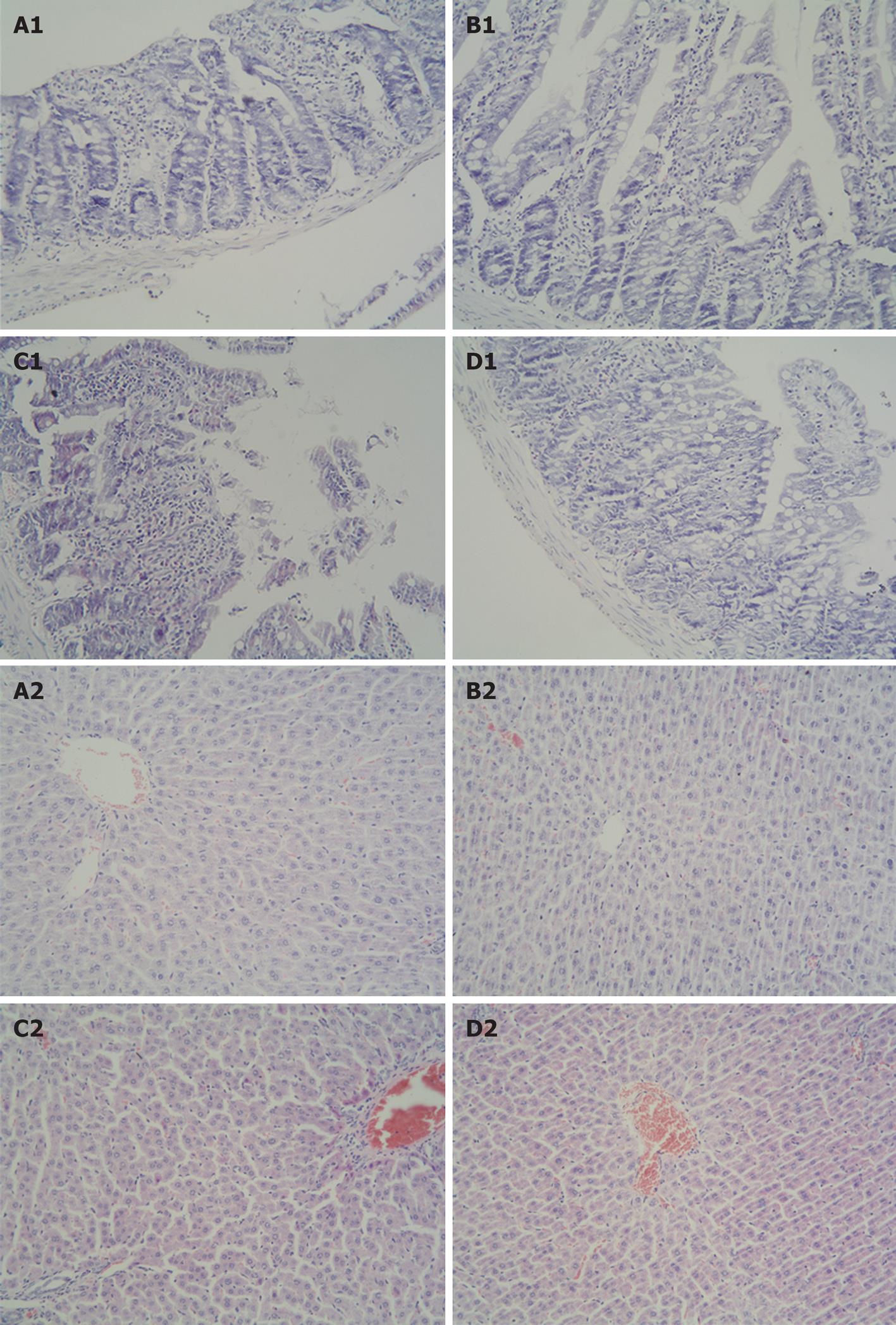
The isolated intestine and liver tissues were harvested and fixed in 10% formalin. After being embedded in paraffin, the tissues were stained with hematoxylin and eosin for light microscopy.
The serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured with an OLYMPUS AU1000 automatic analyzer (AusBio Laboratories Co., Ltd. Beijing, China).
The liver tissues were harvested and homogenized immediately on ice in 5 volumes of normal saline. The homogenates were centrifuged at 1200 r/min for 10 min to remove debris. The superoxide dismutase (SOD), myeloperoxidase (MPO), GSH and GSH-Px activity in the supernatant were determined using an assay kit (Nanjing Jiancheng Corp., China), following the manufacturer’s recommendations.
The SOD activity was determined by hydroxylamine assay developed from xanthine oxidase assay. One unit of SOD sample inhibited the reaction by approximately 50% of the initially measured xanthine oxidase reaction at 37°C. SOD activity in the liver tissues was expressed as units per milligram protein (U/mgprot).
One unit of MPO activity is defined as degrading 1 μmol of hydrogen peroxide at 37°C and MPO activity of tissue was expressed as units per gram (U/g).
Concentrations of GSH were determined by the 5,5’dithio-bis 2-nitrobenzoic acid (DTNB)-GSSG reductase-recycling assay. The amount of GSH was expressed as milligrams per gram protein (mg/gprot).
GSH-Px was measured by the enzymatic reaction which was initiated by addition of H2O2 to the reaction mixture containing reduced glutathione, NADPH and glutathione reductase, and the change in the absorbance at 340 nm was monitored by spectrophotometer. Activity was given in units per milligram protein (U/mgprot).
