Published online Apr 28, 2010. doi: 10.3748/wjg.v16.i16.2010
Revised: February 3, 2010
Accepted: February 10, 2010
Published online: April 28, 2010
AIM: To investigate the changes in renal function at 12-24 mo in patients following sodium phosphate (NaP) preparation for screening colonoscopy.
METHODS: We carried out a retrospective study on the results from patients who received health check-up services as part of an employer-provided wellness program performed between August 2006 and May 2008 and who were followed up for 12-24 mo. Prior to screening colonoscopy, 224 patients underwent bowel cleansing with NaP (NaP group) and 113 patients with polyethylene glycol (PEG group). The control group comprised 672 age-matched patients. We compared the changes in the creatinine levels and the glomerular filtration rates (GFRs) from baseline to 12-24 mo between the NaP, PEG, and control groups using two-way repeated measured analysis of variance. In addition, multivariate linear regression analysis was performed to assess the risk factors for a decreased GFR.
RESULTS: The baseline mean serum creatinine level in the NaP, PEG, and control groups was 1.12 ± 0.15, 1.12 ± 0.16, and 1.12 ± 0.15 mg/dL, which increased to 1.15 ± 0.15, 1.15 ± 0.18, and 1.15 ± 0.15 mg/dL, respectively, after 12-24 mo. The baseline mean GFR in the NaP, PEG, and control groups was 69.0 ± 7.7, 68.9 ± 8.0, and 69.6 ± 6.7 mL/min per 1.73 m2, which decreased to 66.5 ± 7.8, 66.5 ± 8.3, and 67.4 ± 6.4 mL/min per 1.73 m2, respectively, after 12-24 mo. The changes in serum creatinine levels and GFRs were not significantly between the NaP, PEG, and control groups (P = 0.992 and P = 0.233, respectively). Using multivariate linear regression analysis, only the baseline GFR was associated with the change in GFR (P < 0.001). Indeed, the bowel preparations were not associated with the change in GFR (P = 0.297).
CONCLUSION: NaP bowel preparation in subjects with normal renal function was not associated with renal injury, and NaP can thus be used safely for screening colonoscopy.
- Citation: Seol DC, Hong SN, Kim JH, Sung IK, Park HS, Lee JH, Shim CS. Change in renal function after sodium phosphate preparation for screening colonoscopy. World J Gastroenterol 2010; 16(16): 2010-2016
- URL: https://www.wjgnet.com/1007-9327/full/v16/i16/2010.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i16.2010
A bowel cleansing preparation is an essential prerequisite for safe, efficient, and accurate colonoscopy[1]. Oral sodium phosphate (NaP) and polyethylene glycol (PEG) are the most widely used bowel cleansing agents before colonoscopy. Because small-volume NaP is better tolerated than 4-L PEG, NaP is more effective in bowel cleansing than PEG for bowel preparation[2,3].
However, with the widespread use of NaP for bowel preparation, case reports and case series have suggested a possible association between the use of NaP and acute kidney injury, particularly in those patients with potentially compromised renal phosphate handling capacity[4-8]. Several studies have reported an association between NaP exposure and incident kidney injury[9-14]. Indeed, the findings have been mixed, with some studies reporting a strong association[11,12,14] and other studies reporting a non-significant trend toward better kidney outcomes after NaP[9,10,13] compared with other purgatives.
However, the populations evaluated in previous studies had considerable heterogeneity with a variety of indications for colonoscopy and differing health status. The comparisons for renal functional changes have been performed between 2 groups (i.e. NaP vs PEG or NaP vs healthy control). Furthermore, analyses of Asian populations have been limited.
Given the large number of patients exposed to NaP annually, clarification of this association is essential from clinical and public health perspectives. We analyzed the results of health screening services as part of an employer-provided wellness program. The primary end points were the changes in creatinine levels and glomerular filtration rates (GFRs) between 12 and 24 mo after NaP bowel preparation for screening colonoscopy, and the results were compared with those of patients who received PEG or no bowel preparation agents during the same study period.
The Healthcare Center of Konkuk University Medical Center provides medical screening programs to individuals employed at corporations that offer medical screening services annually or biannually as part of their corporate wellness plans. According to the policy of the company, colonoscopy screening may or may not be provided as part of the screening program. Examination data have been recorded electronically in a centralized digital medical record system. The Konkuk University Medical Center Institutional Review Board approved the study protocol.
