Published online May 14, 2010. doi: 10.3748/wjg.v16.i18.2227
Revised: January 27, 2010
Accepted: February 4, 2010
Published online: May 14, 2010
AIM: To determine the short-term cost-utility of molecular adsorbent recirculating system (MARS) treatment in acute liver failure (ALF).
METHODS: A controlled retrospective study was conducted with 90 ALF patients treated with MARS from 2001 to 2005. Comparisons were made with a historical control group of 17 ALF patients treated from 2000 to 2001 in the same intensive care unit (ICU) specializing in liver diseases. The 3-year outcomes and number of liver transplantations were recorded. All direct liver disease-related medical expenses from 6 mo before to 3 years after ICU treatment were determined for 31 MARS patients and 16 control patients. The health-related quality of life (HRQoL) before MARS treatment was estimated by a panel of ICU doctors and after MARS using a mailed 15D (15-dimensional generic health-related quality of life instrument) questionnaire. The HRQoL, cost, and survival data were combined and the incremental cost/quality-adjusted life years (QALYs) was calculated.
RESULTS: In surviving ALF patients, the health-related quality of life after treatmeant was generally high and comparable to the age- and gender-matched general Finnish population. Compared to the controls, the average cost per QALY was considerably lower in the MARS group (64 732€vs 133 858€) within a timeframe of 3.5 years. The incremental cost of standard medical treatment alone compared to MARS was 10 928€, and the incremental number of QALYs gained by MARS was 0.66.
CONCLUSION: MARS treatment combined with standard medical treatment for ALF in an ICU setting is more cost-effective than standard medical treatment alone.
- Citation: Kantola T, Mäklin S, Koivusalo AM, Räsänen P, Rissanen A, Roine R, Sintonen H, Höckerstedt K, Isoniemi H. Cost-utility of molecular adsorbent recirculating system treatment in acute liver failure. World J Gastroenterol 2010; 16(18): 2227-2234
- URL: https://www.wjgnet.com/1007-9327/full/v16/i18/2227.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i18.2227
Since its introduction in 1993[1,2], the molecular adsorbent recirculating system (MARS) has been used in the treatment of both acute liver failure (ALF) and acute-on-chronic liver failure (AOCLF). The MARS device is an extracorporeal albumin dialysis apparatus that removes both albumin-bound and water-soluble toxins from the patient’s blood, enabling native liver regeneration and allowing time to locate a suitable organ for liver transplantation (Ltx)[3-5].
Numerous studies have documented the favorable effects of MARS treatment on clinical and laboratory parameters[4,6-9] and survival[10-13]. However, only three small non-randomized studies[14-16] have focused on the cost-utility of MARS treatment in AOCLF and the health-related quality of life (HRQoL) of MARS-treated AOCLF patients. Currently, there are no studies on the HRQoL or cost-utility of MARS treatment in ALF patients.
In assessing therapy utility, the subjective feelings of the patient should be taken into account in addition to health benefits (e.g. survival) and cost. To ensure that limited resources are utilized in an ethical manner, the impact of a given treatment on the future HRQoL of the patient must be considered[17]. The effectiveness of a given treatment should be assessed by considering the treatment’s impact on both the length and quality of life, which can be combined into the measure quality-adjusted life years (QALYs). In the cost-utility analysis, the QALYs gained by a given treatment are used as the measuring units of efficacy. Thus, the QALYs and cost/QALY-ratios can be used to compare the different treatments in terms of length of life and quality of life. Currently, there is no consensus as to how much a QALY gained can cost, but a 50 000€ threshold has been suggested[17].
The aim of this study was to determine the short-term (3.5 years) cost-utility of MARS treatment in ALF patients.
This study included 90 ALF patients treated with MARS from May 2001 to October 2005 and a historical control group of 17 consecutive ALF patients treated from March 2000 to April 2001. ALF was defined as a rapid deterioration of hepatic synthetic function with or without encephalopathy and no previous history of liver disease[18].
All patients were treated in the same intensive care unit (ICU) specializing in liver disease at the Helsinki University Hospital and according to the same main principles of standard medical therapy (SMT). The SMT in our ICU and the operational principles of the MARS device were reported previously[3-5]. Our liver ICU is the only Ltx center in Finland, and all critical ALF patients are referred to our unit for treatment and transplantation evaluation. The indications for MARS treatment and the treatment protocols are summarized in Table 1. The 3-year survival and the need for Ltx were determined in all patients.
