Published online Apr 28, 2010. doi: 10.3748/wjg.v16.i16.1993
Revised: December 1, 2009
Accepted: December 8, 2009
Published online: April 28, 2010
AIM: To assess and correlate the lipid content of various organs in obese subjects and in persons with a normal body weight.
METHODS: Magnetic resonance spectroscopy and a previously validated gradient echo magnetic resonance imaging method with Dixon’s two point technique were used in this study to quantify fat in liver, pancreas as well as kidney.
RESULTS: In 36 volunteers with body mass index (BMI) ranging from 20.0 to 42.9 kg/m2, the median fat content of liver, pancreas and kidney was 2.3% (interquartile range: 0.2%-7.8%), 2.7% (1.0%-6.5%) and 0.7% (0.1%-1.4%), respectively. BMI and subcutaneous fat correlated significantly with liver and pancreas fat content. We show for the first time the significant correlation of liver and pancreas fat content in healthy controls (r = 0.43, P < 0.01). These observations are related to body weight as measured by BMI and the amount of subcutaneous fat. Kidney fat content is very low and correlates with none of the other fat depots.
CONCLUSION: Renal lipid accumulation, unlike the coupled accumulations of fat in liver and pancreas, is not observed in obese subjects. Unlike suggestions made in previous studies, renal lipid accumulation appears not to be involved in the pathogenesis of renal disease in humans.
- Citation: Sijens PE, Edens MA, Bakker SJ, Stolk RP. MRI-determined fat content of human liver, pancreas and kidney. World J Gastroenterol 2010; 16(16): 1993-1998
- URL: https://www.wjgnet.com/1007-9327/full/v16/i16/1993.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i16.1993
Storage of fat within the peritoneal cavity (visceral fat) and within other tissues (ectopic fat) rather than in subcutaneous adipocytes, is accompanied by adverse metabolic and lipotoxic effects[1,2]. Both visceral fat[1] and liver fat[3] are known for secreting numerous atherogenic factors into the blood system, e.g. lipids and inflammatory cytokines, predisposing to cardiovascular diseases, one of which is atherosclerotic renovascular disease (ARVD)[4,5]. It has been hypothesized that in obesity and metabolic syndrome, ectopic fat causes lipotoxic damage to organs[4,5], e.g. liver (steatohepatitis and cirrhosis)[6], pancreas (β-cell dysfunction)[7] and lipotoxicity of the kidney[4].
Whereas liver steatosis has been quantified in dozens of magnetic resonance imaging (MRI) studies, and pancreatic lipomatosis[8-10] and muscle fattening[8-11] in some, quantitative documentation of the content of fat in human kidneys in situ appears to be lacking entirely. In rats, however, steatosis of the kidney was recently associated with an alteration in renal acidification[12]. In that study, the fat accumulated in the renal cortex, as shown quantitatively by enzymatic triglyceride measurement and qualitatively by oil red O staining[12]. This fits the notion that, within the cortex, the proximal tubule is vulnerable to lipid accumulation due to its role in the reabsorption of free fatty acids bearing albumin[13,14].
The most accurate, and therefore preferable, method for quantifying hepatic fat content is metabolic MRI, i.e. multivoxel magnetic resonance spectroscopy (MRS)[15-17]. Other MRI methods are less reproducible or have systematic errors. In a previously published comparison of subjects examined by MRS and by a Dixon-based dual-echo breath hold gradient echo method, we found strong correlation between the liver fat content according to both methods and concluded that MRI can be used for quantifying fat content, provided that the small systematic overestimation of fat content at the lower end range is corrected for[17]. The comparison is now extended to 36 volunteers, varying from lean to obese, with application of the Dixon-based MRI fat quantification method to pancreas and kidney as well as liver. Only the liver was additionally examined by MRS. Our approach was to apply the equation converting the liver MRI fat values to the MRS values, to the pancreas and kidney MRI values. Additional MRS examinations of pancreas and kidney would have made the total MRI examination times too lengthy. Furthermore, reliable MRS of the renal cortex, a thin layer of tissue sandwiched between the peripheral fat and the fatty core of the kidney, is technically not feasible.
