Published online Dec 14, 2009. doi: 10.3748/wjg.15.5789
Revised: October 1, 2009
Accepted: October 8, 2009
Published online: December 14, 2009
AIM: To study the role of advanced glycation end products (AGE) and their specific receptor (RAGE) in the pathogenesis of liver fibrogenesis.
METHODS: In vitro RAGE expression and extracellular matrix-related gene expression in both rat and human hepatic stellate cells (HSC) were measured after stimulation with the two RAGE ligands, advanced glycation end product-bovine serum albumin (AGE-BSA) and Nε-(carboxymethyl) lysine (CML)-BSA, or with tumor necrosis factor-α (TNF-α). In vivo RAGE expression was examined in models of hepatic fibrosis induced by bile duct ligation or thioacetamide. The effects of AGE-BSA and CML-BSA on HSC proliferation, signal transduction and profibrogenic gene expression were studied in vitro.
RESULTS: In hepatic fibrosis, RAGE expression was enhanced in activated HSC, and also in endothelial cells, inflammatory cells and activated bile duct epithelia. HSC expressed RAGE which was upregulated after stimulation with AGE-BSA, CML-BSA, and TNF-α. RAGE stimulation with AGE-BSA and CML-BSA did not alter HSC proliferation, apoptosis, fibrogenic signal transduction and fibrosis- or fibrolysis-related gene expression, except for marginal upregulation of procollagen α1(I) mRNA by AGE-BSA.
CONCLUSION: Despite upregulation of RAGE in activated HSC, RAGE stimulation by AGE does not alter their fibrogenic activation. Therefore, RAGE does not contribute directly to hepatic fibrogenesis.
- Citation: Lohwasser C, Neureiter D, Popov Y, Bauer M, Schuppan D. Role of the receptor for advanced glycation end products in hepatic fibrosis. World J Gastroenterol 2009; 15(46): 5789-5798
- URL: https://www.wjgnet.com/1007-9327/full/v15/i46/5789.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5789
Advanced glycation end products (AGE) are formed in vitro and in vivo from non-enzymatic glycation of the amino groups of proteins with reducing sugars such as glucose. Although serum levels of AGE are usually low due to constant turnover, they can be detected in vivo once levels of reducing sugars are elevated, as occurs in diabetes[1]. Thus, glycated hemoglobin in the serum of diabetic patients was the first described physiologically relevant AGE[2]. Interest in AGE has increased since several studies suggested that AGE may be responsible for pathological features associated with diabetes. For example, in endothelial cells, AGE were shown to increase the expression of pro-coagulant activity, induce expression of vascular cell adhesion molecule-1, and promote nuclear translocation of nuclear factor-κB (NF-κB). In mononuclear phagocytes, AGE induce the production of platelet-derived growth factor, increase migration, and drive NF-κB activation[3,4].
AGE interact with several receptors, such as the receptor for advanced glycation end products (RAGE), 80K-H phosphoprotein, galectin-3, lactoferrin, scavenger receptors such as SRA or SRB I, and CD36[5,6]. RAGE, a member of the immunoglobulin superfamily of cell surface receptors, is expressed in a variety of tissues and interacts with several AGE ligands, especially with Nε-(carboxymethyl) lysine (CML)[4].
However, while a role for RAGE in the progression of diabetic vasculopathy and kidney failure has been established[7-9], its role in hepatic fibrosis is poorly understood. This is important due to the emerging epidemic of nonalcoholic steatohepatitis (NASH) related to obesity and the metabolic syndrome, conditions that are associated with increased AGE and RAGE and hepatic fibrosis[10-12]. RAGE expression has been described in inflammatory cells[13] and in activated hepatic stellate cells (HSCs)[14], the major fibrogenic effector cells that can undergo activation to myofibroblasts producing the excess extracellular matrix in hepatic fibrosis[15]. Many features of this activation process are mimicked by spontaneously occurring activation on tissue culture plastic in vitro[16,17]. While certain cytokines, growth factors and culture conditions and, as recently demonstrated, AGE[18], can modulate HSC activation and extracellular matrix (ECM) synthesis, the functional contribution of AGE and RAGE expression to fibrogenic activation of HSC and to hepatic fibrosis remains to be elucidated.
We have therefore studied whether physiological AGE concentrations occur in the serum of patients with diabetes[1,19], and whether AGE-RAGE interactions are involved in the progression of liver fibrosis. To this end we investigated RAGE expression in HSC in vitro, and in normal and cirrhotic livers in vivo. Furthermore, the effects of AGE and the key proinflammatory cytokine tumor necrosis factor (TNF)-α on HSC RAGE expression and on the proliferation, kinase activation and profibrogenic and fibrolytic gene expression of HSC were determined.
For preparation of AGE-modified bovine serum albumin (BSA), 0.6 mmol/L BSA and 0.16 mmol/L D-glucose were dissolved in 20 mL PBS, sterile filtered, incubated for 60 d at 37°C and dialyzed against PBS under sterile conditions to remove unreacted D-glucose. Control BSA was incubated in parallel in the absence of D-glucose. Preparation of CML-modified BSA was carried out as previously described[20].
Glycation of AGE-BSA and CML-BSA was determined using the 2,4,6-trinitrobenzenesulfonic acid (TNBS) assay[21], resulting in a 45.6% and 36.5% glycation of lysines for AGE-BSA and CML-BSA, respectively.
After endotoxin removal with the Detoxi-Gel™ (Pierce, Rockford, IL), the final endotoxin concentration determined with the E-toxate® endotoxin detection kit (Sigma, Taufkirchen, Germany) was below 0.04 and 0.02 ng/mL for AGE-BSA and CML-BSA, respectively.
Cell lines were cultured as previously published[20]. The fully activated rat HSC line HSC-T6 (kind donation of Dr. SL Friedman, NY)[22], the moderately activated rat HSC line CFSC-2G (kindly provided by Dr. M Rojkind, Washington, D.C.)[23] and human HSC of passage 3 to 5 (kind gift of Dr. M Pinzani, Florence, Italy)[24] were maintained as previously described (references see below).
Cell culture and animal experimentation: Animals were treated according to the Council of International Organizations of Medical Sciences for the Care and Use of Laboratory Animals in Research. The experimental protocol was approved by the Animal Care Committee of the University of Erlangen-Nuremberg.