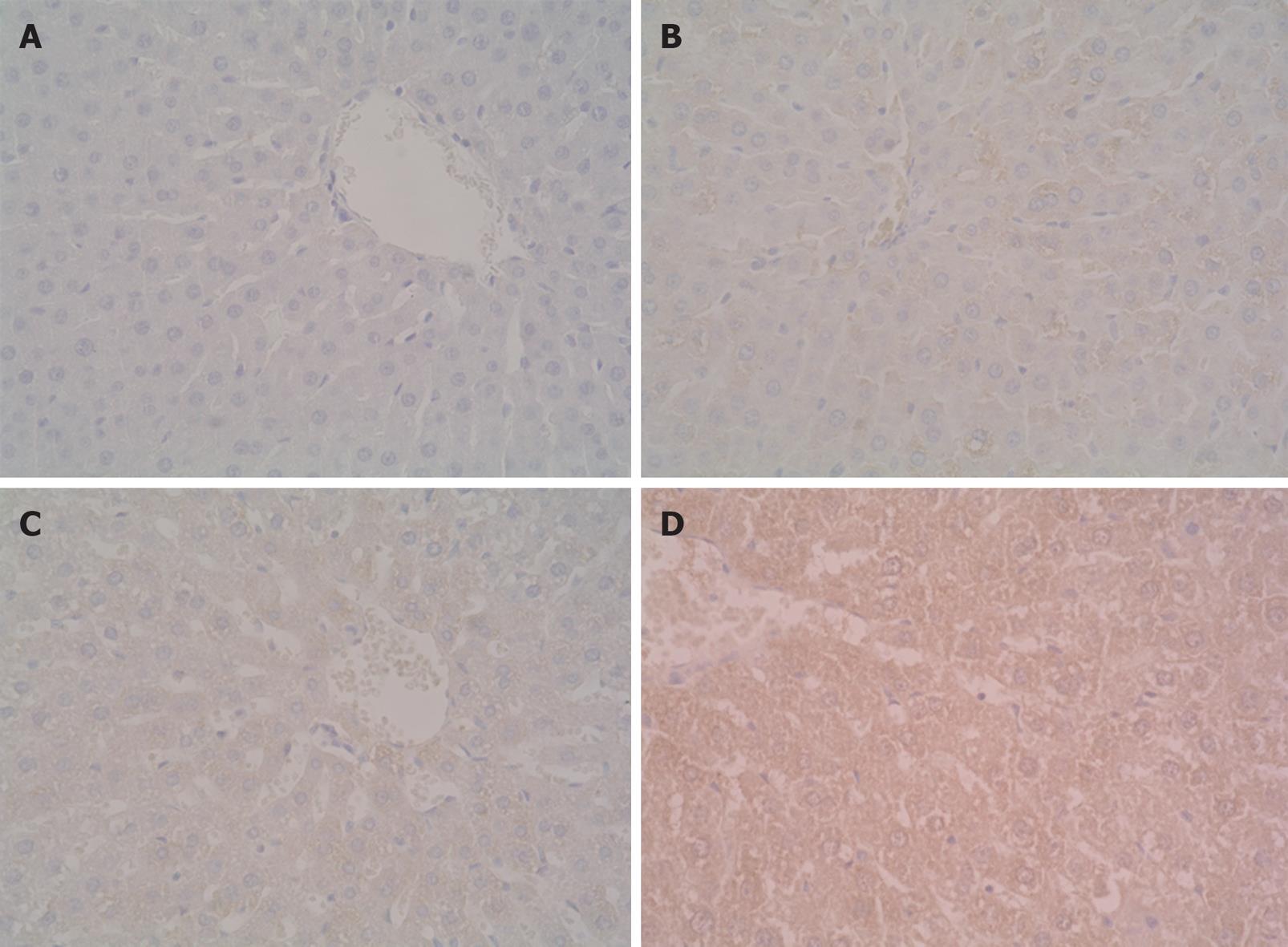
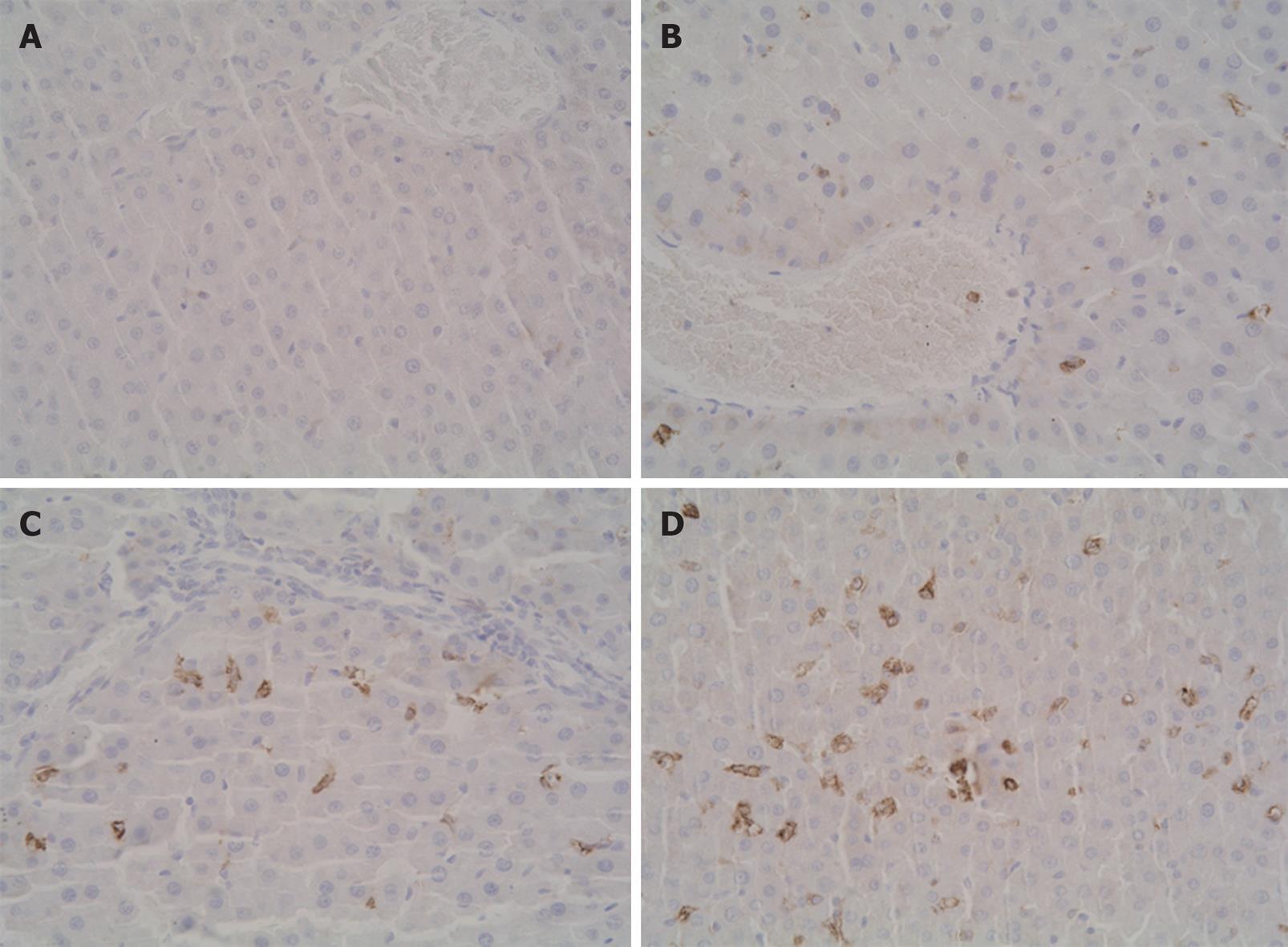
Paraffin-embedded tissue sections, 4 μm in thickness, were stained with SP immunohistochemical technique for Nrf2 and HO-1. The immunohistochemical experiments were performed according to the manufacturer’s recommendations. After being dewaxed or washed in PBS, tissue sections were cultured in 3% hydrogen peroxide to eliminate intrinsic peroxidase, and quenched in normal goat serum for 30 min. The sections were incubated overnight at 4°C with polyclonal rabbit anti-rat Nrf2 antibody (Santa Cruz Corp., Ltd.), followed by the addition of the anti-rabbit immunoglobulin and streptavidin conjugated to horseradish peroxides. Finally, 3,3’-diaminobenzidine was used for color development, and hematoxylin was used for counter staining. The brown or dark brown staining in cytoplasm and/or nucleus was considered to be positive. The results were evaluated semi-quantitatively according to percentage of positive cells in five fields at a 400 multiple signal magnification. The protein expression in tissue sections was graded as 0: less than 5%; 1: from 6% to 25%; 2: from 26% to 50%; 3: from 51% to 75%; and 4: more than 75%[28].
Nrf2 is a nuclear transcriptional factor that binds to the ARE in the promoter region of a number of genes, encoding for antioxidative and phase 2 enzymes such as HO-1 and GSH-Px. The level of Nrf2 in the nucleus was examined by Western blotting to assess Nrf2 activation.
Cellular plasma and nuclear protein were extracted from frozen liver tissues with a protein extraction kit (Pierce, Meridian Road, Rockford, IL, USA) for HO-1 and Nrf2 measurement separately. The protein was separated by 10% SDS-PAGE gel electrophoresis. The protein was electroblotted onto NC membranes (Millipore, Bedford, MA, USA) at 9 V for 30 min. The transferred membranes were then incubated overnight at 4°C with rabbit polyclonal antibody HO-1, Nrf2, GAPDH and PCNA (Santa Cruz Corp., Ltd.) against rat in TBS-T (10 