Published online May 21, 2010. doi: 10.3748/wjg.v16.i19.2417
Revised: December 11, 2009
Accepted: December 18, 2009
Published online: May 21, 2010
AIM: To evaluate the validity of the estimated glomerular filtration rate (eGFR) as a preoperative renal function parameter in patients with gastric cancer.
METHODS: A retrospective study was conducted in 147 patients with gastric cancer. Preoperative creatinine clearance (Ccr), eGFR, and pre- and postoperative serum creatinine (sCr) data were examined. Preoperative Ccr and eGFR were then compared for their reliability in predicting postoperative renal dysfunction.
RESULTS: Among 110 patients with normal preoperative Ccr values, 7 (6.3%) had abnormal postoperative sCr values, and among 112 patients with normal preoperative eGFR values, postoperative sCr was abnormal in 5 (4.5%) (P = 0.53). Among 37 patients with abnormal preoperative Ccr values, 30 (81.1%) had normal postoperative sCr values, and of 35 patients with abnormal preoperative eGFR values, postoperative sCr was normal in 25 (71.4%) (P = 0.34). Preoperative Ccr was significantly correlated with eGFR (r = 0.514), and postoperative sCr was significantly correlated with preoperative Ccr (r = -0.334) and eGFR (r = -0.02).
CONCLUSION: Preoperative eGFR is as effective as Ccr for predicting postoperative renal dysfunction. eGFR should therefore be used as an indicator of preoperative renal function in place of Ccr since it is a cheaper and easier to perform test.
- Citation: Kosuge T, Sawada T, Iwasaki Y, Kita J, Shimoda M, Tagaya N, Kubota K. eGFR is a reliable preoperative renal function parameter in patients with gastric cancer. World J Gastroenterol 2010; 16(19): 2417-2420
- URL: https://www.wjgnet.com/1007-9327/full/v16/i19/2417.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i19.2417
The creatinine clearance (Ccr) test has been used as a gold standard for evaluation of preoperative renal function in patients with gastric cancer[1,2]. However, it is known that Ccr is not strictly equivalent to glomerular filtration rate (GFR) because of the intrinsic secretion of creatinine into urine from tubuli[3]. Furthermore, in order to measure Ccr, it is necessary to collect 2-h or 24-h urine samples, which is inconvenient in older patients and relatively expensive to implement.
Chronic kidney disease (CKD) is defined as a person having GFR less than 60 mL/min, and the frequency of CKD in the Japanese population is around 10%[4]. Therefore, in patients with gastric cancer, the detection of CKD before operation is a crucial matter to prevent postoperative renal complications. If there are other tests which reflect GFR more accurately and that can be performed easily and cheaply, this would be valuable in a clinical setting[5,6].
Although inulin clearance can be used for measuring GFR, it is not a standard preoperative renal function test because of its cumbersome methodology, and alternatively, the Ccr test has been used. In 1999, Levey et al[7] reported an equation for the estimation of GFR. The Modification of Diet in Renal Disease (MDRD) equation includes age, sex, race, serum creatinine (sCr) value, serum blood urea nitrogen value, and serum albumin value. It was suggested that the MDRD equation precisely reflects the GFR in Caucasians but not in Asians, and therefore an equation for estimating the GFR for Japanese individuals (eGFR) was postulated[8,9]. eGFR is calculated using three parameters: sex, age, and sCr. If eGFR is at least as precise as Ccr for estimation of postoperative renal dysfunction, it would be valuable as a preoperative renal function parameter for patients with gastric cancer, as eGFR is easier and cheaper to measure. To clarify this issue, therefore, we analyzed Ccr and eGFR retrospectively in a series of gastric cancer patients.
One hundred and forty-seven patients diagnosed histologically as having gastric cancer who underwent surgery between January 2003 and December 2005, and for whom preoperative Ccr data were available, were enrolled in the present study. The patients’ backgrounds are summarized in Table 1. Gastrectomy was performed based on the guidelines of the Japan Gastric Cancer Study Group[10]. Basically, patients took meals until lunch on the day before operation, and no hyperalimentation was performed. As antibiotics, 2 g/d of cephalosporin was administered from the day of the operation for 3 d.
| Age (yr) | 68.3 ± 9.8 |
| Sex (M/F) | 107/40 |
| Pre-sCr (mg/dL) | 0.74 ± 0.20 |
| Pre-Ccr (mL/min) | 71.5 ± 24.5 |
| Pre-eGFR (mL/min) | 71.1 ± 20.5 |
| Post-max-sCr (mg/dL) | 0.82 ± 0.30 |
| Method of operation | |
| Distal gastrectomy | 79 |
| Total gastrectomy | 59 |
| Others | 9 |
Measurement of sCr was performed by standard enzymatic method. Ccr was measured by 24-h urine collection, and a value of less than 50 mL/min was regarded as abnormal. eGFR was calculated using the following equation[9]: eGFR (mL/min per 1.73 m2) = 194Cr-1.094× Age-0.287 (× 0.739; for female patients). eGFR of less than 60 mL/min was regarded as abnormal. The upper normal limits of sCr for male and female patients were 1.1 mg/dL and 0.8 mg/dL, respectively.