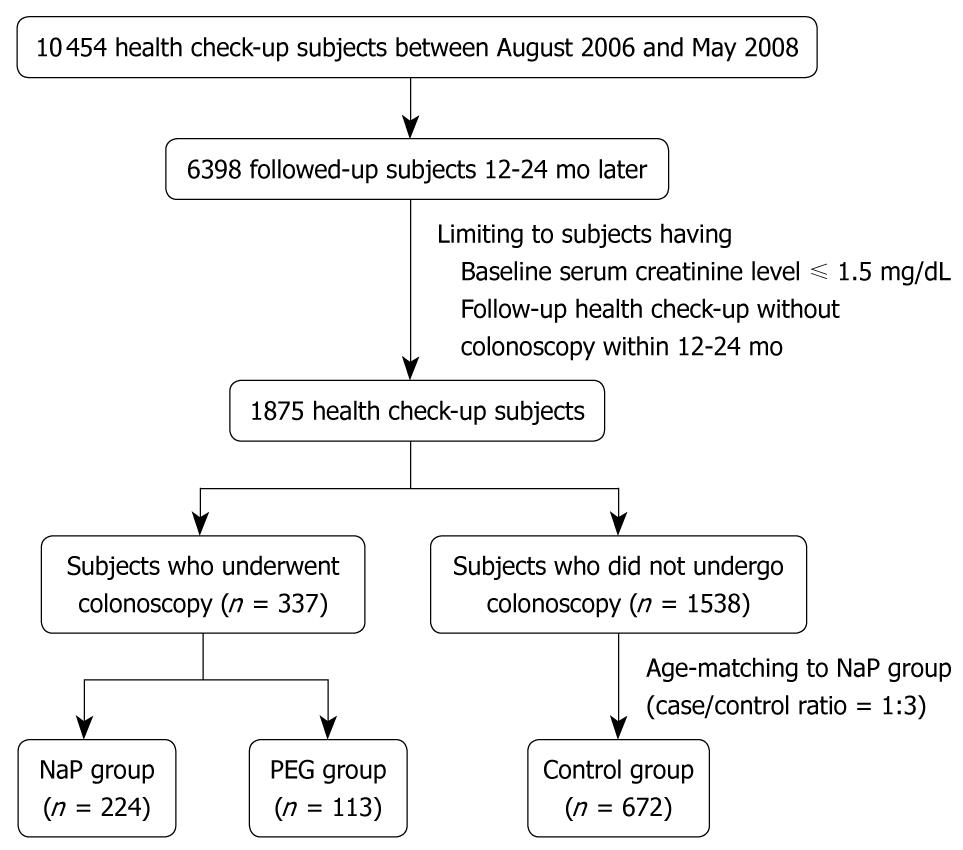
We retrospectively analyzed cohort data of the health screening program in subjects with normal creatinine levels who had undergone a health check-up between August 2005 and May 2008 and follow-up evaluations 12-24 mo later. During this period, 10 454 patients had health check-ups in the Healthcare Center of Konkuk University Medical Center. Of these, 6398 were followed up 12-24 mo later. Two hundred and twenty-four subjects underwent bowel cleansing with NaP for colonoscopy during the initial health check-up and had a follow-up health check-up without a colonoscopy (NaP group). One hundred and thirteen subjects took PEG for colonoscopy preparation and had a follow-up health check-up without a colonoscopy (PEG group). Of 1538 patients who had undergone health check-ups without colonoscopy and follow-up health check-ups without colonoscopy during the same period, 672 subjects, age-matched with the NaP group, were randomly selected as the control group. Figure 1 shows the flow diagram for selection of NaP, PEG, and control subjects.
The patients received written information about the medical screening program and a standard questionnaire, which included questions regarding personal medical history, such as hypertension and diabetes mellitus. Telephone interviews were conducted to ensure that the examinees who called to make an appointment were asymptomatic (i.e. no recent changes in bowel habits, lower abdominal pain, or visible rectal bleeding). Persons with symptoms were urged to seek medical care.