mmol/L Tris-HC1, pH 7.5, 150 mmol/L NaC1, 0.1% Tween-20) containing 5% skim milk. After washing three times in TBS-T, the membranes were incubated for 1 h at 37°C with an anti-rabbit IgG conjugated with horseradish peroxidase. The signals were visualized using the DAB assay kit (Fuzhou Maixin Biological Technology Co., Ltd, Fuzhou, China) and documented with a gel imaging system (UVP Bioimaging System). The signals were analyzed with software Gel-Pro Analyzer 4.0.
All data, which expressed as the mean ± SD, were compared using the paired Student’s t test with the SigmaStat 3.5 statistical software package. One-way analysis of variance (ANOVA) was used to determine significant differences in antioxidant enzyme activities between the groups. A value of P < 0.05 was considered significant.
Intestinal I/R induced apparent intestine and liver injury at 2 h after reperfusion, manifested as histological changes in the intestine and liver with edema, hemorrhage and neutrophil infiltration (Figure 1), as well as a significant increase in serum AST and ALT level (AST: 260.13 ± 40.17 U/L vs 186.00 ± 24.21 U/L, P < 0.01; ALT: 139.63 ± 11.35 U/L vs 48.38 ± 10.73 U/L, P < 0.01, Figure 2) when compared with the control group. Compared with the intestinal I/R group, the intestinal and liver pathological damage was improved in the SFN pretreatment group. In addition, there was a significant difference in the liver function between the intestinal I/R and SFN pretreatment group in serum AST and ALT levels (AST: 260.13 ± 40.17 U/L vs 216.63 ± 22.65 U/L, P < 0.05; ALT: 139.63 ± 11.35 U/L vs 97.63 ± 15.56 U/L, P < 0.01, Figure 2), which indicates that SFN significantly attenuated the intestinal I/R-induced liver injury. There was no significant difference between the SFN control group and the control group in liver pathological damage (Figure 1) and serum AST (AST: 193.38 ± 34.63 U/L vs 186.00 ± 24.21 U/L, P > 0.05, Figure 2) and ALT (ALT: 48.00 ± 8.52 U/L vs 48.38 ± 10.73 U/L, P > 0.05, Figure 2).