The reliability of preoperative Ccr and eGFR for detecting postoperative renal dysfunction was analyzed.
Data were expressed as actual number of patients or mean ± SD. χ2 test was used for two-group comparisons. Statistical analyses were performed by using Graphpad software version 4.0. Significance of correlations was calculated by the Spearman correlation coefficient test. Differences at P < 0.05 were considered significant.
Preoperative Ccr was abnormal in 37 patients (25.2%) and preoperative eGFR was abnormal in 35 patients (23.8%) (Table 2, P = 0.79). Postoperatively, 17 patients had abnormal sCr values. Of these 17 patients, 9 (52.9%) had abnormal preoperative Ccr values and 12 (70.6%) had abnormal preoperative eGFR values (Table 3, P = 0.29).
| Normal | Abnormal | P-value | |
| Pre-Ccr | 37 | 110 | 0.89 |
| Pre-eGFR | 35 | 112 |
| Abnormal pre-Ccr | Abnormal pre-eGFR | P-value | |
| Post-sCr | |||
| Normal | 8 | 5 | 0.29 |
| Abnormal | 9 | 12 |
Among the 110 patients who had normal preoperative Ccr values, 7 (6.3%) had postoperative sCr abnormality, and among the 112 patients who had normal preoperative eGFR values, 5 (4.5%) had postoperative sCr abnormality (Table 4, P = 0.53).
| Post-sCr | P-value | ||
| Normal | Abnormal | ||
| Normal pre-Ccr | 103 | 7 | 0.53 |
| Normal pre-eGFR | 107 | 5 | |
Among the 37 patients who had abnormal preoperative Ccr values, 30 (81.1%) had normal postoperative sCr values, and among the 35 patients who had abnormal preoperative eGFR values, 25 (71.4%) had normal postoperative sCr values (Table 5, P = 0.34).
| Post-sCr | P-value | ||
| Abnormal | Normal | ||
| Abnormal pre-Ccr | 7 | 30 | 0.34 |
| Abnormal pre-eGFR | 10 | 25 | |
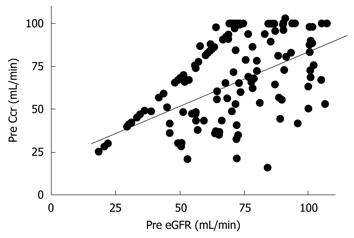
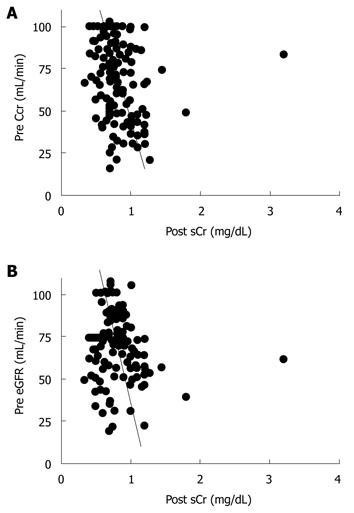
Next, we evaluated the correlation between preoperative Ccr, eGFR, and the maximum postoperative sCr value. Preoperative Ccr and eGFR showed a significant correlation (r = 0.514, P < 0.01, Figure 1). Preoperative Ccr was correlated with the maximum postoperative sCr value (r = -0.334, P < 0.01, Figure 2A). Also, preoperative eGFR was correlated with the maximum postoperative sCr value (r = -0.188, P = 0.02, Figure 2B).
There were no severe postoperative complications, such as anastomotic leakage, bleeding, or infection in the patients who had abnormal preoperative Ccr or eGFR values. None of the patients required postoperative hemodialysis.
The present study showed that eGFR was as equally reliable as Ccr for assessment of preoperative renal function in patients with gastric cancer.
To date, Ccr has been used as a preoperative renal function parameter in patients with gastric cancer. Ccr is measured by 24-h or 2-h urine collection, and it has been suggested that either method can sometimes be inappropriate because of the difficulty involved in implementation, especially in older patients. Furthermore, there is a dissociation between Ccr and GFR in individuals with a low GFR[1]. Although sCr is a simply determined parameter for estimating renal function, it is not a reliable indicator of preoperative renal function because it is easily influenced by sex, muscle volume, and exercise. Also, sCr usually remains normal until GFR decreases to 50 mL/min[11].
GFR has not been used as a gold standard of preoperative renal function because measurement of inulin clearance is more labor-intensive than measurement of Ccr. However, the establishment of the MDRD equation by Levey et al[7] in 1999, and its modification postulated by the Japan Kidney Association, has made it possible to estimate GFR[8,9]. Therefore it is expected that eGFR will replace Ccr in various clinical settings.
In this study, we set the cut-off values of Ccr and eGFR at 50 mL/min and 60 mL/min, respectively. The validity of these cut-off values was endorsed by our finding that there was no significant difference in the number of patients who had abnormal postoperative sCr values between patients having preoperative Ccr abnormality and preoperative eGFR abnormality (Table 3).