An oral NaP solution (Fleet®; Unimed Pharm. Inc., Seoul, Korea) or PEG (Colyte 4L®; Taejun Pharm. Co. Ltd., Seoul, Korea) was used for bowel preparation. Our center used PEG as a purgative for all subjects who underwent colonoscopy until July 2006 and then changed to NaP, except for those patients who had previous renal problems, heart failure, liver cirrhosis, or were > 65 years of age, because of better patient tolerance and cost-effectiveness. The dosing of NaP was as follows. Only liquid foods were consumed on the day of bowel preparation. Two doses of oral NaP solution (45 mL) were given at least 10-12 h apart. Each dose was taken with at least 250 mL of liquid, followed by an additional fluid intake of at least 1 L. The second dose was taken in the same manner and at least 3 h before the procedure. The dosing of PEG was as follows: No solid food was consumed for at least 2 h prior to ingestion of the solution. PEG (240 mL every 10 min) was consumed until the rectal output was clear or 4 L had been consumed. The colonoscopy was performed 4-6 h after bowel cleansing.
Age, race, gender, and clinical data sufficient to calculate an abbreviated Modification of Diet in Renal Disease Study Group (MDRD) glomerular filtration rate (GFR) were collected. The creatinine level on the day of the health check-up was recorded as the patient’s baseline renal function. The creatinine concentration at the follow-up health check-up 12-24 mo later was recorded. The GFR was calculated using the abbreviated MDRD formula: GFR (mL/min per 1.73 m2) = 186 × (serum creatinine) - 1.154 × (age) - 0.203 × 0.742 (if female).
Serum creatinine and GFR at the initial health check-up were considered the baseline levels and at the follow-up health check-up at 12-24 mo were considered the follow-up level. In the current study, we excluded subjects with a baseline creatinine level > 1.5 mg/dL or chronic renal disease.
Continuous variables are expressed as the mean ± SD, while categorical variables are presented as absolute values and percentages. A one-way analysis of variance (ANOVA) was used to examine the differences among the characteristics at baseline of the NaP, PEG, and control groups. The changes in the creatinine levels and GFRs between the NaP, PEG, and control groups were compared using the Student t-test. The changes in the creatinine levels and GFRs between baseline and follow-up among the PEG, NaP and control groups were compared using two-way repeated measures ANOVA. The data for the NaP, PEG and control groups were included in a multivariate linear regression analysis to assess the relationship of the baseline creatinine level, group, age, gender, medication for hypertension, medication for diabetes mellitus, body mass index (BMI), and baseline phosphate level to the decline in renal function. A categorical “group effect” variable was defined to specify whether the patient was in the NaP, PEG, and control groups and was used in the multivariate regression model. For each variable, the odds ratio (OR) and 95% confidence interval (CI) were reported. A two-tailed P-value < 0.05 was considered statistically significant, and all analyses were performed with SPSS (version 12.0K; SPSS Inc., Chicago, IL, USA).
The baseline characteristics of subjects in each group are outlined in Table 1. The mean age of the PEG group, 51.1 ± 10.5 years, was significantly older than in the NaP and control groups (46.9 ± 8.6 years). Although there was a smaller proportion of subjects with diabetes mellitus in the NaP group compared with the PEG or control groups, there was no statistically significant difference among the groups. The phosphate level was significantly higher in the NaP group compared with the PEG and control groups on the day of the baseline health check-up.