Isolation of rat primary hepatocytes: Hepatocytes were freshly isolated from male Wistar rats (190-200 g, Charles River, Sulzfeld, Germany) according to a modified two-step collagenase perfusion method[25] as previously described in detail[26]. Experiments were performed 6 h after plating with cell viabilities ≥ 85% as determined by Trypan Blue exclusion.
Isolation of rat primary hepatic stellate cells: HSC were isolated from male Wistar rats (retired breeders, 400-500 g) as described[27]. Cell viability was usually between 95%-98%. The purity of HSC was confirmed by their stellate shape, and autofluorescence of the cytoplasmic lipid-droplets at 390 nm. Freshly isolated HSC were activated by culture on plastic in the presence of 10% fetal calf serum (FCS) for 1, 5, and 10 d prior to lysis and RNA extraction. HSC plated for 1 d were designated as quiescent, those cultured for 5 and 10 d as partially and fully activated, respectively.
Experimental liver fibrosis: Experimental liver fibrosis was induced in groups of four adult male Wistar rats weighing about 400 g as follows: (1) bile duct ligation (BDL) for 6 wk, (2) thioacetamide (TAA) treatment, 200 mg/g body weight thrice weekly for 12 wk, as previously published[27]. Sham-operated rats served as controls. After sacrifice of all animals, pieces of the right and left liver lobes were removed, fixed in 4% formalin and paraffin embedded, or snap frozen in liquid nitrogen for further analysis.
For RNA analysis 150-200 mg of tissue were homogenized in 1 mL RNAPure for 30 s and an aliquot representing 10 mg of tissue was mixed with 900 μL fresh RNAPure. Morphology of connective tissue was evaluated with hematoxylin-eosin and Sirius Red staining.
Immunohistochemistry: For immunohistochemical analysis specimens from two different liver segments were studied. Sequential deparaffinized sections were blocked with avidin and biotin. After antigen retrieval in a decloaking chamber for 30 s at 120°C in Target Retrieval Solution, pH 6.0 (Dako), sections were incubated overnight at room temperature with monoclonal antibodies to RAGE (1:30, kindly provided by Dr. B Weigle (Dresden, Germany), CD3 (1:10, Serotec MCA 772), CD45 (1:50, Serotec MCA 43R), CD68 (1:30, Serotec MCA 341R), and α-smooth muscle actin (SMA) (1:30, DAKO M 0851), followed by biotinylated horse anti-mouse IgG and streptavidin-biotin alkaline phosphatase[28]. Sections were developed using Fast Red and nuclei counterstained with hematoxylin. RAGE was additionally detected with the catalyzed signal amplification system as previously described[29,20]. For double staining, the slides were treated with a Double Staining Enhancer (Zytomed 50-056) for 30 min before application of the secondary antibody. RAGE-antibody was first applied, followed by the other antibodies developed with Fast Red (RAGE) and with Fast Blue. The co-expressions of RAGE and of CD3, CD68 and of α-SMA inside the liver (portal and lobular areas) were counted in ten randomly selected high-power fields (400 × magnification) using ImageAccess Enterprise 5 software (Imagic Bildverarbeitung, Glattbrugg, Switzerland). The number of immunohistochemically positive cells are given as the percentage of all cells (RAGE) and the respective cell population (CD3, CD68 and of α-SMA) in the studied areas.
Preconfluent (80%) HSC lines, HSC-T6 and CFSC-2G, were seeded at 20 000 cells per well in 24-well plates, washed and incubated with 10-100 μg/mL BSA, AGE-BSA, or CML-BSA, or with 0-10 ng/mL TNF-α (Sigma) in serum-free medium. Protein extraction and Western blotting were performed as previously described[20].
Total RNA was isolated using peqGOLD RNApure reagent (PeqLab Biotechnologie, Erlangen, Germany) and reverse transcribed, followed by real time RT-PCR using a LightCycler instrument (Roche, Mannheim, Germany), as described in detail elsewhere[27,30,31], and the TaqMan principle[32]. Results were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or β2-microglobulin amplified in a parallel reaction. The specific sense and antisense oligonucleotide primers and probes have been published[31,20].
Cell proliferation of CFSC-2G and HSC-T6 cells was determined using BrdU incorporation according to the manufacturer’s protocol (Roche, Manheim, Germany) as recently described[33].
These enzyme activities were determined as previously described[27] using Western Blotting with antibodies to phosphorylated and total ERK1/2 MAPK (Thr202/Tyr204, 1:2000) and anti-phospho-p38 MAPK (Thr180/Tyr182, 1:1000) (from Cell Signaling Technology, Frankfurt, Germany). Phospho-specific signals were normalized to unphosphorylated kinase signals.
Statistical analysis was performed with SPSS v. 16.0 (SPSS GmbH Software, Munich, Germany). Student’s t test and univariate ANOVA (analysis of variance) was used to test for differences between two and more groups, respectively (P < 0.05 was significant). All graphs represent the mean ± SD and were performed at least in triplicate.
Freshly isolated rat HSC, and the rat HSC lines HSC-T6 and CFSC-2G, as well as human HSC, expressed significant RAGE transcripts and protein (Figure 1A and B). During culture, activation of freshly isolated rat HSC RAGE transcripts was upregulated 1.6- and 3.8-fold, respectively, on days 5 and 10 of primary culture (Figure 1C). Culture activation of HSC was associated with highly increased expression of procollagen-α1(I) and α-SMA mRNA which were upregulated > 100-fold and > 50-fold after 5 and 10 d of activation (data not shown).
In cirrhotic livers of rats subjected to BDL, RAGE transcripts were upregulated 4-fold as compared to healthy controls (P < 0.01), whereas in thioacetamide (TAA)-induced cirrhosis RAGE mRNA expression remained unchanged. Fibrosis-related transcripts such as α-SMA, procollagen-α1(I), matrix metalloproteinase (MMP)-13, and tissue inhibitor of metalloproteinase (TIMP)-1 mRNA were highly upregulated in both fibrosis models (Table 1).