High hepatic lipid content is common in obese subjects who are otherwise healthy, i.e. overweight persons not suffering from metabolic disease[15]. In this study we examined 36 volunteers with a wide range of body mass index (BMI) values, using previously validated MRI methods to quantify the fat content of liver, pancreas as well as kidney. The purpose was to quantify the lipid content of these organs in obese subjects and in persons with a normal body weight. Our hypothesis was that in obese persons the lipid content of liver, pancreas and kidney would be higher than in thin persons. Our comparatively large study was also intended to assess possible relationships between the fat content in different organs.
Thirty-six adult volunteers were recruited by advertisement to undergo abdominal MRI and liver MRS. These volunteers had a BMI ranging from 20.0 to 42.9 kg/m2 with a mean value of 27.5 kg/m2 and were average in that they were neither profound exercisers nor sedentary. Eight out of 36 subjects were obese, i.e. they had BMI values exceeding a value of 30. Two-thirds of the participants were men; the median age was 39 years (range: 22-64 years). Everyone was interviewed to assess the status of his health and cases of any disease, including diabetes, were excluded. All volunteers gave informed consent. The studies were approved by the medical ethics committee.
MRI and MRS studies took place in one measurement session, using a 1.5 Tesla whole-body scanner (MAGNETOM Avanto; Siemens Medical Solutions, Erlangen, Germany) equipped with gradients of up to 40 mTm-1 (maximal slew rate = 200 mT m-1/ms) and a six-channel spine array coil. Subjects were in the supine position. In both MRI and MRS a large flex coil placed over the liver was used simultaneously with the spine array coil as receiver. T1-weighted gradient echo images were recorded to assess the anatomy of liver, kidneys and pancreas.
Breath-hold T1-weighted gradient echo MR images, a dual flip angle adaptation of the wide spread gradient-recalled echo MRI method based on Dixon’s two point technique[18], were acquired with 6 mm slice thickness, section gap 0 mm, matrix 256 × 160 and a repetition time (TR) of 155 ms, and TEs of 2.4 ms (OP) and 4.8 ms (IP). Flip angles of 20o and 70o were used to generate intermediate-weighted and T1-weighted images, respectively. Images were corrected for T2* decay: Scorrected = Se(τ/T2*), where τ is the echo time difference between IP and OP images, and S represents the signal intensity in a defined region of interest (ROI)[19]. Under these conditions τ = 2.4 ms combined with T2* = 19.44, calculated from the mean spectral line width of the water peak in human liver measured by MRS in the 36 volunteers (details are given in a next paragraph), gave a correction factor of 1.13 for Sip relative to Sop.
The algorithm used for estimating fat content, modified to prevent occasional mix-ups of water and fat signals[17], consists of: (a) adjustment for T2* relaxation as described above, (b) calculation of the apparent fat content using the following equation %fat = (SIP - SOP)/2SIP× 100% (equation 1) for both intermediate- or hydrogen density-weighted, (%fatHwt at 20o flip angle) and T1-weighted (%fatT1wt at 70o flip angle) image pairs, (c) if fatHwt AND %fatT1wt≤ 20%, then %fat = MIN [%fatHwt, %fatT1wt], if fatHwt AND %fatT1wt > 20% and %fatHwt≤ %fatT1wt, then %fat = %fatHwt, otherwise, %fat = 100% - %fatHwt, where AND is a logical operator, and MIN (a, b) is a mathematical operator computing the minimum value between a and b. A T2* of 19.4 ms for liver tissue was adopted from the results of a previous study[17] for correcting Sip in the above mentioned MRI algorithm. This value was used for correcting all MRI fat content figures in Table 1.
Hybrid 2D-spectroscopic imaging (chemical shift imaging, CSI), point resolved spectroscopy (PRESS) with a repetition time (TR) of 5000 ms and an echo time (TE) of 30 ms, was performed using a field of view of 16 × 16 cm2 and a volume of interest of 5 × 8 × 4 cm3 positioned inside the liver (Figure 1C)[20]. The CSI measurement lasted 16 × 16 × 5 = 1280 s or approximately 21 min. Shimming was automated and water suppression was not applied in order to be able to calculate the fat-water ratio distributions in the liver directly[17]. At the used TR of 5 s, T1 saturation of the water and fat signals is negligible, that is TR > 5T1[21], and at the used TE of 30 ms the correction applied to our data to compensate for the fact that the fat signal has a longer T2 (78 ms) than water (60 ms) was 12.2%[22]. In our MRS method respiratory compensation was not applied as this was previously shown not to affect the quality and composition of our liver spectra[17].