There was no difference in the number of patients who had abnormal sCr values between patients who had normal preoperative Ccr values and normal preoperative eGFR values (Table 4), and also no difference in the number of patients who had normal sCr values between patients with preoperative Ccr abnormality and preoperative eGFR abnormality (Table 5). These results indicate that there is no significant difference in reliability between preoperative Ccr and eGFR for detecting postoperative renal dysfunction.
Preoperative Ccr and eGFR values showed a significant correlation (Figure 1), and Ccr and eGFR were correlated with the maximum postoperative sCr value (Figure 2). Thus, either Ccr or eGFR can be used to predict the maximum postoperative sCr value.
Cystatin C is a cysteine protease and a member of the cystatin super family. Cystatin C is excreted from the glomerulus and reabsorbed by the proximal tubuli, and has been used as a marker for GFR[12,13]. sCr is influenced by prerenal factors, such as muscle volume; on the other hand, cystatin C is not influenced by sex, age, and other prerenal factors. In this retrospective study, cystatin C was not included as a parameter; it would be valuable to evaluate the relationship between eGFR and cystatin C as a preoperative renal function test of gastric cancer.
We conclude that eGFR is as equally valuable as Ccr as an indicator of preoperative renal function in patients with gastric cancer, and that eGFR may now be used in place of Ccr in view of the former’s clear medical and socioeconomic advantages.
Creatinine clearance (Ccr) is not strictly equal to glomerular filtration rate (GFR). It has been accepted that estimated GFR (eGFR) is equal to measured GFR in chronic kidney disease. However, there have been no previous studies regarding the reliability of eGFR as a preoperative renal function test in gastric cancer patients.
If eGFR is as good as Ccr as a preoperative renal function test, eGFR may replace Ccr because of its simplicity of measurement.
eGFR is useful as a preoperative renal function parameter in patients undergoing gastrectomy. Ccr is no longer recommended as a first-choice preoperative renal function test.
eGFR should replace Ccr as a routine preoperative renal function test in various surgical fields.
eGFR is estimated GFR which is calculated from age, sex, serum creatinine value (eGFR3), or by adding serum albumin concentration and blood urea nitrogen value (eGFR5).
I think the conslusion should state "may be as good" not be as categorical as eGFR should be used instead of Ccr. This study based on a 147 patient is a good proof of principle but certainly does not have the power to change the standard of care.
Peer reviewers: Dr. Rabih M Salloum, MD, FACS, Associate Professor of Surgery and Oncology, Department of Surgery, University of Rochester Medical center, 601 Elmwood Avenue, Box SURG, Rochester, NY 14642, United States; Ekmel Tezel, MD, PhD, Associate Professor, Department of General Surgery, Gazi University, Faculty of Medicine, Besevler, Ankara 06500, Turkey
S- Editor Wang YR L- Editor Logan S E- Editor Lin YP
| 1. | Oken DE. Criteria for the evaluation of the severity of established renal disease. Nephron. 1970;7:385-388. [Cited in This Article: ] |
| 2. | Iwasaki Y, Sawada T, Kijima H, Kosuge T, Katoh M, Rokkaku K, Kita J, Shimoda M, Kubota K. Estimated glomerular filtration rate is superior to measured creatinine clearance for predicting postoperative renal dysfunction in patients undergoing pancreatoduodenectomy. Pancreas. 2010;39:20-25. [Cited in This Article: ] |
| 3. | Petri M, Bockenstedt L, Colman J, Whiting-O'Keefe Q, Fitz G, Sebastian A, Hellmann D. Serial assessment of glomerular filtration rate in lupus nephropathy. Kidney Int. 1988;34:832-839. [Cited in This Article: ] |
| 4. | Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621-630. [Cited in This Article: ] |
| 5. | Sawada T, Kita J, Rokkaku K, Kato M, Shimoda M, Kubota K. Hepatectomy in patients with nonuremic minimal renal failure. J Gastrointest Surg. 2006;10:740-745. [Cited in This Article: ] |
| 6. | Iwasaki Y, Sawada T, Mori S, Iso Y, Katoh M, Rokkaku K, Kita J, Shimoda M, Kubota K. Estimating glomerular filtration rate preoperatively for patients undergoing hepatectomy. World J Gastroenterol. 2009;15:2252-2257. [Cited in This Article: ] |
| 7. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [Cited in This Article: ] |
| 8. | Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis. 2007;50:927-937. [Cited in This Article: ] |
| 9. | Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982-992. [Cited in This Article: ] |
| 10. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [Cited in This Article: ] |
| 11. | K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-S266. [Cited in This Article: ] |
| 12. | Tidman M, Sjöström P, Jones I. A Comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the two. Nephrol Dial Transplant. 2008;23:154-160. [Cited in This Article: ] |
| 13. | Zahran A, El-Husseini A, Shoker A. Can cystatin C replace creatinine to estimate glomerular filtration rate? A literature review. Am J Nephrol. 2007;27:197-205. [Cited in This Article: ] |