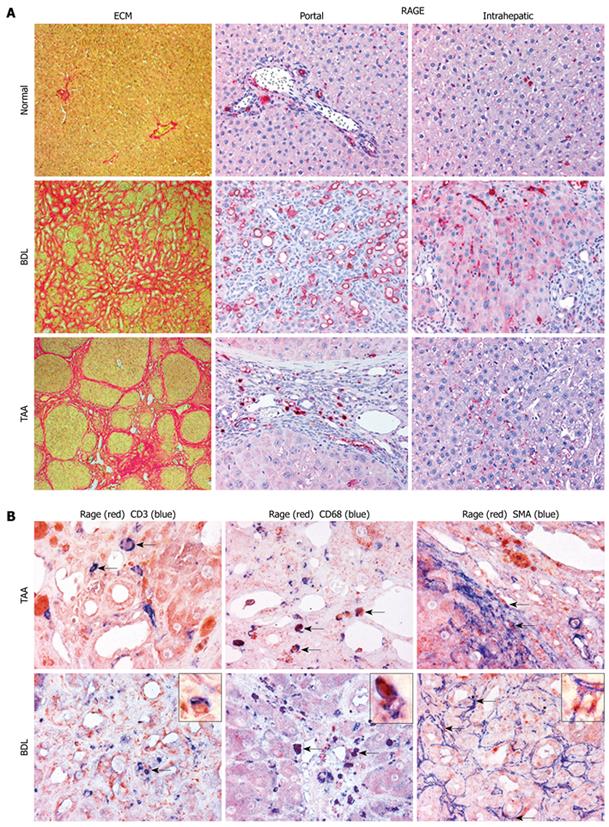
Immunohistochemistry of normal livers showed a significantly lower expression of RAGE protein compared to the fibrotic/cirrhotic livers (P < 0.001, Table 2 and Figure 2A), with predominant expression in portal vein and arterial endothelial cells and sparse expression in lymphocytes and myofibroblasts in the hepatic lobule. Hepatocytes did not express RAGE. In BDL livers RAGE protein was highly expressed in bile duct proliferating epithelia, in periductular α-SMA positive myofibroblasts and in inflammatory cells that were identified as CD3-positive T-lymphocytes and CD68-positive macrophages by use of double staining immunohistochemistry (Table 2, Figure 2A and B). In TAA cirrhosis the number of RAGE-expressing cells was less pronounced than in biliary cirrhosis, in concert with lower RAGE gene expression and fewer inflammatory cell infiltrates, being primarily expressed by macrophages, as opposed to mostly T-lymphocytes and proliferating bile duct epithelia in BDL-cirrhosis (Table 2 and Figure 2B). Only a few α-SMA- and RAGE-positive myofibroblasts were detected in portal areas and septa of both models. The expression of RAGE by endothelial cells in the cirrhotic livers was slightly enhanced in areas of neo-capillarization compared to normal controls (Figure 2A).
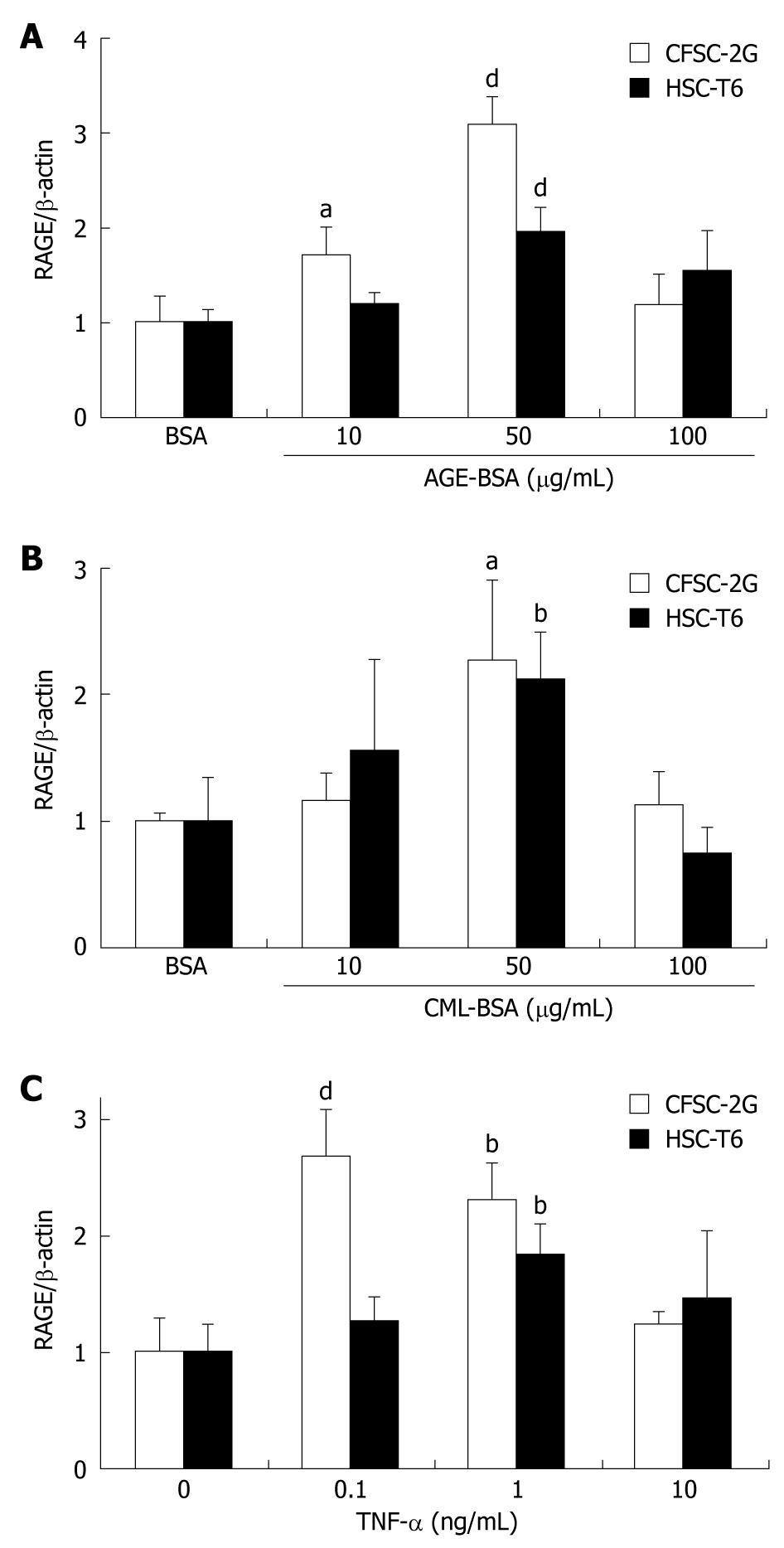
Incubation of CFSC-2G and HSC-T6 HSC with 50 μg/mL AGE-BSA significantly (P < 0.001) upregulated RAGE protein expression by 2-3 fold (Figure 3A). While CFSC-2G cells were more sensitive to AGE-BSA, the highest concentration, 100 μg/mL, was not effective in either cell line. Addition of 50 μg/mL CML-BSA increased RAGE protein expression about 2 fold in both cell lines (P < 0.05) (Figure 3B).