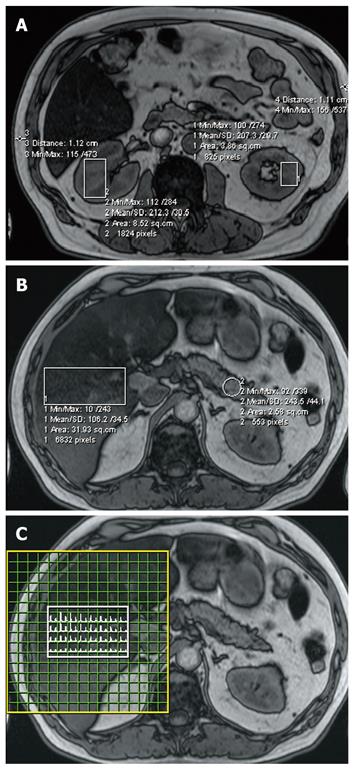
The liver MRS data were co-registered to the MR images in the liver by using the coordinates of the anterior right (AR) and posterior left (PL) at the same height position as regional checkpoints. The kidney and pancreas MRI fat contents were derived from a single slice centered on the organ involved. Figure 1 shows the regions of interest for kidney (a), pancreas and liver tissue (b) and the matching liver MRS spectral map (c) for one of the volunteers examined. In the analysis of kidney data the results for left and right kidney volumes of interest containing medulla and renal column tissue were averaged. MRS measurements of the renal cortex and pancreas were not performed because, apart from measurement time considerations, accurate quantification of small tissue volumes adjacent to the fatty tissue surrounding these organs is impossible.
The thickness of the layer of subcutaneous fat was measured at the two most left and right points on the same MRI slice to obtain a measure of the amount of subcutaneous fat tissue (Figure 1A). A more precise method[23] was not feasible in our study, as the MRI field of view was often too small to depict the entire abdominal body cross section.
The pancreas measurements were confined to the tail (cauda) because that tended to be easier to depict than caput and corpus (Figure 1B). The difference between the liver fat content according to MRS, the gold standard[17], and liver fat content according to MRI was used to correct all MRI content figures determined in this study (kidney, liver, pancreas). This approach is reasonable because with the combined use of the flex and spine array as receiver MRI coils, the B1 field in the positions corresponding with the locations of these organs did not show significant differences. In other words, the outcome of phantom experiments gave us reason to expect that equivalent Dixon’s two point technique MRI signal intensities in liver, pancreas and kidney should correspond to the same fat content.
The distributions of the obtained fat contents were not normal, thus non-parametric correlations were calculated using Spearman’s correlation coefficients (with 2-tailed testing of significance).
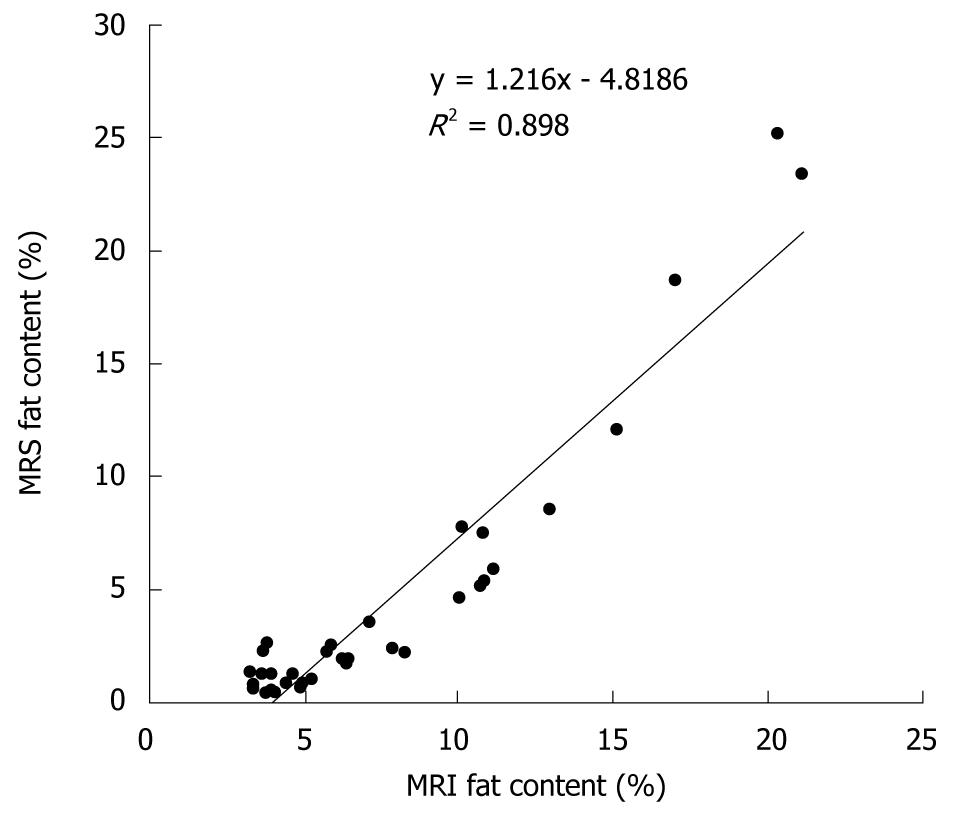
Mean liver fat content according to MRS was 3.2% lower than that according to MRI (4.4% vs 7.6%). The linear correlation between liver fat content according to MRI and MRS is shown in Figure 2 (r = 0.95, P < 0.0001). The obtained slope (y = 1.216x - 4.82) was used to correct all MRI-determined tissue fat contents.