| Etiology | MARS treatment initiation criteria | Treatment protocol |
| Acute liver failure | Rapid deterioration of hepatic synthetic function and clinical condition despite conservative standard medical therapy | 22-h sessions daily until: |
| And one of the following criteria: | (1) Native liver recovers | |
| (1) Ingestion of a lethal dose of a known hepatotoxin (e.g. Amanita, paracetamol) | (2) Suitable transplant organ is found | |
| (2) The patient fulfills the criteria for highly urgent liver transplantation | (3) Irreversible multi-organ damage occurs |
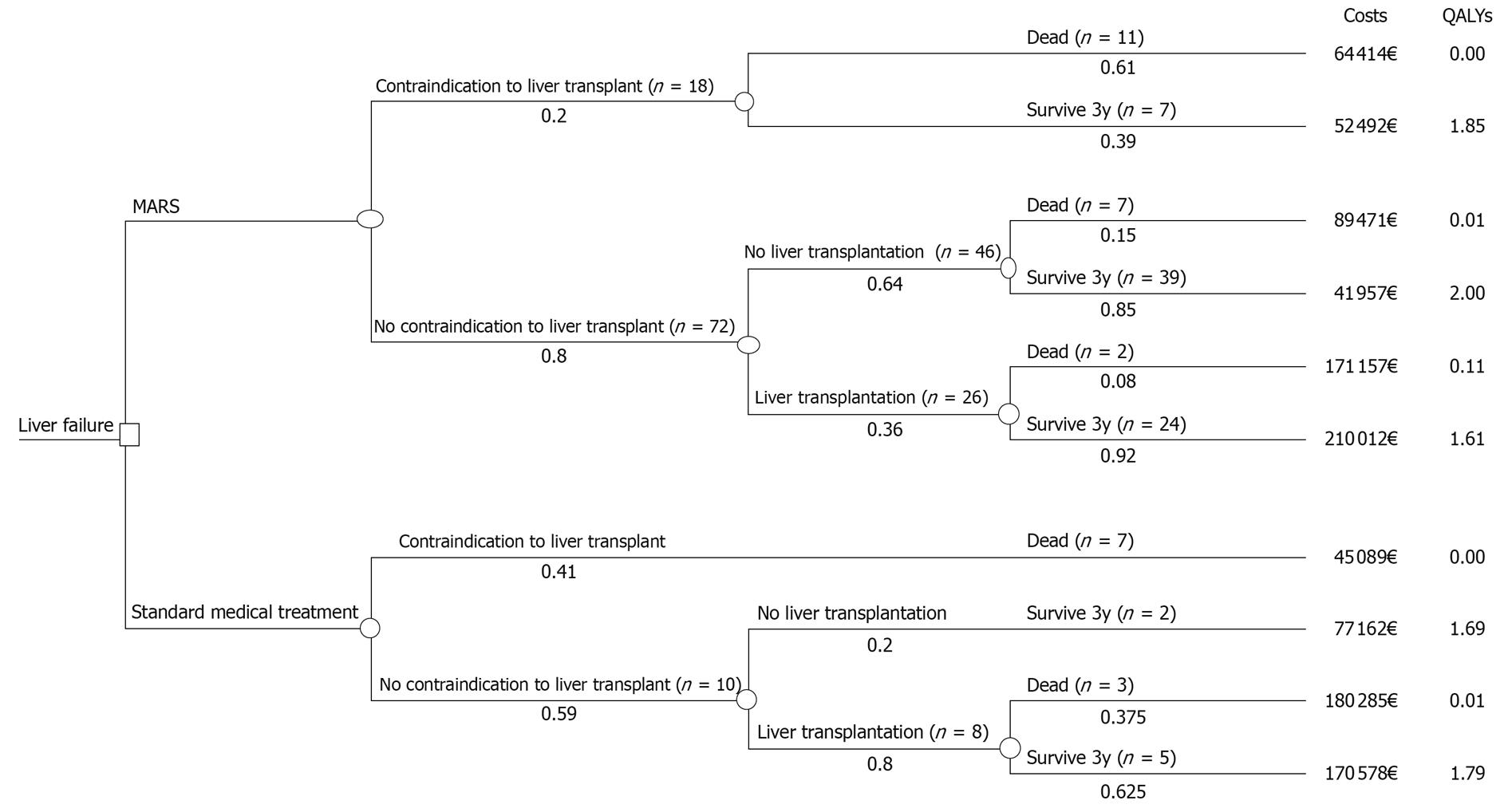
In the cost-utility analysis, effectiveness was measured as QALYs gained. The costs and outcomes of MARS treatment were compared with those of SMT in the control group over a 3-year time horizon from the perspective of the health care provider. For this comparison, a deterministic decision model was developed using TreeAge Pro HealthCare software (TreeAge, Williamstown, MA, USA). The model was used to combine the data on costs and effectiveness and to incorporate data variability and uncertainty into the analysis (Figure 1). The pathways presented in Figure 1 are mutually exclusive sequences of events, and the expected values are based on the pathway values weighted by the pathway probabilities. The probabilities show the proportion of the patient cohort that is expected to experience the event at any particular point in the tree.
The incremental cost-effectiveness ratio (ICER) was determined by dividing the difference in costs by the difference in QALYs. Both costs and QALYs were discounted 5% as currently recommended in the Finnish guidelines for pharmacoeconomic evaluation. The base-case analysis estimated the cost-effectiveness based on the mean values from the data in the simplified model structure. The effect of model input parameter uncertainty on the results was tested in a one-way and probabilistic sensitivity analysis.
From the year 2000 onwards, the Helsinki University Central Hospital district began to use the clinical patient-administration database Ecomed® for registering all treatment costs. In this study all direct medical costs were obtained from the Ecomed®-database (Datawell Ltd., Espoo, Finland). The total costs included all relevant liver disease-related expenses incurred at the Helsinki University Hospital. All costs incurred between 6 mo before the first MARS treatment (or liver ICU admission in the control group) and 3 years after the treatment were included. Complete cost data were available only for those patients who were living (31 MARS and 16 control patients) and received all hospital care in the catchment area of the Helsinki and Uusimaa Hospital District. Therefore, patients referred from outside the catchment were excluded from the cost analysis. Direct non-medical costs (transportation, domestic help, productivity costs due to absences from work, etc.) were not included. The mean total cost within the specified time period was used in the base-case analysis. All costs in Euros were inflated to the 2006 price level.
The HRQoL was measured by the 15D (15-dimensional generic health-related quality of life instrument)[19-21], which is a generic, self-administered questionnaire for adults using criteria related to 15 dimensions: moving, seeing, hearing, breathing, sleeping, eating, speech, eliminating, usual activities, mental function, discomfort and symptoms, depression, distress, vitality, and sexual activity. For each dimension, the patient chooses one of five levels that best describes his/her current state of health. A set of utility weights is used to generate a single index number, the 15D score, which ranges from 0 to 1 (1 = full health, 0 = dead)[22]. For most of the important properties (i.e. reliability, content validity, discriminatory power, and responsiveness to change), the 15D is at least equal to other similar HRQoL instruments, such as the EQ-5D, SF-6D, HUI3, and AqoL[19-21,23-25].