Similarly, TNF-α upregulated RAGE protein expression significantly (P < 0.01) in both CFSC-2G and HSC-T6 HSC (Figure 3C). Again, a greater RAGE induction was observed in CFSC-2G cells, where 0.1 ng/mL TNF-α resulted in a nearly 3-fold (P < 0.001) upregulation of RAGE expression.
Incubation of CFSC-2G HSC with 50 g/mL AGE-BSA or CML-BSA did not significantly modify transcript levels of transforming growth factor (TGF)-β1, α-SMA, and MMP-13, or RAGE itself, except for a marginal (23%, P < 0.05) but reproducible upregulation of procollagen-α1(I) mRNA by AGE-BSA (Table 3). These results could be confirmed in HSC-T6 and human HSC (data not shown).
| BSA | AGE-BSA | CML-BSA | |
| RAGE | 1.00 ± 0.14 | 1.20 ± 0.11 | 1.26 ± 0.07 |
| TGF-β1 | 1.00 ± 0.06 | 1.12 ± 0.21 | 0.99 ± 0.07 |
| Procollagen-α1(I) | 1.00 ± 0.08 | 1.23 ± 0.06a | 1.12 ± 0.19 |
| α-SMA | 1.00 ± 0.14 | 1.20 ± 0.20 | 1.16 ± 0.04 |
| MMP-13 | 1.00 ± 0.05 | 1.09 ± 0.14 | 0.97 ± 0.09 |
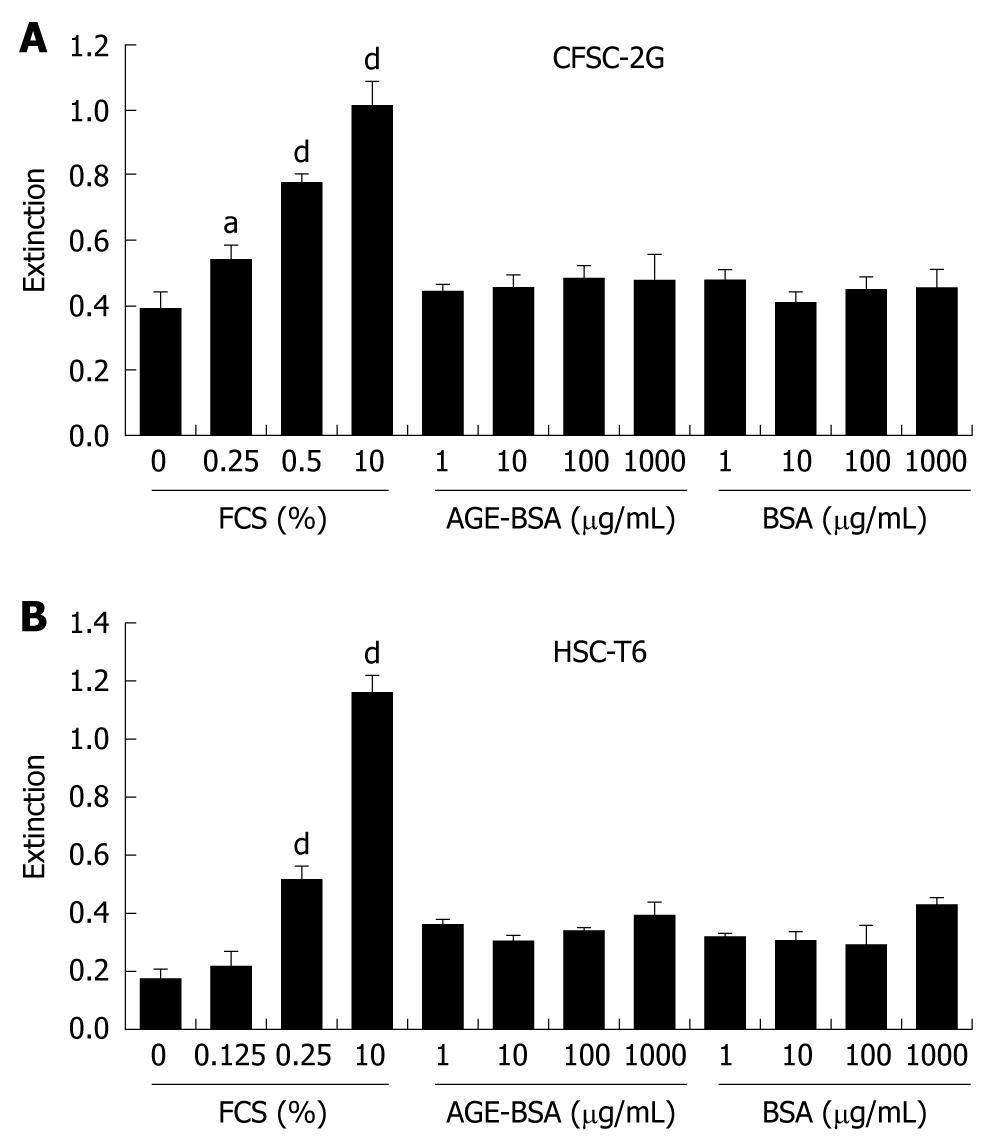
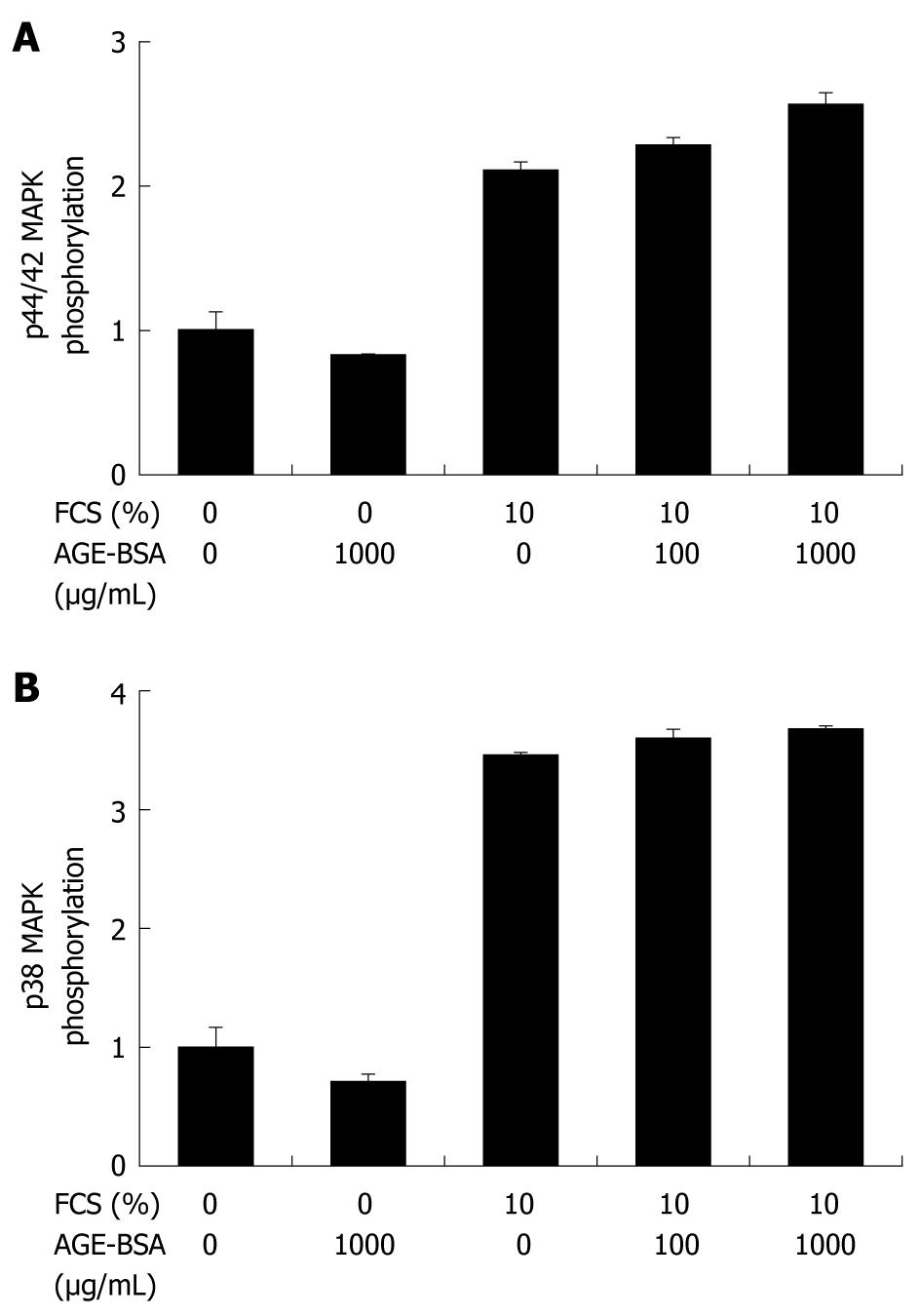
CFSC-2G and HSC-T6 cells were exposed to 1-1000 μg/mL AGE-BSA for 24 h, and DNA synthesis was assessed by BrdU incorporation. As opposed to the mitogen FCS, AGE-BSA did not induce DNA synthesis in these cells (Figure 4). In addition, cell numbers remained unchanged after addition of AGE-BSA (data not shown). In line with the proliferation data, AGE-BSA did not induce p44/42 (Figure 5A) or p38 (Figure 5B) MAPK activation when compared to 10% FCS as positive control.