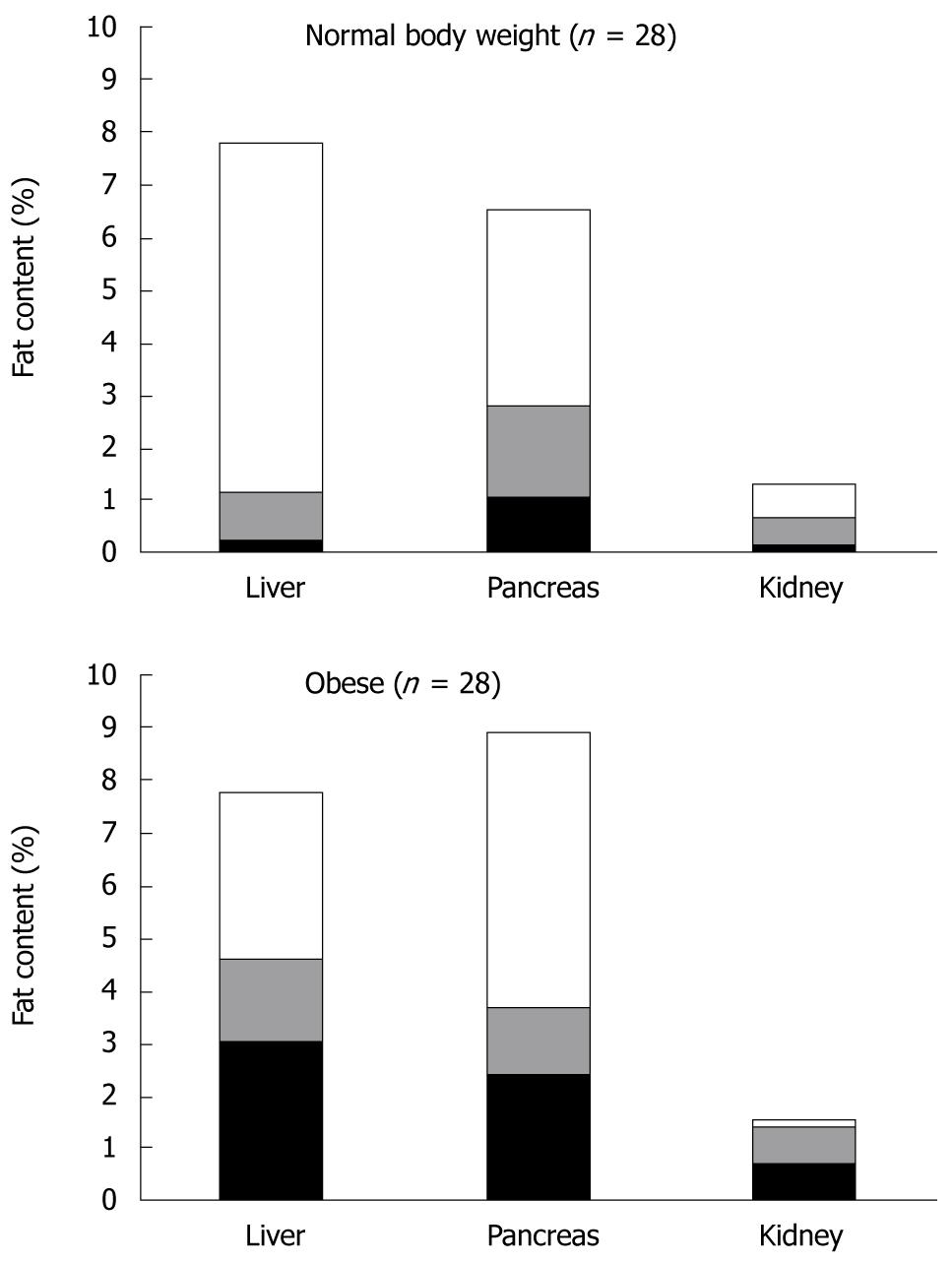
After correction for this systematic overestimation, which is inherent to the MRI method[13], calculated fat content of liver, pancreas and kidney was 2.3% (interquartile range: 0.2%-7.8%), 2.7% (1.0%-6.5%) and 0.7% (0.1%-1.4%), respectively. For the kidney fat measurements the variance between subjects was lower (interquartile range: 1.8%) than in liver (7.6%) and pancreas (5.5%). Illustrated by Figure 3 is the observation that, compared with the normal body weight subjects, the subgroup of obese subjects tended to have comparatively high fat in the liver (median 4.57% vs 1.11%), slight elevation in the pancreas (3.60% vs 2.26%) and equally low fat contents in the kidney (1.35 vs 0.64). The interquartile ranges for these organs in obese and normal weight persons showed great overlap and were of comparable magnitude, i.e. 2.93-7.70 (0.18-7.76), 2.32-8.88 (0.62-5.45) and 0.61-1.49 (0.10-1.17), respectively. Table 1 shows that BMI, a measure of overall fat deposits in the human body, correlated significantly with the amount of subcutaneous fat (r = 0.77, P < 0.01), liver fat content (r = 0.52, P < 0.01) and pancreas fat content (r = 0.35, P < 0.05). The amount of subcutaneous fat also correlated significantly with liver fat content and pancreas fat content (r = 0.45 and r = 0.44, respectively; P < 0.01). Liver and pancreas fat contents were significantly correlated (r = 0.43, P < 0.01). Kidney fat content was comparatively low, showed little intersubject variation and correlated with none of the other fat depots.