Most ALF patients admitted to our liver ICU are seriously ill and encephalopathic, if not unconscious, and thus unable to fill out the HRQoL questionnaires. Therefore, we used expert opinion to assess the pre-treatment HRQoL. Three ICU doctors separately and retrospectively estimated the HRQoL of 30 ALF patients using the 15D instrument and the patients’ clinical documents. All patients were divided into five groups according to their pre-treatment encephalopathy grade, which was represented as a number from 0 to 4. The HRQoL was assessed in all patients who were conscious and not intubated. The HRQoL was evaluated for six randomly selected non-intubated patients from each encephalopathy grade 0-3, and their average 15D score was assumed to represent the approximate HRQoL of the entire group (Table 2). Unconscious and intubated patients, which included some encephalopathy grade 3 patients and all encephalopathy grade 4 patients, received a 15D score of 0.0162[22].
| Pre-treatment encephalopathy grade | 15D score |
| 0 | 0.532 ± 0.151 |
| 1 | 0.461 ± 0.188 |
| 2 | 0.403 ± 0.177 |
| 3 | 0.079 ± 0.077 |
| 4 | 0.016 |
In July 2007, the 15D questionnaire was sent to the 68 MARS-treated ALF patients who were still alive (one patient was not found). Two reminder letters were sent to those who did not return the first questionnaire. In total, 79% (54/68) completed the post-treatment 15D questionnaire; 37% of these patients (20/54) had undergone Ltx. At the time of the survey, the time elapsed after the first MARS treatment varied among individual patients (median 49 mo, range 22-72 mo). Thus, a 15D score 3 years after the first MARS treatment was individually estimated for each patient using linear regression analysis with the patient’s age, time since MARS treatment, and the etiology of liver failure as explanatory variables.
The mean 15D scores and resulting QALYs were calculated separately for each pathway in the decision tree (Figure 1). For patients who died within the time horizon, QALYs were calculated based on survival, assuming the 15D score declined linearly from the baseline value to zero at the time of death.
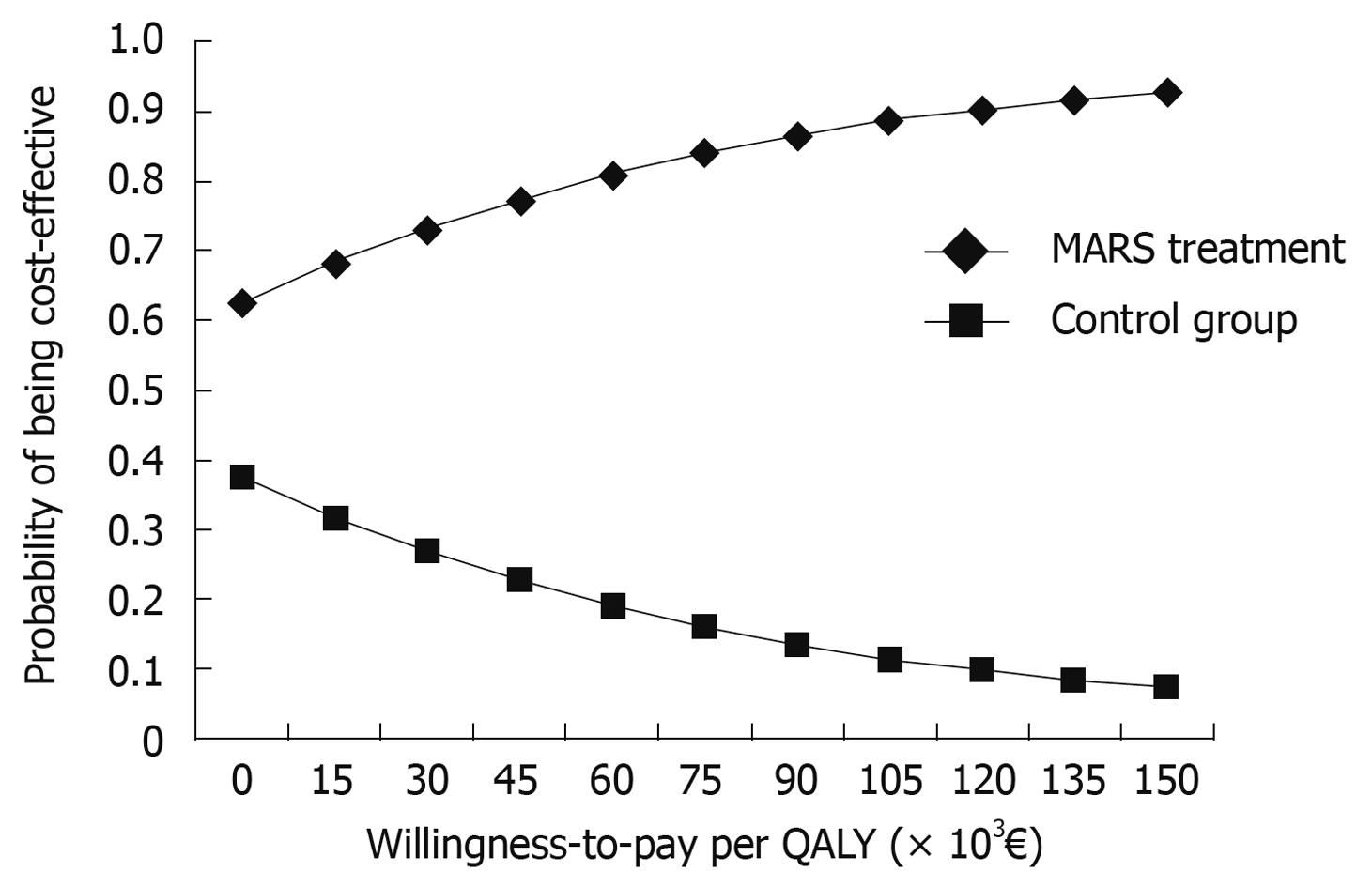
The effect that model parameter uncertainty had on the results was examined first in a one-way analysis and further in a probabilistic sensitivity analysis. In the one-way sensitivity analysis, the discount rate, the mean cost and QALYs of both the MARS and control groups, the probability of Ltx, and survival rates varied. For the probabilistic sensitivity analysis, probability distributions for tree probabilities, cost, and utility input parameters were estimated. The results of the probabilistic sensitivity analysis are presented as a cost-effectiveness acceptability curve (Figure 2). Probability and utility values are represented by beta distributions (limiting values between 0 and 1 for utility values), the parameters of which were estimated from the patient data. For cost estimates, a gamma distribution was determined. Because the actual patient level cost data were limited, regression analysis was used to estimate costs for all patients. Sex, Ltx and its contraindication, and the number of MARS treatments received were used as explanatory variables in the regression analysis.