| Characteristics | NaP group (n = 224) | PEG group (n = 113) | Control group (n = 672) | P |
| Age (yr) | 46.9 ± 8.6 | 51.1 ± 10.5 | 46.9 ± 8.6 | < 0.001 |
| Gender | 0.453 | |||
| Male | 149 (67) | 81 (72) | 475 (71) | |
| Female | 75 (33) | 32 (28) | 197 (29) | |
| Body mass index (kg/m2) | 23.4 ± 3.1 | 23.8 ± 2.7 | 23.6 ± 2.9 | 0.496 |
| Hypertension | 50 (22) | 34 (30) | 188 (28) | 0.186 |
| Diabetes mellitus | 29 (13) | 23 (20) | 134 (20) | 0.056 |
| Serum phosphate1 (mg/dL) | 88.9 ± 14.5 | 95.3 ± 17.4 | 91.6 ± 17.4 | 0.004 |
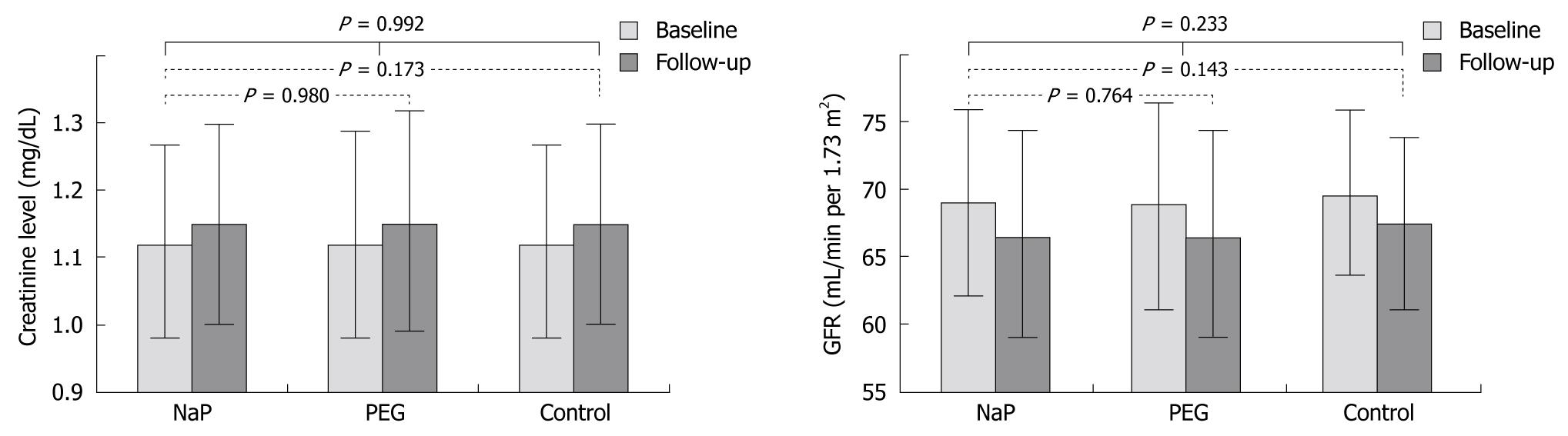
Table 2 illustrates the changes in renal function between the groups. There was no significant difference between the baseline and follow-up levels of mean creatinine levels and the calculated MDRD GFR between groups. The baseline creatinine level for the NaP group ranged from 0.5-1.5 mg/dL (mean, 1.12 ± 0.15 mg/dL), with a calculated MDRD GFR ranging from 35 to 143 mL/min per 1.73 m2 (mean, 69.03 ± 7.74 mL/min per 1.73 m2). Serum creatinine at 12-24 mo follow-up ranged from 0.4-3.0 mg/dL (mean, 1.01 ± 0.15 mg/dL), and GFR was 22-175 mL/min per 1.73 m2 (mean, 66.46 ± 7.78 mL/min per 1.73 m2). There was no significant difference in the changes in serum creatinine between the NaP and PEG group (P = 0.980) or the NaP and control group (P = 0.173). No significant difference was not found in the changes in GFR between the NaP and PEG group (P = 0.764) or the NaP and control group (P = 0.143). In addition, although creatinine level and GFR deterioration occurred at the 12-24 mo interval, there was no significant difference in the changes in creatinine levels and GFRs between the NaP, PEG, and control groups (P = 0.992 and P = 0.233, respectively; Figure 2).
| Evaluation time | Creatinine level (mg/dL) | GFR (mL/min per 1.73 m2) | ||||||
| NaP group | PEG group | Control group | P | NaP group | PEG group | Control group | P | |
| Baseline | 1.12 ± 0.15 | 1.12 ± 0.16 | 1.12 ± 0.15 | 0.985 | 69.03 ± 7.74 | 68.91 ± 7.95 | 69.57 ± 6.67 | 0.467 |
| Follow-up 12–24 mo later | 1.15 ± 0.15 | 1.15 ± 0.18 | 1.15 ± 0.15 | 0.928 | 66.46 ± 7.78 | 66.47 ± 8.26 | 67.42 ± 6.39 | 0.118 |
To further identify the variables involved in the deterioration of renal function in patients who were exposed to the NaP preparation, we applied a linear regression model with the dependent variables (change in creatinine and GFR from baseline to 12-24 mo) and the following independent variables: age, creatinine or GFR at baseline, hypertension, diabetes, BMI, and serum phosphate. No significant risk factor for changes in serum creatinine (Table 3) was found. The NaP bowel preparation also was not associated with the creatinine change (P = 0.366). On the other hand, results of multivariate linear regression analysis indicated that the GFR change was only associated with the baseline GFR (P < 0.001). Based on the multiple linear regression model in Table 3, when all other variables remained constant, the change in GFR decreased by 0.14 units for every 1-unit increase in GFR at baseline. The NaP bowel preparation was not associated with the GFR change (P = 0.269). However, the model was used to select the significant variables for the change in the GFR rather than predicting the change in the GFR level since the R2 value was low.