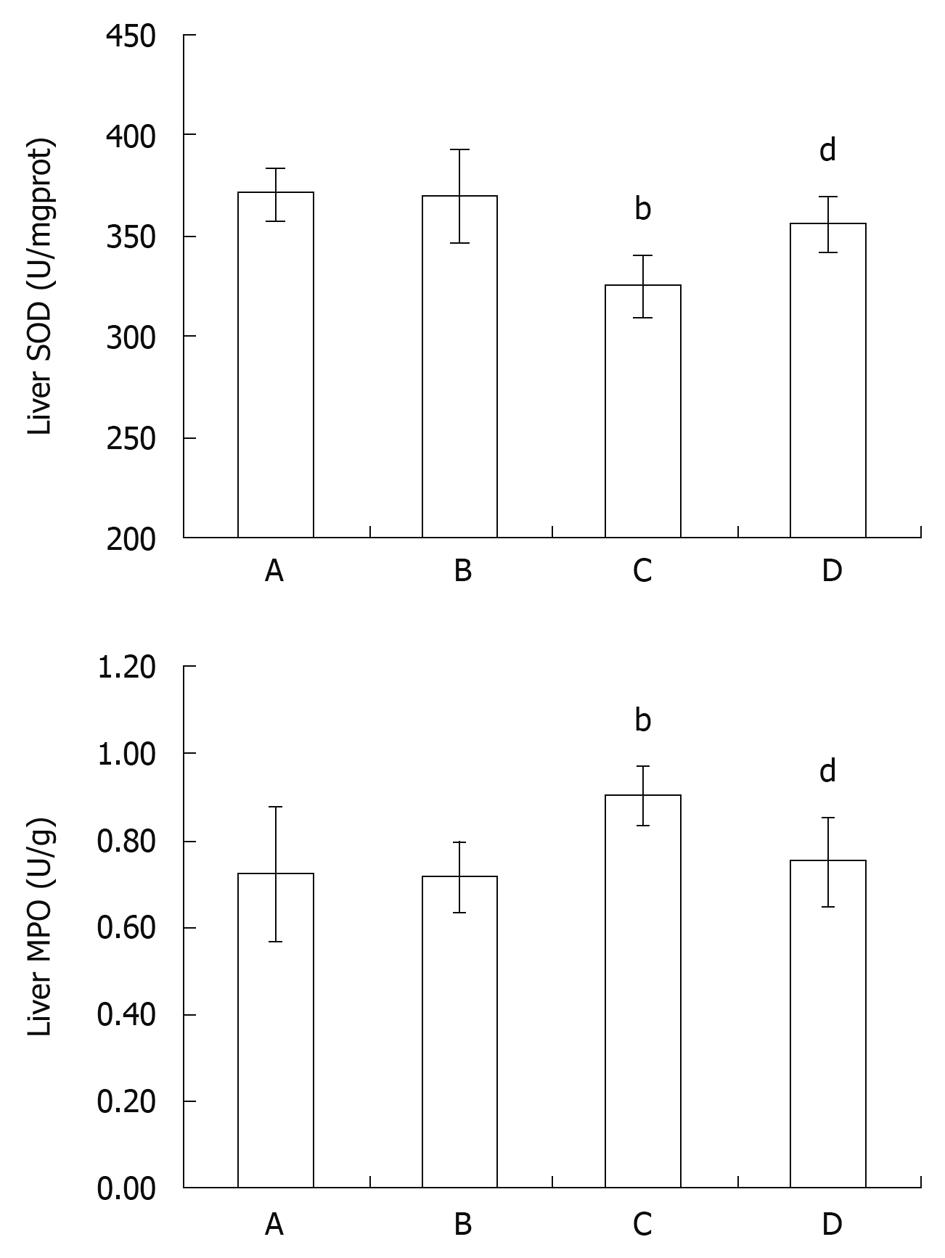
SOD is the major enzyme for scavenging ROS, and its activity can reflect its functional status. The liver homogenate SOD activity in the intestinal I/R group decreased significantly at 2 h of reperfusion in comparison with the control group (P < 0.01, Figure 3). SOD activity was elevated markedly after SFN pretreatment compared with the intestinal I/R group (P < 0.05, Figure 3).
MPO activity in the liver homogenate was measured for estimating the leukocyte recruitment to liver tissues. The liver tissue MPO activity increased significantly after intestinal I/R at 2 h of reperfusion compared with the control group (P < 0.05, Figure 3). The administration of SFN reduced the MPO activity in liver tissues significantly in comparison with the intestinal I/R group (P < 0.05, Figure 3), thus indicating that SFN alleviated the leukocyte recruitment to liver tissues.
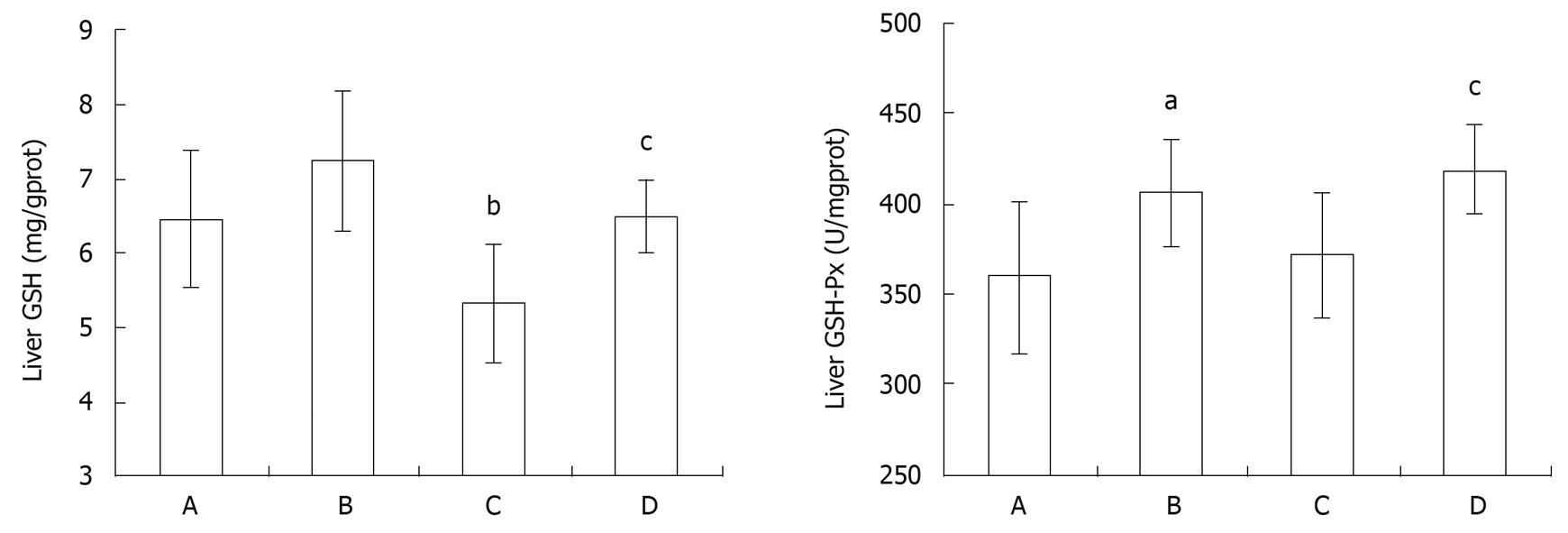
GSH is a kind of antioxidant which can scavenge ROS. Phase 2 enzyme GSH-Px present in the cell can catalyze this reaction. The liver homogenate GSH activity in the intestinal I/R group decreased significantly at 2h of reperfusion as compared with the control group (P < 0.01, Figure 4). GSH activity was elevated markedly after SFN pretreatment in comparison with the intestinal I/R group (P < 0.05, Figure 4).