Our findings show that RAGE, a prominent receptor for AGE, is expressed in hepatic stellate cells (HSC) derived from various species. Our data are in line with previous studies showing the RAGE upregulation in single culture-activated HSC either of rat or human origin[14,18]. Using different experimental hepatic fibrosis models in rats we found that transcript levels of RAGE correlate well with RAGE protein expression in agreement with our immunohistochemical co-localization studies. Compared with earlier studies of RAGE expression in other organs[4], we could detect RAGE not only in myofibroblasts (HSC) and endothelial cells, but also in lymphocytes, macrophages/small Kupffer cells and proliferating bile duct epithelial cells. These results are in contrast to prior studies that either identified RAGE expression in bovine hepatocytes in vivo[34], or exclusively in HSC and myofibroblasts, but not in hepatocytes, sinusoidal endothelial or Kupffer cells[14]. The reasons for these discrepancies may be the use of different antibody reagents with different specificities. We used an antibody that has been characterized thoroughly and found to react specifically with cells on tissue sections[28]. In addition, we could partly confirm our in vivo data with our in vitro studies using various types of HSC.
During culture-activation of HSC, RAGE expression was increased significantly. This increase was paralleled by the known upregulation of major transcripts related to fibrosis progression, i.e. TGF-β1, the most prominent profibrogenic cytokine; procollagen α1(I), a precursor of the major fibrillar collagen; TIMP-1, the central inhibitor of matrix metalloproteinases (MMP); and α-SMA, a marker for HSC activation[16,17].