| Variable | Creatinine (R2 = 0.016) | GFR (R2 = 0.053) | ||
| Correlation coefficient (95% CI) | P | Correlation coefficient (95% CI) | P | |
| Baseline | -0.018 (-0.057 to 0.021) | 0.366 | -0.135 (-0.187 to -0.083) | < 0.001 |
| Group effect (NaP, PEG and control groups) | 0.002 (-0.004 to 0.009) | 0.489 | -0.247 (-0.685 to 0.191) | 0.269 |
| Age at baseline creatinine and GFR measurement | 0.000 (0.000 to 0.001) | 0.433 | -0.036 (-0.077 to 0.006) | 0.094 |
| Hypertension | 0.007 (-0.009 to 0.023) | 0.365 | -0.748 (-1.774 to 0.277) | 0.152 |
| Diabetes mellitus | -0.016 (-0.034 to 0.002) | 0.079 | 1.082 (-0.076 to 2.240) | 0.067 |
| Body mass index | 0.001 (-0.001 to 0.003) | 0.208 | -0.038 (-0.162 to 0.086) | 0.545 |
| Serum phosphate | -0.001 (-0.004 to 0.003) | 0.733 | 0.020 (-0.205 to 0.246) | 0.859 |
The demand for colonoscopy has steadily increased, with improved colorectal cancer screening uptake[15]. Most patients undergoing colonoscopy receive NaP- or PEG-based purgatives. Multiple studies have shown that NaP is better tolerated and more cost-effective, and at least as efficacious as PEG[2,3]. In a meta-analysis that compared NaP with PEG for bowel preparation in 25 randomized studies, the occurrence of serious adverse events with oral NaP was zero[16,17]. Thus, there may be a reason to preferentially use NaP.
However, recent case reports and case series have suggested a possible association between NaP and acute kidney injury[4-8,18], termed acute phosphate nephropathy. These cases presented to their medical care providers with impaired renal function and elevated serum creatinine levels from a few days up to a few weeks after ingestion of NaP. All of these patients had histopathologic evidence of phosphate nephropathy. Although the mechanism by which NaP may cause renal injury is unknown, acute phosphate nephropathy is characterized histologically by deposition of calcium phosphate crystals in tubular epithelial cells and lumens, interstitial inflammation and fibrosis, and evidence of tubular cell apoptosis[6]. In many reported cases, the serum creatinine levels have remained increased long after disease recognition, leading some investigators to conclude that NaP may be an under-recognized cause of chronic kidney disease[18].
The present study examined changes in renal functions in patients who had received oral NaP as a colon cleansing agent before colonoscopy and compared the changes with a group who received PEG or did not receive any purgatives. If a potent association existed, use of NaP would be inadvisable. If a weak, but significant, association existed, the risk of NaP must be weighed against such potential benefits as improved visualization of bowel mucosa and perhaps increased willingness of patients to undergo colonoscopy. If no association exists, NaP may be used without reservation. In this study, during the 12 to 24 mo follow-up, the changes in serum creatinine and MDRD GFRs were similar between the NaP, PEG, and control groups.
A number of previous controlled human studies have reported an association between NaP exposure and incident kidney injury[9-14]. Table 4 summarizes the design, demographic characteristics, and effect estimates across previous studies[9-14]. Unfortunately, it is impossible to determine whether an association between NaP and deterioration of renal function exists because of interstudy heterogeneity[19].