The increased activity of the liver tissue GSH-Px was significant after SFN pretreatment as against the control group (P < 0.05, Figure 4). GSH-Px activity was elevated markedly after SFN pretreatment in comparison with the intestinal I/R group (P < 0.05, Figure 4), thus supporting the hepatoprotective effect of SFN.
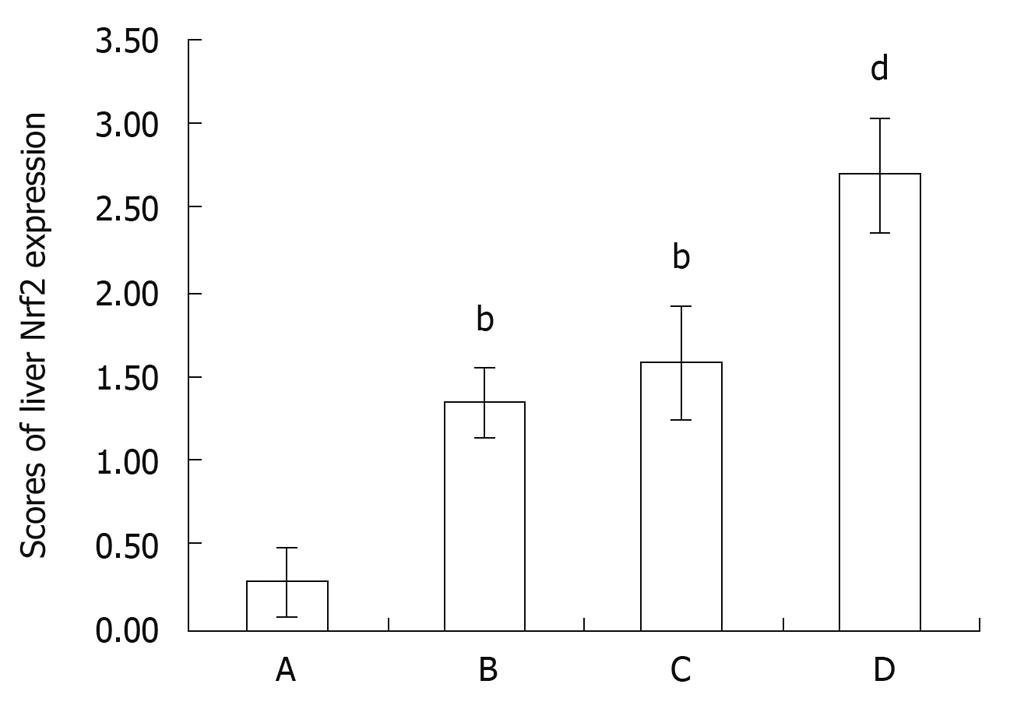
The expression of Nrf2 in control group showed light brown immunostaining in cytoplasm and no staining in the nuclei. The significantly positive expressions of Nrf2 as strong brown staining in cytoplasm and nuclei were observed in the intestinal I/R group and SFN control groups (P < 0.01, P < 0.01, Figures 5 and 6). Compared with the intestinal I/R group, the positive rates of Nrf2 expression increased significantly in SFN pretreatment group (P < 0.01, Figures 5 and 6).
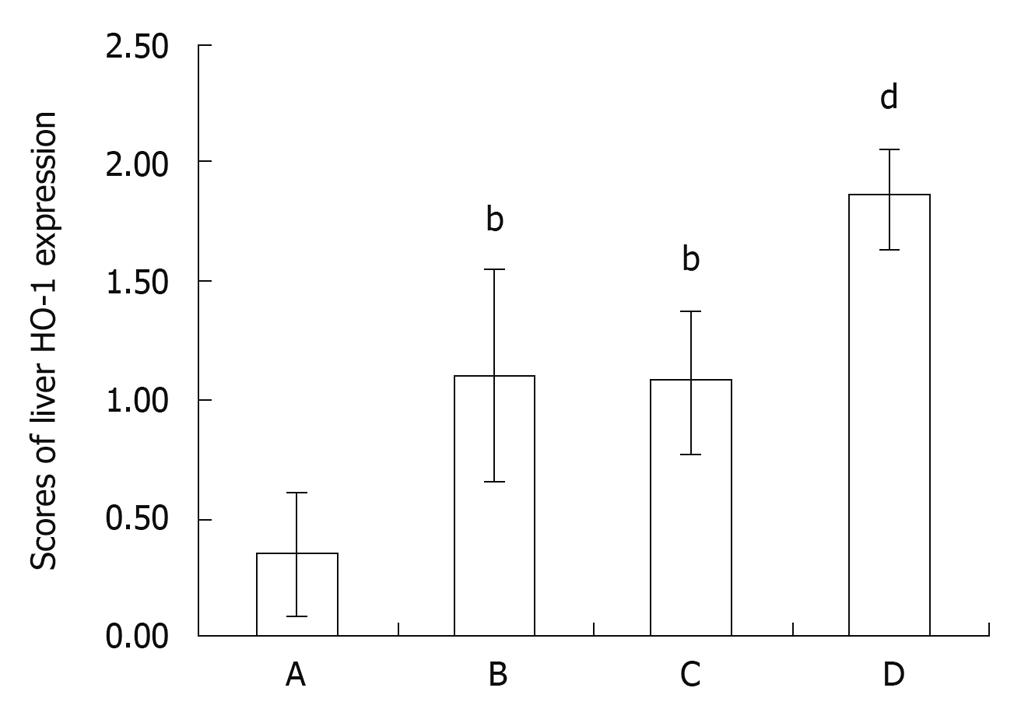
The expression of HO-1 in the control group showed little brown immunostaining in cytoplasm while significant positive expression of HO-1 as brown staining in cytoplasm was observed in the intestinal I/R group and SFN control groups (P < 0.01, P < 0.01, Figures 7 and 8). Compared with the intestinal I/R group, the positive rates of HO-1 expression increased significantly in cytoplasm in SFN pretreatment group (P < 0.01, Figures 7 and 8).