All data were analyzed using SPSS for Windows version 15.0 (SPSS, Inc., Chicago, IL, USA). The Wilcoxon signed-rank test, the Mann-Whitney U-test, Pearson’s χ2, and Fisher’s exact tests were used when appropriate. P≤ 0.05 was considered significant, and a ≥ 0.03 (absolute value) difference in the HRQoL scores was considered clinically important[26].
The demographic and clinical characteristics of the MARS-treated and control ALF patients did not differ significantly, with the exception of a need for vasoactive infusion (Table 3). The grade of encephalopathy before treatment was also comparable between the two groups. The etiological distribution of ALF patients differed between the groups (P = 0.002). Control patients had mostly ALF of unknown etiology (65%), whereas the majority of MARS-treated patients (57%) had ALF due to known toxicity (e.g. paracetamol or other drugs).
| MARS group | Historical control group | P value | |
| Pre-treatment demographic & clinical data | |||
| Number of patients | 90 | 17 | |
| Age (yr) | 45 (14-81) | 42 (21-72) | 0.714 |
| Sex (male) | 37 (41%) | 7 (41%) | 0.996 |
| Body mass index (kg/m2) | 26 (17-40) | 26 (21-37) | 0.372 |
| MARS sessions/patient | 2 (1-9) | 0 | |
| Mechanically ventilated | 30 (33%) | 6 (35%) | 0.875 |
| Vasoactive infusion used | 27 (30%) | 9 (56%) | 0.041 |
| Renal insufficiency | 29 (32%) | 7 (41%) | 0.474 |
| MELD-score | 31 (5-50) | 30 (19-51) | 0.225 |
| Mean encephalopathy grade | 1.7 ± 1.6 | 2.1 ± 1.7 | 0.335 |
| Contra-indication to Ltx prior to treatment | 11 (12%) | 4 (24%) | 0.253 |
| Became untransplantable during treatment | 7 (8%) | 3 (18%) | 0.195 |
| Number of transplanted patients | 26 (29%) | 8 (47%) | 0.162 |
| Pre-treatment key laboratory values | |||
| Platelets (× 109/L) | 138 (11-410) | 142 (51-448) | 0.919 |
| NH4 ion (μmol/L) | 68 (8-512) | 90 (14-241) | 0.559 |
| Bilirubin (μmol/L) | 215 (4-761) | 381 (38-880) | 0.057 |
| Creatinine (μmol/L) | 75 (35-1318) | 78 (38-275) | 0.946 |
| FV (%) | 32 (5-142) | 38 (7-71) | 0.865 |
The percentage of transplanted patients was lower (29% vs 47%, P = 0.14), and the percentage of patients who survived 3 years after treatment was higher (78% vs 41%, P = 0.002) in the MARS-treated group compared to controls. A similar survival benefit favoring MARS treatment compared to controls was noted in both transplanted (92% vs 63%, P = 0.072) and non-transplanted patients (72% vs 22%, P = 0.006).
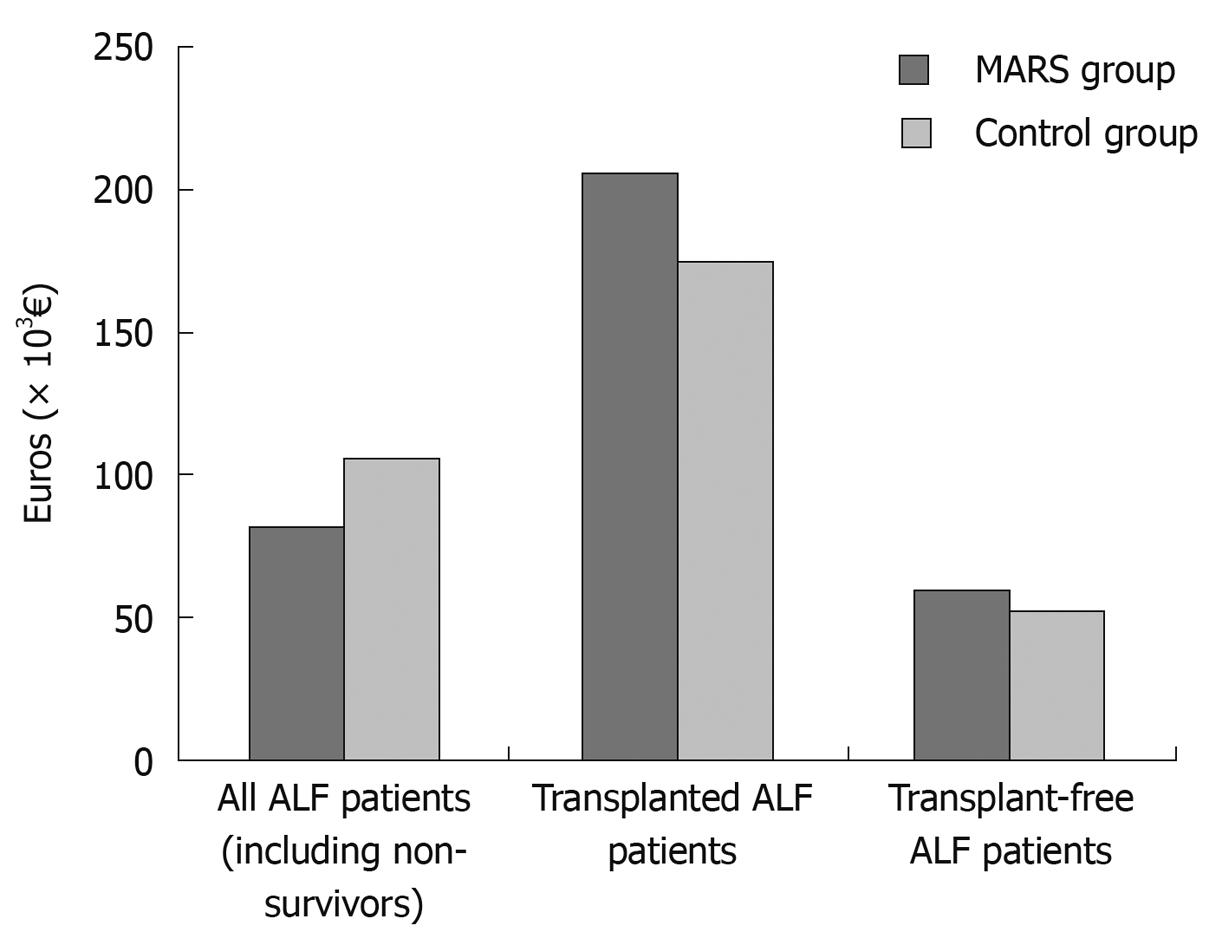
The total mean direct medical costs related to liver disease were 79 745€ for the MARS group and 105 820€ for the controls (Table 4). The total mean direct medical costs for transplanted and non-transplanted MARS and control patients are shown in Figure 3 and the expected costs weighted by the pathway probabilities are shown in Table 5.