| Design | Population | Race (%) | Control | Timing of Follow-up | Association between NaP exposure and incident kidney injury | |
| Abaskharoun et al[9] | RC | Any patients who had outpatient CFS | Not reported | PEG | 3 mo-9 yr | No |
| Brunelli et al[10] | CC | Any patients who had outpatient CFS | White 46%, Non-white 52%, Unknown 2.3% | Any non-NaP preparation | Within 6 mo | No |
| Hurst et al[11] | RC | Any patients who had screening CFS | White 54%, Black 19%, Unknown 27% | PEG | Within 1 yr | Yes |
| Khurana et al[12] | RC | Any patients1 | White 84%, Black 8%, Hispanic 7%, Asian 0.5% | No bowel preparation | 6-9 mo/ 12-18 mo | Yes |
| Russmann et al[13] | RC | Any patients who had CFS | Not reported | PEG | Within 6 mo | No |
| Singal et al[14] | RC | Any patients who had CFS | White 27%, Black 53%, Hispanic 20%, Asian 0.3% | PEG | Within 1 yr | Yes |
Previous studies, except one study by Hurst et al[11], enrolled any colonoscopy recipients[9,10,12-14]. Inpatient colonoscopy recipients are more likely to have an underlying disease and more likely to develop kidney injury compared with asymptomatic healthy persons. In addition, because serum assays were not routinely used in previous studies, the authors had to derive this information by retrospectively identifying those patients whose conditions required the measurement of serum creatinine levels before and after bowel preparation. Therefore, the previous study population was not representative of all patients who received a bowel preparation for colonoscopy, especially for the purpose of screening colonoscopy. On the other hand, our participants had scheduled serum assays and colonoscopies as a part of health check-up services for an employer-provided wellness program, and this examination served the employees annually or biannually. Thus, all of our participants were an asymptomatic and relatively healthy homogenous group. In addition, studies in Asian subjects have been very rare, and our analysis could be relevant for this population.
Another noteworthy point is the role of the control group. Some studies considered PEG-treated controls[9,11,13,14], whereas other studies also included any non-NaP bowel purgative[10,12]. Because there is negligible systemic absorption of PEG[20] and experimental data have not shown kidney lesions, even after prolonged daily exposure[21], the PEG-treated group is a reasonable control for the NaP-treated group. However, because there was no recognizable possibility of purgative-induced renal impairment, such as volume depletion or patient condition necessitating PEG instead of NaP (renal impairment, heart failure, hepatic dysfunction, electrolyte imbalance, acute colitis, or inflammatory bowel disease), the subjects who were not exposed to PEG or NaP were also an appropriate control. In our study, both control groups were used and we found no significant differences in renal functional deterioration in the NaP group compared with either control group.
Lastly, the follow-up timing was important to compare the renal functional deterioration because purgative-induced volume depletion (expected to be greater with NaP, which is hyperosmolar, than with PEG, which is iso-osmolar) may have impaired renal function early after exposure without causing irreparable kidney damage. However, previous studies, apart from that of Abaskharoun et al[9], followed the serum creatinine level up to 12 mo[10-14]. Although the choice of a 12-24 mo follow-up interval was not based on firm evidence, we believe that it was a reasonable choice because the resultant kidney injury may take longer than 12 mo to become clinically manifest, and NaP exposure might result in a transient rise in serum creatinine[18].
Our multivariate analysis identified that baseline renal function was an independent predictor of deterioration in renal function. This is a well-established risk factor for renal disease[22]. On the other hand, in our multivariate analysis, the deterioration of GFR was not associated with NaP use or the serum phosphate level. A typical bowel preparation regimen for colonoscopy using NaP contains 5-10 times the usual daily intake of inorganic phosphate, and studies have shown a significant increase in serum phosphate levels[23-25]. Hyperphosphatemia has been shown to predispose to acute kidney injury in rat models[26], and an analogy to tumor lysis syndrome suggests a similar effect in humans. In addition, data from rat models suggest that hyperphosphatemia causes progressive deterioration in kidney function[27,28]. An experimental model suggested that hyperphosphatemia resulted in calcium phosphate deposition in tubular cells and lumens, with subsequent apoptosis of tubular epithelial cells and interstitial fibrosis, consistent with histologic findings from case reports of acute phosphate nephropathy[6,18]. Differences in predisposition to NaP-related kidney injury may result from allelic variations in sodium phosphate transporters or factors influencing their expression and cellular trafficking[28].
With respect to exposure misclassification, our patients lacked data on nephrotoxic medications, including renin angiotensin system inhibitors, diuretics, non-steroidal anti-inflammatories, and radio-contrast material. There was unavoidable selection bias involving patients who received PEG because of our purgative selection. Thus, the presumably high-risk population was a lower proportion of the NaP group compared with the PEG group.