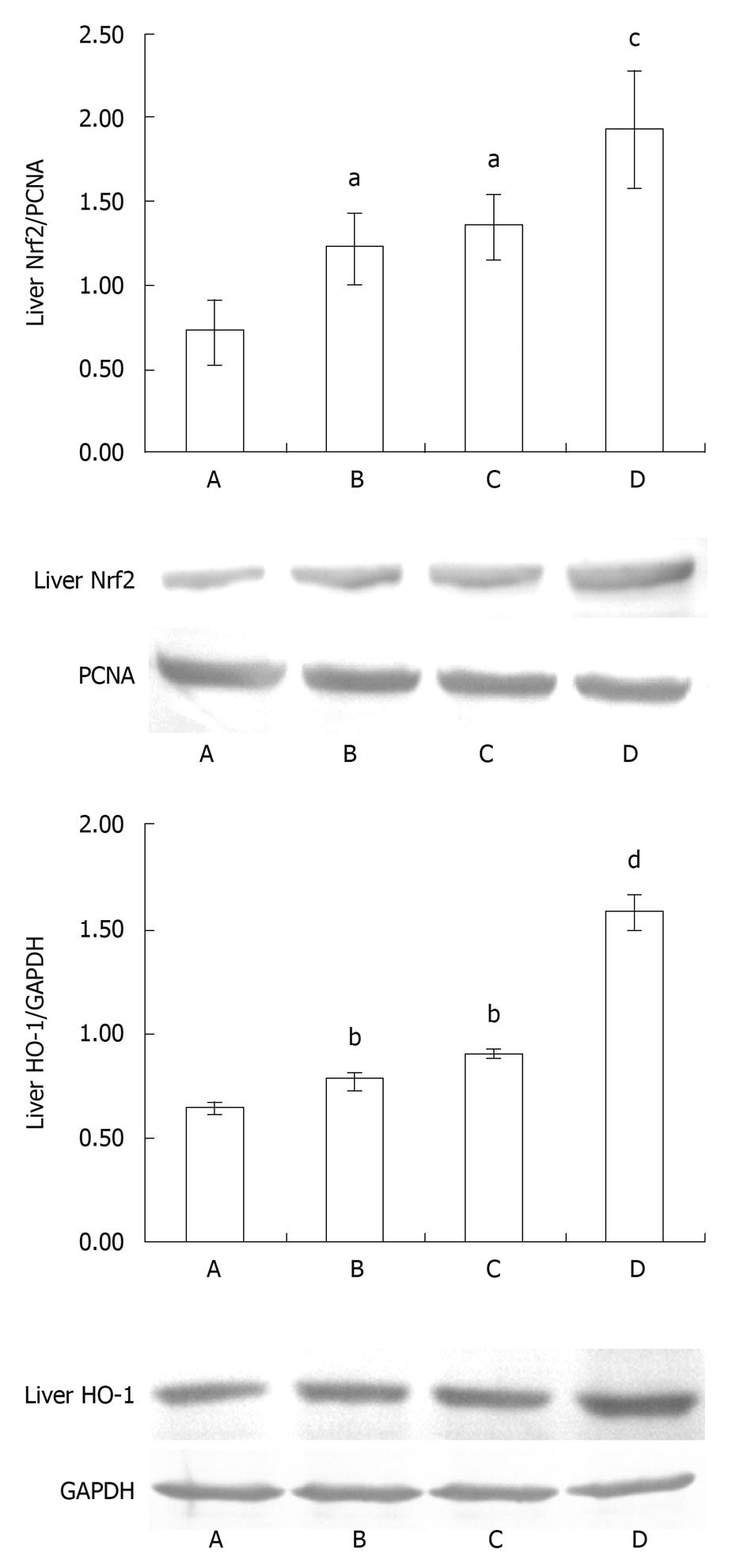
The expression of nuclear Nrf2 of the liver tissues in animals with 2-h reperfusion is shown in Figure 9. The results showed a weak Nrf2 positive signal in the liver of the control group. However, a strong Nrf2 protein signal was found in the intestinal I/R group (P < 0.01, Figure 9). The signal enhanced significantly in the SFN pretreatment group in comparison with the intestinal I/R group (P < 0.01, Figure 9), thereby indicating that SFN could increase the Nrf2 activation in liver tissues.
Western blotting showed weak positive staining for HO-1 in the liver in the control group. However, a significant HO-1 signal was observed in the intestinal I/R group. Compared with the control group, IOD level of HO-1 increased markedly (P < 0.01, Figure 9). The signal enhanced significantly in the SFN pretreatment group in comparison with the intestinal I/R group (P < 0.01, Figure 9), hinting that HO-1 was activated in liver tissues caused by SFN.
Intestinal I/R is not only necessary to the intestine itself, but involves severe distant organ dysfunction. The liver is the first distant organ involved in the severe attack from intestinal I/R[8] due to its vasculature being coupled in series with that of the intestine[29]. During the reperfusion, ROS formed as participants in the I/R-induced leukocyte-mediated liver inflammation responses[30-32], and many clinical and experimental studies showed that ROS plays a crucial role in the pathogenesis of I/R[7,33].
ROS can be restrained by endogenous free radical scavengers such as SOD, catalase, the glutathione peroxidase/glutathione/glutathione reductase system and the thioredoxin peroxidase/thioredoxin/thioredoxin reductase system[34]. SOD catalyses the dismutation of the superoxide anion (O2·) into H2O2, which can be transformed into H2O and O2 by catalase (CAT). GSH as a kind of endogenous free radical scavenger can be regulated by GSH-Px. Ros also oxidizes proteins, induces lipid peroxidation and initiates DNA stand breaks, which can be blocked by the above-mentioned antioxidative agents.