These healthy volunteers, who varied in body weight from lean to obese, had kidney fat contents that were comparatively low and showed little variation between subjects. The kidney fat content correlated with none of the other fat depots, indicating that in otherwise healthy subjects obesity does not affect kidney fat content. In other words, renal lipid accumulation, unlike the accumulation of fat in liver and pancreas, is not observed in obese healthy subjects.
In this study, we used a previously validated gradient echo MRI method[17] for determining the fat content in liver pancreas and kidney. In our liver measurements we compared the MR spectroscopy results in the thirty-six volunteers with the MRI liver content results to refine the correction equation needed to adapt the MRI method for quantitative use. Specifically, we determined the coefficient of correlation between the two-point Dixon-based MRI method and the MRS results in liver and used the result to correct all MRI data for systematic overestimation at the lower end range, a phenomenon also observed by others[24] and most probably related to Rician noise distribution-related overestimation of the magnitude images of weak fat signals. Thus we have used our liver data for correcting all MRI data based on the assumption that the MRI method works out the same for liver and other organs (pancreas, kidney). This is the best one can do, considering that (1) MRS examinations of small fat embedded organs are inaccurate; and that (2) it is not an option to collect tissue biopsies from healthy volunteers. Furthermore, as stated in Materials and Methods, control experiments had made sure that with the radiofrequency coils used, the MRI signal intensities in positions corresponding with those of liver, pancreas and kidney corresponded to the same fat contents.