| Subgroups according to outcome | 3-yr survival | Cost (€) | HRQoL | ||||||||
| n | % | Mean (d) | n | Mean | Min | Max | n | Pre-treatment mean | Post-treatment mean | ||
| MARS group | All ALF patients | 90 | 78 | 31 | 79 745 | 11 961 | 370 573 | 90 | 0.30 | 0.70 | |
| Alive at 3 yr | 70 | 24 | 78 724 | 11 961 | 370 573 | 70 | 0.34 | 0.89 | |||
| Dead | 20 | 63 | 7 | 83 243 | 32 496 | 171 157 | 20 | 0.15 | 0.00 | ||
| Contraindication to Ltx | 18 | 39 | |||||||||
| Alive at 3 yr | 7 | 4 | 52 492 | 30 325 | 112 585 | 7 | 0.38 | 0.85 | |||
| Dead | 11 | 14 | 5 | 64 414 | 32 496 | 95 666 | 11 | 0.13 | 0.00 | ||
| No contraindication - no Ltx | 46 | 85 | |||||||||
| Alive at 3 yr | 39 | 15 | 41 957 | 11 961 | 137 235 | 39 | 0.40 | 0.93 | |||
| Dead | 7 | 110 | 1 | 89 471 | 7 | 0.09 | 0.00 | ||||
| Transplanted | 26 | 92 | |||||||||
| Alive at 3 yr | 24 | 5 | 210 012 | 96 984 | 370 573 | 24 | 0.22 | 0.84 | |||
| Dead | 2 | 169 | 1 | 171 157 | 2 | 0.47 | 0.00 | ||||
| Control group | All ALF patients | 17 | 41 | 16 | 105 820 | 16 862 | 262 481 | 17 | 0.27 | 0.36 | |
| Contraindication to Ltx | 0 | ||||||||||
| Dead | 7 | 9 | 7 | 45 089 | 17 591 | 105 917 | 7 | 0.27 | 0.00 | ||
| No contraindication - no Ltx | 100 | ||||||||||
| Alive at 3 yr | 2 | 2 | 77 162 | 16 862 | 137 462 | 2 | 0.27 | 0.85 | |||
| Transplanted | 63 | ||||||||||
| Alive at 3 yr | 5 | 4 | 170 578 | 117 444 | 262 481 | 5 | 0.32 | 0.87 | |||
| Dead | 3 | 28 | 3 | 180 285 | 129 120 | 250 772 | 3 | 0.19 | 0.00 | ||
| Cost (€) | Incremental cost (€) | QALYs | Incremental QALYs | Cost per QALY (€) | Incremental cost per QALY | |
| MARS | 93 214 | 1.44 | 0.66 | 64 732 | MARS dominates SMT | |
| Control group (SMT only) | 104 142 | 10 928 | 0.78 | 133 858 |
In all patient groups, most of the costs were incurred within a year of the first MARS or standard medical treatment. The costs during the second and third years were negligible. In all groups, the highest costs were observed in transplanted patients.