ARE is an enhancer element that initiates the transcription of a battery of genes encoding phase 2 enzymes[35]. Activation of gene transcription through the ARE is mediated primarily by Nrf2[36]. Under basal conditions, Nrf2 is sequestered in the cytoplasm by Keap1, and upon exposure of cells to inducers such as oxidative stress and certain chemopreventive agents, Nrf2 dissociates from Keap1, translocates to the nucleus, binds to ARE, and transactivates phase 2 detoxifying and antioxidant genes[37]. Recent studies have implied that the activation of Nrf2/ARE pathway attenuate ischemia/reperfusion injury of the heart, brain and kidney[26,38,39]. And SFN induces Nrf2-driven phase 2 enzyme expression by modulating the activation in kidney ischemia reperfusion injury[40].
In this study, we evaluated the effect of SFN on regulating Nrf2/ARE in liver injury of the animal intestinal I/R model. We found that Nrf2 activation by sulforaphane pretreatment protected liver against injury induced by intestinal I/R, which was characterized by improved alternation in liver tissue pathology and liver function, and an enhanced antioxidant capacity, being consistent with the Nrf2 expression and content changes.
GSH-Px and HO-1 are two kinds of phase 2 enzymes. HO-1 is a ubiquitous heat shock protein (HSP32) that is highly induced by diverse stress-related conditions[41]. It is upregulated in response to oxidative stress in many tissues, providing generalized endogenous protection against oxidative stress[42]. Our results indicated that GSH-Px and HO-1 can be upregulated by SFN pretreatment through Nrf2-ARE pathway. Therefore, development of a kind of strategy to reduce oxidative stress by inducing endogenous phase 2 enzymes is attractive, which can be regulated by Nrf2. In order to clarify the mechanism comprehensively, further researches should determine the indexes of cell death such as apoptosis and inflammation in this pathophysiology process.
In conclusion, ROS plays an important role in the pathogenesis of liver injury induced by intestinal I/R. SFN exerted the protective effect on the liver injury induced by intestinal I/R, attributable to the antioxidant effect through Nrf2-ARE pathway.
Sulforaphane (SFN) is a natural product derived from isothiocyanate which is present in cruciferous vegetables such as broccoli that has been used as a chemopreventive compound. The cytoprotective effect exerted by this compound is mediated by transcription factor NF-E2-related factor-2 (Nrf2), which binds to the antioxidant response element (ARE) in the promoter region of a number of genes, encoding for antioxidative and phase 2 enzymes. SFN can disrupt the Nrf2-Keap1 complex, and permit Nrf2 to translocate into the nucleus to activate the ARE-driven genes.
Studies suggest that Nrf2 activation by SFN results in effective protection from cancers and renal ischemia/reperfusion (I/R), but no one has evaluated SFN to determine if it can protect liver injury induced by intestinal I/R.
The study for the fist time showed that SFN exerted the protective effect on the liver injury induced by intestinal I/R, attributable to the antioxidant effect through Nrf2-ARE pathway.
This study has indicated that SFN pretreatment attenuates liver injury induced by intestinal I/R in rats, attributable to the antioxidant effect through Nrf2-ARE pathway.
Nrf2 is sequestered in the cytoplasm by Keap1, and upon exposure of cells to inducers such as oxidative stress and certain chemopreventive agents, Nrf2 dissociates from Keap1, translocates to the nucleus, binds to ARE, and transactivates phase 2 detoxifying and antioxidant genes. The activation of Nrf2/ARE pathway attenuates ischemia/reperfusion injury.
The paper is well-written and data are convincing. All data confirm the hypothesis and adequately discussed.
Peer reviewer: Natalia A Osna, MD, PhD, Liver Study Unit, Research Service (151), VA Medical Center, 4101 Woolworth Avenue, Omaha, NE 68105, United States
S- Editor Tian L L- Editor Ma JY E- Editor Lin YP
| 1. | Kubes P, Hunter J, Granger DN. Ischemia/reperfusion-induced feline intestinal dysfunction: importance of granulocyte recruitment. Gastroenterology. 1992;103:807-812. [Cited in This Article: ] |
| 2. | Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1-10. [Cited in This Article: ] |
| 3. | Pierro A, Eaton S. Intestinal ischemia reperfusion injury and multisystem organ failure. Semin Pediatr Surg. 2004;13:11-17. [Cited in This Article: ] |
| 4. | Li JY, Yin HZ, Gu X, Zhou Y, Zhang WH, Qin YM. Melatonin protects liver from intestine ischemia reperfusion injury in rats. World J Gastroenterol. 2008;14:7392-7396. [Cited in This Article: ] |
| 5. | Homer-Vanniasinkam S, Crinnion JN, Gough MJ. Post-ischaemic organ dysfunction: a review. Eur J Vasc Endovasc Surg. 1997;14:195-203. [Cited in This Article: ] |
| 6. | Radhakrishnan RS, Radhakrishnan GL, Radhakrishnan HR, Xue H, Adams SD, Moore-Olufemi SD, Harting MT, Cox CS Jr, Kone BC. Pretreatment with bone morphogenetic protein-7 (BMP-7) mimics ischemia preconditioning following intestinal ischemia/reperfusion injury in the intestine and liver. Shock. 2008;30:532-536. [Cited in This Article: ] |
| 7. | Yao JH, Zhang XS, Zheng SS, Li YH, Wang LM, Wang ZZ, Chu L, Hu XW, Liu KX, Tian XF. Prophylaxis with carnosol attenuates liver injury induced by intestinal ischemia/reperfusion. World J Gastroenterol. 2009;15:3240-3245. [Cited in This Article: ] |
| 8. | Yao JH, Li YH, Wang ZZ, Zhang XS, Wang YZ, Yuan JC, Zhou Q, Liu KX, Tian XF. Proteasome inhibitor lactacystin ablates liver injury induced by intestinal ischaemia-reperfusion. Clin Exp Pharmacol Physiol. 2007;34:1102-1108. [Cited in This Article: ] |