Enhanced RAGE expression in hepatic fibrogenesis was further shown in rats with cirrhosis induced by BDL and TAA treatment, which was in line with prior findings in CCl4-induced hepatic fibrosis where RAGE transcript and protein levels were upregulated until 6 wk after the completion of CCl4 treatment[35]. Differences in RAGE expression may be due to the lack of inflammation in TAA-treated animals, since TAA treatment was stopped one week prior to tissue removal. Progressive injury, however, was still present in bile duct-ligated animals at the time of sacrifice, with enhanced numbers of CD68-positive macrophages/small Kupffer cells and CD3-positive T-lymphocytes as compared to the TAA-cirrhosis model. Furthermore, we showed that α-SMA-positive HSC/myofibroblasts of the septal or portal interface, representing the prominent fibrogenic effectors, expressed RAGE in both fibrosis models.
In studies of diabetes-associated cardiovascular and renal disease, an upregulation of RAGE has been linked to enhanced levels of AGE[7,9], in association with epithelial-myofibroblast transdifferentiation[36] and induction of fibrogenesis[37-39]. Additionally, inhibition of the interaction of AGE-RAGE with neutralizing monoclonal anti-RAGE antibodies or the AGE cross-link disrupting agent ALT-711 prevented pathological effects of hyperglycemia in blood vessels and kidneys[8,40].
Our results show that AGE-BSA, as well as the specific RAGE ligand CML-BSA, upregulated the expression of the receptor itself in HSC. This phenomenon was reported previously in human vascular and umbilical vein endothelial cells[41], where 50 μg/mL AGE-BSA induced a 2- and 2.5-fold increased RAGE protein expression, respectively, compared to untreated cells. Other studies showed that certain vascular domains, renal glomeruli or intima media and adventitia of the aorta exhibit increased RAGE expression[42] and that interactions of AGE with RAGE resulted in autoinduction of RAGE expression[43]. Our observed reduction of response using higher concentrations of AGE-BSA could reflect receptor saturation, downregulation, or an antagonist effect on RAGE expression.
Addition of the proinflammatory cytokine TNF-α to HSC upregulated RAGE protein and mRNA expression up to 3-fold, in accordance with previous studies showing that RAGE is upregulated in inflamed tissues[44]. Interestingly, the blockade of RAGE by murine-soluble RAGE as decoy could decrease acetaminophen-induced hepatotoxicity[45] and liver injury in an ischemia and reperfusion model in mice[46]. Furthermore, an approximately 2-fold increase of RAGE protein expression after incubation of human microvascular endothelial cells with up to 100 ng/mL TNF-α has been reported[41]. In the present study, however, RAGE expression peaked at 0.1 and 1 ng/mL TNF-α and decreased to baseline levels at 10 ng/mL TNF-α, apparently due to receptor downregulation at high concentrations and this indicated a greater sensitivity of HSC than that of human microvascular endothelial cells to TNF-α. Increased RAGE expression under inflammatory conditions, such as those triggered by TNF-α, may result in enhanced binding of AGEs to RAGE, further increasing RAGE expression and expression of proinflammatory cytokines. This could be relevant for patients with insulin resistance, overt diabetes, and obesity, who frequently present with hepatic inflammation and fibrosis, i.e. patients with NASH.
The upregulated RAGE expression in HSC may lead to the conclusion that AGE-RAGE interactions play a role in hepatic fibrogenesis. However, in contrast to upregulation of RAGE by AGE/CML-AGE and TNF-α, we clearly showed that AGE-BSA and CML-BSA were unable to induce expression of fibrosis or fibrolysis-related genes in CFSC-2G or HSC-T6 HSC. Using another experimental approach Xia et al[35] showed that targeting of RAGE by specific siRNA downregulated fibrogenesis-related transcripts in vitro and in vivo. Of note, we took great care to synthesize AGE-BSA and CML-BSA in a sterile environment and to remove any remaining endotoxin contamination in the products. Possible discrepancies between previously published cellular effects of AGE and the present lack of induction may be explained by the presence of endotoxin in previously synthesized AGE preparations.
Another pathogenic feature of HSC activation, besides migration, apoptosis, ECM synthesis, or contractility, is increased proliferation[47]. According to our experimental setup, we can conclude that AGE do not alter hepatic fibrogenesis through induction of HSC proliferation.
Previous studies of endothelial cells and monocytes, especially mononuclear phagocyte-derived dendritic cells of the liver after massive liver injury[13], showed that interactions of AGE with RAGE induce, besides RAGE, the expression of proinflammatory cytokines, such as TNF-α and interleukin-1 and -6[41]. These events are mediated by activation of redox-sensitive signaling pathways involving NADPH oxidase, or mitogenic pathways involving the small G-protein Ras that lead to activation of mitogen-activated protein kinases (MAPK), or involving nuclear factor-κB (NF-κB). Since no data on AGE-induced MAPK activation in HSCs exist, we aimed to investigate whether RAGE upregulation by AGE may be due to stimulation of MAPK signal transduction pathways. We could not find any stimulatory effect of endotoxin-free AGE-BSA or CML-BSA on activation of p44/42 MAPK, which mediates cellular growth and differentiation, and p38 MAPK, which regulates cytokine expression and controls cellular responses to cytokines and stress. Again, this contrasts with previous reports showing that interaction of AGE with RAGE induced intracellular signaling pathways involved in inflammatory responses including MAPK or NF-κB activation[48]. Moreover, downregulation of RAGE by specific siRNAs was associated with NF-κB degradation supporting the linkage of RAGE to the NF-κB pathway[35]. Only a single study indicated that AGE may not uniformly play a role in cellular activation[49].
In summary, the present data do not support a direct role of AGE and AGE-RAGE axis in the fibrogenic activation of HSC, such as profibrogenic ECM-related gene expression, signal transduction, or proliferation. However, the finding that RAGE in HSC is upregulated during their activation in vitro and in HSC/myofibroblasts, macrophages/small Kupffer cells, endothelia of neo-capilliarization and proliferating bile duct epithelia during fibrogenesis in vivo does not exclude the possibility that RAGE may drive fibrogenesis indirectly, e.g. via soluble factors that are released from these non-HSC cell types. This could still have relevance for patients with insulin resistance and NASH who display elevated serum and tissue levels of AGE[1,50]. Whether the observed RAGE upregulation may contribute to fibrosis via these indirect pathways needs to be investigated in further studies using either co-culture experiments, gene modified animals, or the administration of AGEs to animals with experimental liver fibrosis.