In conclusion, the changes in renal function in patients who used NaP was similar to healthy controls or PEG-treated patients. Further studies are warranted to validate and generalize our findings. Nonetheless, careful attention must be taken with patient selection, especially in patients with impaired renal function, and appropriate dosing of oral NaP (45 mL dose taken twice daily, 6-12 h apart) to prevent renal toxicity. Adequate hydration (at least 2-3 L of clear fluid throughout the cleansing period) is important during colon cleansing with oral NaP.
Case reports and case series have suggested a potential association between use of oral sodium phosphate (NaP) bowel preparations and kidney injury. Given the large numbers of patients exposed to NaP and the large and increasing incidence of chronic kidney disease, this association is of great public health importance.
In the past year, a number of controlled human studies have evaluated the association between NaP exposure and incident kidney injury. However, results of these studies have been mixed, because considerable heterogeneity among population, control, and timing of follow-up.
As a potential risk of NaP is suggested, it is unethical to evaluate the association between NaP exposure and incident kidney injury using a prospective, randomized, controlled study. Thus, the selection of the study population is most important to reduce bias. Unlike previous studies, the present study evaluated the results from routine health check-ups for an employer-provided wellness program. Thus, although the analyses were retrospective, the selection bias was small and follow-up timing was consistent compared with previous studies. In addition, the changes in creatinine level and glomerular filtration rates (GFRs) in the NaP-treated group were compared not only to the polyethylene glycol-treated group, but also the healthy control group who did not use any purgatives.
Bowel preparation with oral NaP in patients who have normal renal function seems safe and effective; however, adequate hydration must be maintained before and after colonoscopy. To optimize safety, other agents should be considered in patients with increased serum creatinine or decreased GFR at baseline and in those predisposed to nephropathy.
The study by Seol et al has been designed to answer a relevant clinical question: is the bowel cleaning NaP-prep safe in general population? Although retrospective, due to the large sample size and adequate control population, the study clearly reassures on this issue.
Peer reviewer: Vito Annese, MD, Department of Internal Medicine, Unit of Gastroenterology, Hospital, Viale Cappuccini, 1, San Giovanni Rotondo 71013, Italy
S- Editor Wang YR L- Editor Cant MR E- Editor Lin YP
| 1. | Bond JH. Should the quality of preparation impact postcolonoscopy follow-up recommendations? Am J Gastroenterol. 2007;102:2686-2687. [Cited in This Article: ] |
| 2. | Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy - a meta-analysis. Colorectal Dis. 2006;8:247-258. [Cited in This Article: ] |
| 3. | Hsu CW, Imperiale TF. Meta-analysis and cost comparison of polyethylene glycol lavage versus sodium phosphate for colonoscopy preparation. Gastrointest Endosc. 1998;48:276-282. [Cited in This Article: ] |
| 4. | Carl DE, Sica DA. Acute phosphate nephropathy following colonoscopy preparation. Am J Med Sci. 2007;334:151-154. [Cited in This Article: ] |
| 5. | Fine A, Patterson J. Severe hyperphosphatemia following phosphate administration for bowel preparation in patients with renal failure: two cases and a review of the literature. Am J Kidney Dis. 1997;29:103-105. [Cited in This Article: ] |
| 6. | Markowitz GS, Nasr SH, Klein P, Anderson H, Stack JI, Alterman L, Price B, Radhakrishnan J, D'Agati VD. Renal failure due to acute nephrocalcinosis following oral sodium phosphate bowel cleansing. Hum Pathol. 2004;35:675-684. [Cited in This Article: ] |
| 7. | Orias M, Mahnensmith RL, Perazella MA. Extreme hyperphosphatemia and acute renal failure after a phosphorus-containing bowel regimen. Am J Nephrol. 1999;19:60-63. [Cited in This Article: ] |
| 8. | Ullah N, Yeh R, Ehrinpreis M. Fatal hyperphosphatemia from a phosphosoda bowel preparation. J Clin Gastroenterol. 2002;34:457-458. [Cited in This Article: ] |