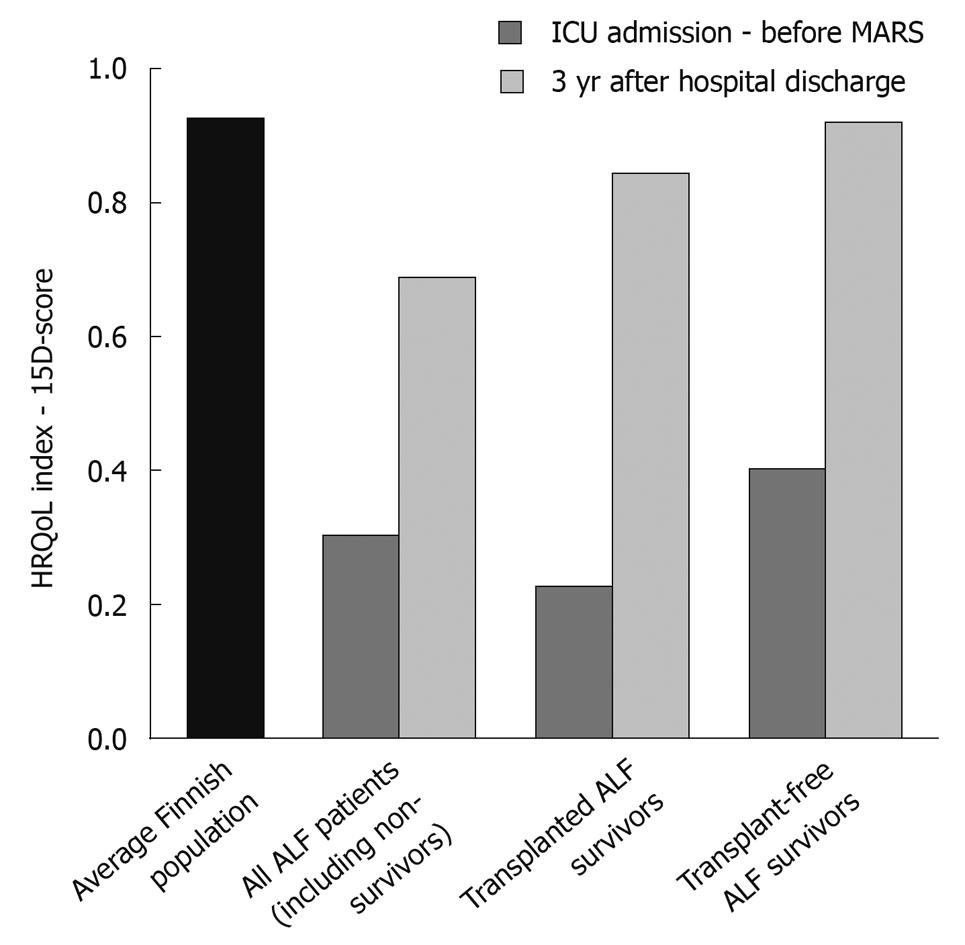
The pre- and post-treatment 15D scores are shown in Figure 4 and Table 4. The estimated HRQoL for all patients prior to treatment was very low compared to an age-standardized reference population in Finland (0.30 vs 0.92)[27]. The highest post-treatment 15D scores were observed in MARS-treated patients who did not have a contraindication to Ltx and recovered without transplantation. The same patients also experienced the highest mean number of QALYs (2.00, within the 3-year follow-up). The groups with the smallest mean number of QALYs were patients with a contraindication to Ltx and those who died within 3 years of treatment.
In the base-case analysis, the MARS group strongly dominated the control group receiving only SMT; MARS treatment was both less costly and more effective than SMT in ALF patients (Table 5). Using the 3-year time horizon, the expected outcome of MARS patients was 1.44 QALYs and the expected costs were 93 214€. The corresponding figures for the controls were 0.78 and 104 142€, respectively. Compared to MARS, the incremental cost of SMT in the control group was 10 928€. The incremental number of QALYs gained by MARS was 0.66.
MARS remained the dominant strategy throughout most of the one-way sensitivity analyses. Neither increasing the proportion of patients with a contraindication to Ltx in the MARS group nor varying the survival rates of patients after Ltx or MARS-treated patients with a contraindication, mitigated the dominance of MARS treatment. MARS also remained the dominant strategy throughout QALY estimate variation in the one-way sensitivity analysis. Using a discount rate of 0%, 3%, or 5% did not eliminate the dominance.
The results were more sensitive to variation in the cost estimates. In the one-way sensitivity analysis, increasing the costs for MARS-treated patients who had no contraindication and survived three years with Ltx removed the dominance of MARS and resulted in an ICER of 63 206€ per QALY gained.
The proportion of liver disease with unknown etiology was significantly higher in the control group (P = 0.002), which can lead to a higher probability of Ltx. We varied the probability of Ltx in the MARS group from 0.89 (observed in controls) to 0.36 (observed in the MARS-treated group); throughout which, MARS remained the more effective alternative. However, when the probability of Ltx in the MARS group was increased to 0.42 or higher, the expected cost of MARS exceeded that of the control group receiving SMT, and its dominance was eliminated. When the probability of Ltx in the MARS group was set to the same level as the control group (0.89), the ICER was 98 686€ per QALY gained.
The cost-effectiveness acceptability curve (Figure 2) of MARS vs controls showed the probability that MARS is cost-effective given a range of willingness-to-pay thresholds per QALY gained. If the decision-maker is willing to pay 50 000€ per QALY, the probability of MARS being cost-effective is 78%. Furthermore, the probability of MARS being cost-effective is 95% if the willingness-to-pay threshold is 200 000€ per QALY.
To our knowledge, there are no previous studies evaluating the cost-utility of MARS treatment in ALF patients. We found that MARS treatment was both less costly and more effective than SMT in ALF. Compared to the controls, the average cost per QALY was significantly lower in the MARS group (64 732€vs 133 858€), mainly due to the significantly higher 3-year overall survival rate and fewer transplantations in the MARS group.
The average overall 3.5-year costs associated with the treatment of ALF were substantial with or without MARS treatment due to the long ICU stay and, in some cases, Ltx costs. The fewer Ltx in the MARS group resulted in reduced overall average costs compared to conservative treatment. Because the costs associated with the Ltx procedure are high, any intervention that decreases the number of transplantations is bound to have a profound effect on the total expense.
As reported previously[28,29], we found that, even though the HRQoL of surviving transplanted patients was generally very good, it was still somewhat lower than that of a person in the age-standardized general population (0.84 vs 0.92)[27]. In ALF patients who recovered without Ltx, the HRQoL after treatment was similar to that of the age-standardized Finnish reference population (0.93 vs 0.92)[27].
Only a handful of HRQoL and cost-effectiveness studies have been completed on MARS patients, and all have been limited to AOCLF patients treated in the same center[14-16]. Until now, MARS-treated ALF patients have not been evaluated in terms of total cost and QALYs, possibly owing to the rarity of the condition, which makes it difficult to enroll enough patients for statistical analysis. Furthermore, comparing results from different studies can be difficult because transplant organ availability varies, and centers may have different criteria for MARS treatment. In addition, the heterogeneity of the etiology of ALF in different countries markedly affects survival rates and the percentage of patients who may experience native liver recovery[30,31].