Advanced glycation end products (AGE) and their specific receptor (RAGE) play an important role in the pathogenesis of inflammation and fibrosis in diabetes mellitus. While RAGE has been detected in numerous tissues, its role in organs such as the liver which are also exposed to (circulating) AGE remains largely unexplored.
The study of liver fibrosis is a challenging research area due to the enormous socio-epidemiologic and medical-therapeutical impact of chronic liver diseases which frequently progress to cirrhosis. We have made tremendous progress in our understanding of the pathomechanisms underlying liver fibrosis progression, including the structural components of the hepatic scar tissue (extracellular matrix), the molecules that are central to its excess deposition and to its removal, and the direct and indirect effector cells that drive fibrosis progression, i.e. fibrogenesis (Friedman, Gastro 08; Schuppan and Afdhal, Lancet 08). Recent studies suggested an association of an activated AGE-RAGE-axis with fibrogenesis, but clear functional data were lacking.
The findings suggest a more indirect than direct effect of the AGE-RAGE-axis on liver fibrogenesis and rule out a direct effect of AGE on the fibrogenic effector cells, i.e. hepatic stellate cells.
Future applications depend on additional studies that would explore the putative indirect effects of RAGE on fibrogenesis, e.g. via cytokines produced by AGE-activated macrophages, biliary duct epithelia or endothelial cells. This could facilitate the development of specific AGE-RAGE-inhibitors that could generate a novel class of therapeutics, especially in conditions where AGE play a prominent role, such as non-alcoholic fatty liver disease.
AGE are formed in vitro and in vivo from non-enzymatic glycation of the amino groups of proteins with reducing sugars such as glucose. AGE can be detected in vivo once levels of reducing sugars are elevated as occurs in diabetes. AGE interact with several receptors, most specifically with the receptor for advanced glycation end products (RAGE). RAGE is a member of the immunoglobulin superfamily of cell surface receptors which are expressed in a variety of tissues.
The originality of this study resides in the broad and exhaustive study of RAGE expression in two well-defined and complementary rodent models of liver fibrosis, the use of different cells and cell lines, and extensive in vitro stimulation studies of hepatic stellate cells with AGE to assess their effect on the expression of extracellular matrix component and matrix dissolving metalloproteinases. This broad approach is novel and has for the first time provided clear results as to the effect of stimulation of the AGE-RAGE axis in hepatic stellate cells and the putative fibrogenic role it plays in fibrogenic activation of macrophages/Kupffer cells, endothelia or biliary duct epithelia.
Peer reviewers: Mark D Gorrell, PhD, Professor, Centenary Institute of Cancer Medicine and Cell Biology, Locked bag No. 6, Newtown, NSW 2042, Australia; Devanshi Seth, PhD, Senior Scientist, Centenary Institute & Drug Health Services, RPAH & Clinical Senior Lecturer, Clinical School of Medicine, University of Sydney, Camperdown, NSW 2050, Australia
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
| 1. | Schmidt AM, Yan SD, Stern DM. The dark side of glucose. Nat Med. 1995;1:1002-1004. [Cited in This Article: ] |
| 2. | Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun. 1969;36:838-843. [Cited in This Article: ] |
| 3. | Kirstein M, Brett J, Radoff S, Ogawa S, Stern D, Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci USA. 1990;87:9010-9014. [Cited in This Article: ] |
| 4. | Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740-31749. [Cited in This Article: ] |
| 5. | Horiuchi S, Sakamoto Y, Sakai M. Scavenger receptors for oxidized and glycated proteins. Amino Acids. 2003;25:283-292. [Cited in This Article: ] |
| 6. | Iacobini C, Amadio L, Oddi G, Ricci C, Barsotti P, Missori S, Sorcini M, Di Mario U, Pricci F, Pugliese G. Role of galectin-3 in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S264-S270. [Cited in This Article: ] |
| 7. | Jerums G, Panagiotopoulos S, Forbes J, Osicka T, Cooper M. Evolving concepts in advanced glycation, diabetic nephropathy, and diabetic vascular disease. Arch Biochem Biophys. 2003;419:55-62. [Cited in This Article: ] |
| 8. | Forbes JM, Thallas V, Thomas MC, Founds HW, Burns WC, Jerums G, Cooper ME. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J. 2003;17:1762-1764. [Cited in This Article: ] |
| 9. | Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol. 2005;289:F645-F659. [Cited in This Article: ] |
| 10. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [Cited in This Article: ] |
| 11. | Wanless IR, Shiota K. The pathogenesis of nonalcoholic steatohepatitis and other fatty liver diseases: a four-step model including the role of lipid release and hepatic venular obstruction in the progression to cirrhosis. Semin Liver Dis. 2004;24:99-106. [Cited in This Article: ] |
| 12. | Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183-1192. [Cited in This Article: ] |
| 13. | Cataldegirmen G, Zeng S, Feirt N, Ippagunta N, Dun H, Qu W, Lu Y, Rong LL, Hofmann MA, Kislinger T. RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB. J Exp Med. 2005;201:473-484. [Cited in This Article: ] |
| 14. | Fehrenbach H, Weiskirchen R, Kasper M, Gressner AM. Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology. 2001;34:943-952. [Cited in This Article: ] |
| 15. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [Cited in This Article: ] |
| 16. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [Cited in This Article: ] |
| 18. | Iwamoto K, Kanno K, Hyogo H, Yamagishi S, Takeuchi M, Tazuma S, Chayama K. Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J Gastroenterol. 2008;43:298-304. [Cited in This Article: ] |
| 19. | Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53:131-142. [Cited in This Article: ] |
| 20. | Lohwasser C, Neureiter D, Weigle B, Kirchner T, Schuppan D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J Invest Dermatol. 2006;126:291-299. [Cited in This Article: ] |