| 9. | Abaskharoun R, Depew W, Vanner S. Changes in renal function following administration of oral sodium phosphate or polyethylene glycol for colon cleansing before colonoscopy. Can J Gastroenterol. 2007;21:227-231. [Cited in This Article: ] |
| 10. | Brunelli SM, Lewis JD, Gupta M, Latif SM, Weiner MG, Feldman HI. Risk of kidney injury following oral phosphosoda bowel preparations. J Am Soc Nephrol. 2007;18:3199-3205. [Cited in This Article: ] |
| 11. | Hurst FP, Bohen EM, Osgard EM, Oliver DK, Das NP, Gao SW, Abbott KC. Association of oral sodium phosphate purgative use with acute kidney injury. J Am Soc Nephrol. 2007;18:3192-3198. [Cited in This Article: ] |
| 12. | Khurana A, McLean L, Atkinson S, Foulks CJ. The effect of oral sodium phosphate drug products on renal function in adults undergoing bowel endoscopy. Arch Intern Med. 2008;168:593-597. [Cited in This Article: ] |
| 13. | Russmann S, Lamerato L, Marfatia A, Motsko SP, Pezzullo JC, Olds G, Jones JK. Risk of impaired renal function after colonoscopy: a cohort study in patients receiving either oral sodium phosphate or polyethylene glycol. Am J Gastroenterol. 2007;102:2655-2663. [Cited in This Article: ] |
| 14. | Singal AK, Rosman AS, Post JB, Bauman WA, Spungen AM, Korsten MA. The renal safety of bowel preparations for colonoscopy: a comparative study of oral sodium phosphate solution and polyethylene glycol. Aliment Pharmacol Ther. 2008;27:41-47. [Cited in This Article: ] |
| 15. | Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology. 2005;129:1151-1162. [Cited in This Article: ] |
| 16. | Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2007;25:373-384. [Cited in This Article: ] |
| 17. | Shawki S, Wexner SD. How safe is bowel preparation with oral sodium phosphate solution? Nat Clin Pract Gastroenterol Hepatol. 2008;5:482-483. [Cited in This Article: ] |
| 18. | Markowitz GS, Stokes MB, Radhakrishnan J, D'Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389-3396. [Cited in This Article: ] |
| 19. | Brunelli SM. Association between oral sodium phosphate bowel preparations and kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:448-456. [Cited in This Article: ] |
| 20. | Brady CE 3rd, DiPalma JA, Morawski SG, Santa Ana CA, Fordtran JS. Urinary excretion of polyethylene glycol 3350 and sulfate after gut lavage with a polyethylene glycol electrolyte lavage solution. Gastroenterology. 1986;90:1914-1918. [Cited in This Article: ] |
| 21. | Tusing TW, Elsea JR, Sauveur AB. The chronic dermal toxicity of a series of polyethylene glycols. J Am Pharm Assoc Am Pharm Assoc (Baltim). 1954;43:489-490. [Cited in This Article: ] |
| 22. | Russmann S, Lamerato L, Motsko SP, Pezzullo JC, Faber MD, Jones JK. Risk of further decline in renal function after the use of oral sodium phosphate or polyethylene glycol in patients with a preexisting glomerular filtration rate below 60 ml/min. Am J Gastroenterol. 2008;103:2707-2716. [Cited in This Article: ] |
| 23. | Lieberman DA, Ghormley J, Flora K. Effect of oral sodium phosphate colon preparation on serum electrolytes in patients with normal serum creatinine. Gastrointest Endosc. 1996;43:467-469. [Cited in This Article: ] |
| 24. | Casais MN, Rosa-Diez G, Pérez S, Mansilla EN, Bravo S, Bonofiglio FC. Hyperphosphatemia after sodium phosphate laxatives in low risk patients: prospective study. World J Gastroenterol. 2009;15:5960-5965. [Cited in This Article: ] |
| 25. | Ainley EJ, Winwood PJ, Begley JP. Measurement of serum electrolytes and phosphate after sodium phosphate colonoscopy bowel preparation: an evaluation. Dig Dis Sci. 2005;50:1319-1323. [Cited in This Article: ] |
| 26. | Zager RA. Hyperphosphatemia: a factor that provokes severe experimental acute renal failure. J Lab Clin Med. 1982;100:230-239. [Cited in This Article: ] |
| 27. | Ibels LS, Alfrey AC, Haut L, Huffer WE. Preservation of function in experimental renal disease by dietary restriction of phosphate. N Engl J Med. 1978;298:122-126. [Cited in This Article: ] |
| 28. | Nagano N, Miyata S, Obana S, Kobayashi N, Fukushima N, Burke SK, Wada M. Sevelamer hydrochloride, a phosphate binder, protects against deterioration of renal function in rats with progressive chronic renal insufficiency. Nephrol Dial Transplant. 2003;18:2014-2023. [Cited in This Article: ] |