Renal lipotoxicity and its role in the pathogenesis of renal disease are not fully understood[4,5]. It has been assumed that renal disease progression is promoted by the accumulation of lipids in the kidneys, a phenomenon in which triglyceride-rich lipoproteins, free fatty acids and their metabolites, and albumin-loaded free fatty acids appear to be involved. Quantitative documentation of the content of fat in human kidneys in situ appears not to exist, so we cannot compare our MRI-determined kidney fat content figures to literature data. In a bovine growth hormone transgenic mouse line, kidney triglycerides, while lower than those found in liver, recently showed a similar trend of reduced levels as compared with non-transgenic littermate controls showing overall fat mass increases[25]. Furthermore, in rats, steatosis of the kidney was recently associated with an alteration in renal acidification[12]. The fat accumulated in the renal cortex, as shown quantitatively by enzymatic triglyceride measurement and qualitatively by oil red O staining[12]. This fits the notion that, within the cortex, the proximal tubule is vulnerable to lipid accumulation due to its role in the reabsorption of free fatty acids bearing albumin[13,14]. Our result in humans, with regard to the finding of very low fat content (up to 2%) in the cortex of kidneys of both lean and obese individuals, is not in line with the findings of the above experimental studies.
Our observation of significant correlation between liver and pancreas fat content according to MRI (r = 0.43, P < 0.01) is also in disagreement with a recent study involving a smaller number of volunteers than we included here (17 vs 36)[10], and with another small study (n = 15) in which the existence of significant correlation between liver and pancreas fat content is not mentioned[8]. The small scale of both earlier studies in humans probably explains their failure to demonstrate the correlation between the fat content in liver and pancreas. The presence of such a correlation does fit a previous demonstration that obese nondiabetic subjects have increased fat in the pancreas[8]. It is also in line with a notion that lipomatosis of the pancreas reflects early events in the pathogenesis of diabetes[9], confirming what has been shown in numerous publications dealing with the fattening of the liver.
The significant correlations of BMI with both liver and pancreas fat content in this study are in agreement with a previous report[8]. Why would obese subjects tend to accumulate fat in liver and pancreas and not in the kidney, despite the observation that obesity and the metabolic syndrome are involved with initiation of chronic kidney disease[4]? It seems that due to specific requirements, such as albumin loading needed to facilitate the uptake of fat into kidney tissue, obese but otherwise healthy subjects do not accumulate fat in the kidneys.
In conclusion, this is the first demonstration of the use of an MRI method for determining kidney fat content. Observed for the first time are significantly correlated liver and pancreas fat contents in (otherwise) healthy persons varying in body weight from lean to obese. These observations are related to body weight as measured by BMI and the amount of subcutaneous fat. The amount of fat in the kidney in obese persons is small and not related to the amount of body fat or the fat content of liver and pancreas. We have thus shown that in obesity, the first step in the pathogenesis of renal disease, lipid is not accumulated in the kidney. Therefore, the role of lipid accumulation in the pathogenesis of renal disease, diabetes and metabolic disease in humans should be reconsidered. That is not to say that fat metabolites, rather than the triglyceride levels detected here by MRI, may have profound effects. In future studies we propose to examine patients suffering from the above illnesses in order to validate our current hypothesis that the lipid content is always low in the kidneys.
Worldwide obesity is on the rise and so are diseases associated with accumulation of fat in various organs.
This is the first study providing quantitative fat content data for human liver, pancreas and kidney in situ.
Shown here for the first time is significant correlation of the liver and pancreas fat contents in persons with body weights varying between thin and obese.
This study shows that magnetic resonance imaging can be used to study metabolic disease noninvasively. The fat content of multiple organs can thus be quantified and correlated.
The fat content of human tissues can be quantified by using magnetic resonance imaging methods to quantify and compare the signals of water and fat. The fat content of human tissues is typically expressed as fat/(water+fat).
The study assessed renal lipid accumulation, as well as fat accumulation in the liver and pancreas of a series of volunteers varying in body weight from thin to obese. Results confirmed the correlation between liver and subcutaneous fat content as well as showing the absence of fat storage in the kidney. The latter is the real novel finding of the study.