| 9. | Tomita M, Kishimoto H, Takizawa Y, Hayashi M. Effects of intestinal ischemia/reperfusion on P-glycoprotein mediated biliary and renal excretion of rhodamine123 in rat. Drug Metab Pharmacokinet. 2009;24:428-437. [Cited in This Article: ] |
| 10. | Yildiz F, Terzi A, Coban S, Celik H, Aksoy N, Bitiren M, Cakir H, Ozdogan MK. Protective effects of resveratrol on small intestines against intestinal ischemia-reperfusion injury in rats. J Gastroenterol Hepatol. 2009;24:1781-1785. [Cited in This Article: ] |
| 11. | Deitch EA, Rutan R, Waymack JP. Trauma, shock, and gut translocation. New Horiz. 1996;4:289-299. [Cited in This Article: ] |
| 12. | Sun Z, Olanders K, Lasson A, Dib M, Annborn M, Andersson K, Wang X, Andersson R. Effective treatment of gut barrier dysfunction using an antioxidant, a PAF inhibitor, and monoclonal antibodies against the adhesion molecule PECAM-1. J Surg Res. 2002;105:220-233. [Cited in This Article: ] |
| 13. | Brooks HJ, McConnell MA, Corbett J, Buchan GS, Fitzpatrick CE, Broadbent RS. Potential prophylactic value of bovine colostrum in necrotizing enterocolitis in neonates: an in vitro study on bacterial attachment, antibody levels and cytokine production. FEMS Immunol Med Microbiol. 2006;48:347-354. [Cited in This Article: ] |
| 14. | Guven A, Tunc T, Topal T, Kul M, Korkmaz A, Gundogdu G, Onguru O, Ozturk H. Alpha-lipoic acid and ebselen prevent ischemia/reperfusion injury in the rat intestine. Surg Today. 2008;38:1029-1035. [Cited in This Article: ] |
| 15. | Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227-C241. [Cited in This Article: ] |
| 16. | Nitescu N, Ricksten SE, Marcussen N, Haraldsson B, Nilsson U, Basu S, Guron G. N-acetylcysteine attenuates kidney injury in rats subjected to renal ischaemia-reperfusion. Nephrol Dial Transplant. 2006;21:1240-1247. [Cited in This Article: ] |
| 17. | Yu D, Sekine-Suzuki E, Xue L, Fujimori A, Kubota N, Okayasu R. Chemopreventive agent sulforaphane enhances radiosensitivity in human tumor cells. Int J Cancer. 2009;125:1205-1211. [Cited in This Article: ] |
| 18. | de Vries HE, Witte M, Hondius D, Rozemuller AJ, Drukarch B, Hoozemans J, van Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45:1375-1383. [Cited in This Article: ] |
| 19. | Kwak MK, Wakabayashi N, Kensler TW. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat Res. 2004;555:133-148. [Cited in This Article: ] |
| 20. | Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385-394. [Cited in This Article: ] |
| 21. | Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Doré S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147:53-59. [Cited in This Article: ] |
| 22. | Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O'Farrelly C, Rabb H, Taylor CT. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20:2624-2626. [Cited in This Article: ] |
| 23. | Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37:139-143. [Cited in This Article: ] |
| 24. | Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105-1127. [Cited in This Article: ] |
| 25. | Shen G, Khor TO, Hu R, Yu S, Nair S, Ho CT, Reddy BS, Huang MT, Newmark HL, Kong AN. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67:9937-9944. [Cited in This Article: ] |
| 26. | Yoon HY, Kang NI, Lee HK, Jang KY, Park JW, Park BH. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol. 2008;75:2214-2223. [Cited in This Article: ] |
| 27. | Megison SM, Horton JW, Chao H, Walker PB. A new model for intestinal ischemia in the rat. J Surg Res. 1990;49:168-173. [Cited in This Article: ] |
| 28. | Song M, Xia B, Li J. Effects of topical treatment of sodium butyrate and 5-aminosalicylic acid on expression of trefoil factor 3, interleukin 1beta, and nuclear factor kappaB in trinitrobenzene sulphonic acid induced colitis in rats. Postgrad Med J. 2006;82:130-135. [Cited in This Article: ] |
| 29. | Turnage RH, Bagnasco J, Berger J, Guice KS, Oldham KT, Hinshaw DB. Hepatocellular oxidant stress following intestinal ischemia-reperfusion injury. J Surg Res. 1991;51:467-471. [Cited in This Article: ] |
| 30. | Leister I, Mbachu EM, Post S, Samel ST, Stojanovic T, Gutt CN, Becker H, Markus PM. Vasoactive intestinal polypeptide and gastrin-releasing peptide attenuate hepatic microvasculatory disturbances following intestinal ischemia and reperfusion. Digestion. 2002;66:186-192. [Cited in This Article: ] |
| 31. | Kaplan N, Yagmurdur H, Kilinc K, Baltaci B, Tezel S. The protective effects of intravenous anesthetics and verapamil in gut ischemia/reperfusion-induced liver injury. Anesth Analg. 2007;105:1371-1378, table of contents. [Cited in This Article: ] |
| 32. | Horie Y, Wolf R, Anderson DC, Granger DN. Hepatic leukostasis and hypoxic stress in adhesion molecule-deficient mice after gut ischemia/reperfusion. J Clin Invest. 1997;99:781-788. [Cited in This Article: ] |
| 33. | Itoh M, Guth PH. Role of oxygen-derived free radicals in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology. 1985;88:1162-1167. [Cited in This Article: ] |
| 34. | McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159-163. [Cited in This Article: ] |
| 35. | Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61-69. [Cited in This Article: ] |
| 36. | Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291-13295. [Cited in This Article: ] |
| 37. | Jeong WS, Jun M, Kong AN. Nrf2: a potential molecular target for cancer chemoprevention by natural compounds. Antioxid Redox Signal. 2006;8:99-106. [Cited in This Article: ] |
| 38. | Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365-374. [Cited in This Article: ] |
| 39. | Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116-1131. [Cited in This Article: ] |
| 40. | Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526-1539. [Cited in This Article: ] |
| 41. | Dragun D, Hoff U, Park JK, Qun Y, Schneider W, Luft FC, Haller H. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001;60:1173-1181. [Cited in This Article: ] |