The most recent cost-utility evaluation of MARS treatment included 79 alcohol-related AOCLF patients[14]; their 3-year survival was 52% with mean direct medical costs of 40 032€ and an ICER of 31 448€ per life-year gained. However, transplanted patients and those with serious co-morbidities were excluded from this analysis, which had a huge impact on the total costs. Another study focused on the impact of MARS treatment on the hospitalization costs and 1-year survival of cirrhotic AOCLF patients[32]; the in-hospital cost for surviving MARS-treated patients was $32 036, $4000 less than in the control group.
The main limitation of this study was the nonrandomized design. Also, due to patient incapacitation, the pre-treatment HRQoL was retrospectively assessed by an expert panel rather than by the patients themselves. Studies have shown that the subjective feelings of a patient, such as pain or HRQoL, are usually misjudged or underestimated by the attending doctors, other treatment staff, or even close relatives[33,34]. Another factor affecting the positive outcomes of MARS-treated ALF patients in this study was the uneven distribution of liver failure etiologies between treatment groups. The prognosis and need for Ltx is strongly related to the underlying cause of ALF[30]; the MARS treatment group was at an advantage in that it included many patients with a good prognosis compared to controls. This predisposition must be taken into account when interpreting the results of this study. The sensitivity analysis was used to estimate the effect of this bias, and it seems that even if both the MARS and control groups had a similar distribution of etiologies, MARS treatment would remain the more effective strategy, although more expensive in terms of total cost.
One must bear in mind that this study dealt with a 3.5-year time window, thus severely underestimating the QALYs to be gained over the remaining lifetime. Alternatively, we could have extrapolated the expected lifetime costs and outcomes; however, this would have necessitated further assumptions and resulted in greater uncertainty.
In conclusion, cost-utility analysis found that MARS treatment plus SMT is both less costly and more effective than SMT alone in ALF patients. Although some uncertainty exists in the model input parameters, probabilistic sensitivity analysis showed that the probability of MARS being cost-effective is higher than that of SMT alone.
The molecular adsorbent recirculating system (MARS) device is an extracorporeal albumin dialysis apparatus which can be used to treat liver failure patients. MARS removes both albumin-bound and water-soluble toxins from the patient’s blood, enabling native liver regeneration and allowing time to locate a suitable organ for liver transplantation. Numerous studies have documented the favorable effects of MARS treatment on clinical and laboratory parameters and survival. However, only a few small non-randomized studies have focused on the cost-utility of MARS treatment in patients with acute-on-chronic liver failure.
The cost-utility of the MARS treatment and its impact on the health-related quality of life in acute liver failure patients has never been investigated previously.
The results from the current study suggest that MARS treatment is both less costly and more effective than standard medical therapy in the treatment of acute liver failure patients. Compared to the controls, the average cost per quality-adjusted life year (QALY) was significantly lower in the MARS group, mainly due to the significantly higher 3-year overall survival rate and fewer transplantations in the MARS group. The authors also found that in MARS-treated acute liver failure patients who recovered without liver transplantation, the health-related quality of life after treatment was similar to that of the age-standardized Finnish reference population.
The cost-utility analysis suggests that MARS treatment plus standard medical therapy is both less costly and more effective than standard medical therapy alone in acute liver failure patients. Therefore, the additional costs which are associated with the use of the MARS machine seem justifiable in this patient group.
This paper deals with the important topic of the cost-utility of molecular adsorbent recirculating system treatment in acute liver failure. The report is concise and informative.
Peer reviewer: Hon-Yi Shi, PhD, Associate Professor, Graduate Institute of Healthcare Administration, Kaohsiung Medical University, 100, Shih-Chuan 1st Road, San Ming District, Kaohsiung 807, Taiwan, China
S- Editor Wang YR L- Editor Webster JR E- Editor Lin YP
| 1. | Stange J, Mitzner S, Ramlow W, Gliesche T, Hickstein H, Schmidt R. A new procedure for the removal of protein bound drugs and toxins. ASAIO J. 1993;39:M621-M625. [Cited in This Article: ] |
| 2. | Stange J, Ramlow W, Mitzner S, Schmidt R, Klinkmann H. Dialysis against a recycled albumin solution enables the removal of albumin-bound toxins. Artif Organs. 1993;17:809-813. [Cited in This Article: ] |
| 3. | Kantola T, Koivusalo AM, Höckerstedt K, Isoniemi H. The effect of molecular adsorbent recirculating system treatment on survival, native liver recovery, and need for liver transplantation in acute liver failure patients. Transpl Int. 2008;21:857-866. [Cited in This Article: ] |
| 4. | Lahdenperä A, Koivusalo AM, Vakkuri A, Höckerstedt K, Isoniemi H. Value of albumin dialysis therapy in severe liver insufficiency. Transpl Int. 2005;17:717-723. [Cited in This Article: ] |
| 5. | Kantola T, Koivusalo AM, Parmanen S, Höckerstedt K, Isoniemi H. Survival predictors in patients treated with a molecular adsorbent recirculating system. World J Gastroenterol. 2009;15:3015-3024. [Cited in This Article: ] |
| 6. | Pugliese F, Novelli G, Poli L, Levi Sandri GB, Di Folco G, Ferretti S, Morabito V, Ruberto F, Berloco PB. Hemodynamic improvement as an additional parameter to evaluate the safety and tolerability of the molecular adsorbent recirculating system in liver failure patients. Transplant Proc. 2008;40:1925-1928. [Cited in This Article: ] |