Peer reviewer: Giovanni Maconi, MD, Department of Gastroenterlogy, ‘L.Sacco’ University Hospital, Via G.B. Grassi, 74, Milan 20157, Italy
S- Editor Wang YR L- Editor Logan S E- Editor Ma WH
| 1. | Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83-92. [Cited in This Article: ] |
| 2. | Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159-5165. [Cited in This Article: ] |
| 3. | Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27-38. [Cited in This Article: ] |
| 4. | Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2:550-562. [Cited in This Article: ] |
| 5. | Weinberg JM. Lipotoxicity. Kidney Int. 2006;70:1560-1566. [Cited in This Article: ] |
| 6. | Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000-3004. [Cited in This Article: ] |
| 7. | Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351-366. [Cited in This Article: ] |
| 8. | Kovanlikaya A, Mittelman SD, Ward A, Geffner ME, Dorey F, Gilsanz V. Obesity and fat quantification in lean tissues using three-point Dixon MR imaging. Pediatr Radiol. 2005;35:601-607. [Cited in This Article: ] |
| 9. | Raeder H, Haldorsen IS, Ersland L, Grüner R, Taxt T, Søvik O, Molven A, Njølstad PR. Pancreatic lipomatosis is a structural marker in nondiabetic children with mutations in carboxyl-ester lipase. Diabetes. 2007;56:444-449. [Cited in This Article: ] |
| 10. | Schwenzer NF, Machann J, Martirosian P, Stefan N, Schraml C, Fritsche A, Claussen CD, Schick F. Quantification of pancreatic lipomatosis and liver steatosis by MRI: comparison of in/opposed-phase and spectral-spatial excitation techniques. Invest Radiol. 2008;43:330-337. [Cited in This Article: ] |
| 11. | Sinha S, Misra A, Rathi M, Kumar V, Pandey RM, Luthra K, Jagannathan NR. Proton magnetic resonance spectroscopy and biochemical investigation of type 2 diabetes mellitus in Asian Indians: observation of high muscle lipids and C-reactive protein levels. Magn Reson Imaging. 2009;27:94-100. [Cited in This Article: ] |
| 12. | Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294:F1315-F1322. [Cited in This Article: ] |
| 13. | Gekle M. Renal tubule albumin transport. Annu Rev Physiol. 2005;67:573-594. [Cited in This Article: ] |
| 14. | Riazi S, Khan O, Tiwari S, Hu X, Ecelbarger CA. Rosiglitazone regulates ENaC and Na-K-2Cl cotransporter (NKCC2) abundance in the obese Zucker rat. Am J Nephrol. 2006;26:245-257. [Cited in This Article: ] |
| 15. | Sijens PE. Parametric exploration of the liver by magnetic resonance methods. Eur Radiol. 2009;19:2594-2607. [Cited in This Article: ] |
| 16. | Edens MA, van Ooijen PM, Post WJ, Haagmans MJ, Kristanto W, Sijens PE, van der Jagt EJ, Stolk RP. Ultrasonography to quantify hepatic fat content: validation by 1H magnetic resonance spectroscopy. Obesity (Silver Spring). 2009;17:2239-2244. [Cited in This Article: ] |
| 17. | Irwan R, Edens MA, Sijens PE. Assessment of the variations in fat content in normal liver using a fast MR imaging method in comparison with results obtained by spectroscopic imaging. Eur Radiol. 2008;18:806-813. [Cited in This Article: ] |
| 18. | Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189-194. [Cited in This Article: ] |
| 19. | Hussain HK, Chenevert TL, Londy FJ, Gulani V, Swanson SD, McKenna BJ, Appelman HD, Adusumilli S, Greenson JK, Conjeevaram HS. Hepatic fat fraction: MR imaging for quantitative measurement and display--early experience. Radiology. 2005;237:1048-1055. [Cited in This Article: ] |
| 20. | Sijens PE, Smit GP, Borgdorff MA, Kappert P, Oudkerk M. Multiple voxel 1H MR spectroscopy of phosphorylase-b kinase deficient patients (GSD IXa) showing an accumulation of fat in the liver that resolves with aging. J Hepatol. 2006;45:851-855. [Cited in This Article: ] |
| 21. | Longo R, Ricci C, Masutti F, Vidimari R, Crocé LS, Bercich L, Tiribelli C, Dalla Palma L. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol. 1993;28:297-302. [Cited in This Article: ] |
| 22. | Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487-495. [Cited in This Article: ] |
| 23. | Fishbein MH, Mogren C, Gleason T, Stevens WR. Relationship of hepatic steatosis to adipose tissue distribution in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2006;42:83-88. [Cited in This Article: ] |
| 24. | Kim H, Taksali SE, Dufour S, Befroy D, Goodman TR, Petersen KF, Shulman GI, Caprio S, Constable RT. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point dixon and three-point IDEAL. Magn Reson Med. 2008;59:521-527. [Cited in This Article: ] |
| 25. | Palmer AJ, Chung MY, List EO, Walker J, Okada S, Kopchick JJ, Berryman DE. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology. 2009;150:1353-1360. [Cited in This Article: ] |