| 21. | Fields R. The rapid determination of amino groups with TNBS. Methods in Enzymology. 1972;25:464-468. [Cited in This Article: ] |
| 22. | Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, Blaner WS. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882-893. [Cited in This Article: ] |
| 23. | Rojkind M, Novikoff PM, Greenwel P, Rubin J, Rojas-Valencia L, de Carvalho AC, Stockert R, Spray D, Hertzberg EL, Wolkoff AW. Characterization and functional studies on rat liver fat-storing cell line and freshly isolated hepatocyte coculture system. Am J Pathol. 1995;146:1508-1520. [Cited in This Article: ] |
| 24. | Casini A, Pinzani M, Milani S, Grappone C, Galli G, Jezequel AM, Schuppan D, Rotella CM, Surrenti C. Regulation of extracellular matrix synthesis by transforming growth factor beta 1 in human fat-storing cells. Gastroenterology. 1993;105:245-253. [Cited in This Article: ] |
| 25. | Seglen PO. Hepatocyte suspensions and cultures as tools in experimental carcinogenesis. J Toxicol Environ Health. 1979;5:551-560. [Cited in This Article: ] |
| 26. | Dan Z, Popov Y, Patsenker E, Preimel D, Liu C, Wang XD, Seitz HK, Schuppan D, Stickel F. Hepatotoxicity of alcohol-induced polar retinol metabolites involves apoptosis via loss of mitochondrial membrane potential. FASEB J. 2005;19:845-847. [Cited in This Article: ] |
| 27. | Popov Y, Patsenker E, Bauer M, Niedobitek E, Schulze-Krebs A, Schuppan D. Halofuginone induces matrix metalloproteinases in rat hepatic stellate cells via activation of p38 and NFkappaB. J Biol Chem. 2006;281:15090-15098. [Cited in This Article: ] |
| 28. | Srikrishna G, Huttunen HJ, Johansson L, Weigle B, Yamaguchi Y, Rauvala H, Freeze HH. N -Glycans on the receptor for advanced glycation end products influence amphoterin binding and neurite outgrowth. J Neurochem. 2002;80:998-1008. [Cited in This Article: ] |
| 29. | van Gijlswijk RP, Wiegant J, Raap AK, Tanke HJ. Improved localization of fluorescent tyramides for fluorescence in situ hybridization using dextran sulfate and polyvinyl alcohol. J Histochem Cytochem. 1996;44:389-392. [Cited in This Article: ] |
| 30. | Benten D, Kumaran V, Joseph B, Schattenberg J, Popov Y, Schuppan D, Gupta S. Hepatocyte transplantation activates hepatic stellate cells with beneficial modulation of cell engraftment in the rat. Hepatology. 2005;42:1072-1081. [Cited in This Article: ] |
| 31. | Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045-1054. [Cited in This Article: ] |
| 32. | Dötsch J, Hogen N, Nyúl Z, Hänze J, Knerr I, Kirschbaum M, Rascher W. Increase of endothelial nitric oxide synthase and endothelin-1 mRNA expression in human placenta during gestation. Eur J Obstet Gynecol Reprod Biol. 2001;97:163-167. [Cited in This Article: ] |
| 33. | Patsenker E, Popov Y, Wiesner M, Goodman SL, Schuppan D. Pharmacological inhibition of the vitronectin receptor abrogates PDGF-BB-induced hepatic stellate cell migration and activation in vitro. J Hepatol. 2007;46:878-887. [Cited in This Article: ] |
| 34. | Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, Nowygrod R, Neeper M, Przysiecki C, Shaw A. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699-1712. [Cited in This Article: ] |
| 35. | Xia JR, Liu NF, Zhu NX. Specific siRNA Targeting the Receptor for Advanced Glycation End Products Inhibits Experimental Hepatic Fibrosis in Rats. Int J Mol Sci. 2008;9:638-661. [Cited in This Article: ] |
| 36. | Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest. 2001;108:1853-1863. [Cited in This Article: ] |
| 37. | Twigg SM, Chen MM, Joly AH, Chakrapani SD, Tsubaki J, Kim HS, Oh Y, Rosenfeld RG. Advanced glycosylation end products up-regulate connective tissue growth factor (insulin-like growth factor-binding protein-related protein 2) in human fibroblasts: a potential mechanism for expansion of extracellular matrix in diabetes mellitus. Endocrinology. 2001;142:1760-1769. [Cited in This Article: ] |
| 38. | Nakamura S, Niwa T. Advanced glycation end-products and peritoneal sclerosis. Semin Nephrol. 2004;24:502-505. [Cited in This Article: ] |
| 39. | Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem. 2001;81:102-113. [Cited in This Article: ] |
| 40. | De Vriese AS, Flyvbjerg A, Mortier S, Tilton RG, Lameire NH. Inhibition of the interaction of AGE-RAGE prevents hyperglycemia-induced fibrosis of the peritoneal membrane. J Am Soc Nephrol. 2003;14:2109-2118. [Cited in This Article: ] |
| 41. | Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H. The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-alpha through nuclear factor-kappa B, and by 17beta-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem. 2000;275:25781-25790. [Cited in This Article: ] |
| 42. | Soulis T, Thallas V, Youssef S, Gilbert RE, McWilliam BG, Murray-McIntosh RP, Cooper ME. Advanced glycation end products and their receptors co-localise in rat organs susceptible to diabetic microvascular injury. Diabetologia. 1997;40:619-628. [Cited in This Article: ] |
| 43. | Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84:489-497. [Cited in This Article: ] |
| 44. | Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070-1077. [Cited in This Article: ] |
| 45. | Ekong U, Zeng S, Dun H, Feirt N, Guo J, Ippagunta N, Guarrera JV, Lu Y, Weinberg A, Qu W. Blockade of the receptor for advanced glycation end products attenuates acetaminophen-induced hepatotoxicity in mice. J Gastroenterol Hepatol. 2006;21:682-688. [Cited in This Article: ] |
| 46. | Zeng S, Feirt N, Goldstein M, Guarrera J, Ippagunta N, Ekong U, Dun H, Lu Y, Qu W, Schmidt AM. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology. 2004;39:422-432. [Cited in This Article: ] |
| 47. | Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311-335. [Cited in This Article: ] |
| 48. | Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889-901. [Cited in This Article: ] |
| 49. | Valencia JV, Mone M, Zhang J, Weetall M, Buxton FP, Hughes TE. Divergent pathways of gene expression are activated by the RAGE ligands S100b and AGE-BSA. Diabetes. 2004;53:743-751. [Cited in This Article: ] |
| 50. | Hyogo H, Yamagishi S, Iwamoto K, Arihiro K, Takeuchi M, Sato T, Ochi H, Nonaka M, Nabeshima Y, Inoue M. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:1112-1119. [Cited in This Article: ] |