| 7. | Donati G, Piscaglia F, Colì L, Silvagni E, Righini R, Donati G, Pini P, Stefoni S, Bolondi L. Acute systemic, splanchnic and renal haemodynamic changes induced by molecular adsorbent recirculating system (MARS) treatment in patients with end-stage cirrhosis. Aliment Pharmacol Ther. 2007;26:717-726. [Cited in This Article: ] |
| 8. | Doria C, Mandalá L, Scott VL, Gruttadauria S, Marino IR. Fulminant hepatic failure bridged to liver transplantation with a molecular adsorbent recirculating system: a single-center experience. Dig Dis Sci. 2006;51:47-53. [Cited in This Article: ] |
| 9. | Koivusalo AM, Teikari T, Höckerstedt K, Isoniemi H. Albumin dialysis has a favorable effect on amino acid profile in hepatic encephalopathy. Metab Brain Dis. 2008;23:387-398. [Cited in This Article: ] |
| 10. | El Banayosy A, Kizner L, Schueler V, Bergmeier S, Cobaugh D, Koerfer R. First use of the Molecular Adsorbent Recirculating System technique on patients with hypoxic liver failure after cardiogenic shock. ASAIO J. 2004;50:332-337. [Cited in This Article: ] |
| 11. | Heemann U, Treichel U, Loock J, Philipp T, Gerken G, Malago M, Klammt S, Loehr M, Liebe S, Mitzner S. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology. 2002;36:949-958. [Cited in This Article: ] |
| 12. | Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, Berger ED, Lauchart W, Peszynski P, Freytag J. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277-286. [Cited in This Article: ] |
| 13. | Saliba F, Camus C, Durand F, Mathurin P, Delafosse B, Barange K, Belnard M, Letierce A, Ichai P, Samuel D. Randomized controlled multicenter trial evaluating the efficacy and safety of albumin dialysis with MARS in patients with fulminant and subfulminant hepatic failure. The liver meeting 2008, 50th anniversary meeting of the international association for the study of liver. San Francisco 2008. Hepatology. 2008;48:4 suppl. [Cited in This Article: ] |
| 14. | Hessel FP. Economic evaluation of the artificial liver support system MARS in patients with acute-on-chronic liver failure. Cost Eff Resour Alloc. 2006;4:16. [Cited in This Article: ] |
| 15. | Hessel FP, Mitzner SR, Rief J, Gress S, Guellstorff B, Wasem J. Economic evaluation of MARS--preliminary results on survival and quality of life. Liver. 2002;22 Suppl 2:26-29. [Cited in This Article: ] |
| 16. | Hessel FP, Mitzner SR, Rief J, Guellstorff B, Steiner S, Wasem J. Economic evaluation and 1-year survival analysis of MARS in patients with alcoholic liver disease. Liver Int. 2003;23 Suppl 3:66-72. [Cited in This Article: ] |
| 17. | Talmor D, Shapiro N, Greenberg D, Stone PW, Neumann PJ. When is critical care medicine cost-effective? A systematic review of the cost-effectiveness literature. Crit Care Med. 2006;34:2738-2747. [Cited in This Article: ] |
| 18. | Garcia G, Keeffe E. Acute liver failure. Handbook of Liver Disease. New York: Churchill Livingstone 1998; 15-26. [Cited in This Article: ] |
| 19. | Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med. 2001;33:328-336. [Cited in This Article: ] |
| 20. | Sintonen H. The 15D-measure of health-related quality of life. II. Feasibility, reliability and validity of its valuation system. National Centre for Health Program Evaluation, Working Paper 42, Melbourne, 1995. Available from: http://www.buseco.monash.edu.au/centres/che/pubs/wp42.pdf. [Cited in This Article: ] |
| 21. | Sintonen H. The 15D-measure of health-related quality of life. I. Reliability, validity and sensitivity of its health state descriptive system. National Centre for Health Program Evaluation, Working Paper 41, Melbourne, 1994. Available from: http://www.buseco.monash.edu.au/centres/che/pubs/wp41.pdf. [Cited in This Article: ] |
| 22. | Sintonen H. The health-related quality of life (HRQoL) instrument. Available from: http://www.15d-instrument.net/15d. [Cited in This Article: ] |
| 23. | Stavem K. Reliability, validity and responsiveness of two multiattribute utility measures in patients with chronic obstructive pulmonary disease. Qual Life Res. 1999;8:45-54. [Cited in This Article: ] |
| 24. | Hawthorne G, Richardson J, Day NA. A comparison of the Assessment of Quality of Life (AQoL) with four other generic utility instruments. Ann Med. 2001;33:358-370. [Cited in This Article: ] |
| 25. | Moock J, Kohlmann T. Comparing preference-based quality-of-life measures: results from rehabilitation patients with musculoskeletal, cardiovascular, or psychosomatic disorders. Qual Life Res. 2008;17:485-495. [Cited in This Article: ] |
| 26. | Sintonen H. Outcome measurement in acid-related diseases. Pharmacoeconomics. 1994;5:17-26. [Cited in This Article: ] |
| 27. | Aromaa A, Koskinen S. Health and functional capacity in Finland. baseline results of the health 2000 health examination survey. 2004. Report No.: Publications of National Public Health Institute, Series B 12/2004. Available from: http://www.ktl.fi/attachments/suomi/julkaisut/julkaisusarja_b/2004b12.pdf. [Cited in This Article: ] |
| 28. | Tome S, Wells JT, Said A, Lucey MR. Quality of life after liver transplantation. A systematic review. J Hepatol. 2008;48:567-577. [Cited in This Article: ] |
| 29. | Aberg F, Rissanen AM, Sintonen H, Roine RP, Höckerstedt K, Isoniemi H. Health-related quality of life and employment status of liver transplant patients. Liver Transpl. 2009;15:64-72. [Cited in This Article: ] |
| 30. | Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947-954. [Cited in This Article: ] |
| 31. | Khashab M, Tector AJ, Kwo PY. Epidemiology of acute liver failure. Curr Gastroenterol Rep. 2007;9:66-73. [Cited in This Article: ] |
| 32. | Hassanein T, Oliver D, Stange J, Steiner C. Albumin dialysis in cirrhosis with superimposed acute liver injury: possible impact of albumin dialysis on hospitalization costs. Liver Int. 2003;23 Suppl 3:61-65. [Cited in This Article: ] |
| 33. | Räsänen P, Roine E, Sintonen H, Semberg-Konttinen V, Ryynänen OP, Roine R. Use of quality-adjusted life years for the estimation of effectiveness of health care: A systematic literature review. Int J Technol Assess Health Care. 2006;22:235-241. [Cited in This Article: ] |