Published online Dec 7, 2009. doi: 10.3748/wjg.15.5669
Revised: October 19, 2009
Accepted: October 26, 2009
Published online: December 7, 2009
AIM: To evaluate the anti-viral effect of emodin plus Astragalus polysaccharide (APS) in hepatitis B virus (HBV) transgenic mice.
METHODS: Sixty HBV transgenic mice (HBV TGM) whose weight varied between 18 and 24 g were randomly divided into 3 groups, with 20 mice in each group. Group A was the normal control, where the mice were treated with physiological saline; group B was the positive control where the mice were treated with lamivudine solution (100 mL/kg per day). Group C was the experimental group where the mice were treated with physiological saline containing emodin and APS (57.59 mg/kg per day and 287.95 mg/kg per day, respectively). The mice were treated daily for 3 wk. After 1 wk recovery time, the mice were sacrificed and serum as well as liver tissues were collected for ELISA and histological examination.
RESULTS: After 21 d treatment, HBV DNA levels in group B and group C significantly declined when compared with group A (P < 0.05). However, a significant increase in HBV DNA content was observed in group B, whereas this phenomenon was not observed in group C. A reduction in the contents of HBsAg, HBeAg and HBcAg in the mice from group B and C was observed when compared with group A.
CONCLUSION: Emodin and APS have a weak but persistent inhibitory effect on HBV replication in vivo, which may function as a supplementary modality in the treatment of hepatitis B infection.
- Citation: Dang SS, Jia XL, Song P, Cheng YA, Zhang X, Sun MZ, Liu EQ. Inhibitory effect of emodin and Astragalus polysaccharide on the replication of HBV. World J Gastroenterol 2009; 15(45): 5669-5673
- URL: https://www.wjgnet.com/1007-9327/full/v15/i45/5669.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5669
Hepatitis B is a global health problem, and affects about 400 million people worldwide, particularly in Asian and Western Pacific countries[1]. It is the leading cause of cirrhosis and liver cancer in China[2]. The current treatment strategy for hepatitis B is to eradicate replication and infection of hepatitis B virus (HBV) in vivo using anti-virus drugs such as interferon and nucleoside analogs such as lamivudine[3]. However, because of their side effects, low antiviral potency, and long treatment period, the actual effects of these treatments are neither ideal nor adequate[4].
Chinese herbal medicine has been used for chronic liver diseases for thousands of years in China, and their efficacy has been confirmed by modern biological technology in recent years[5,6]. Emodin (1,3,8-tri-hydroxy-6-methylanthraquinone) is an active component of herbal medicine derived from the genera Rheum, Polygonum, Rhamnus and Senna[7]. Our previous study showed that emodin could inhibit the replication of HBV in human hepatoma cells in vitro[8], suggesting its potential antiviral effect in hepatitis B treatment. Astragalus polysaccharide (APS) is a bioactive chemical in Astragalus membranaceus (AM) which has been used in medicine for centuries in China and is believed to exhibit immune-stimulatory, anti-viral, anti-oxidation and anti-tumor effects[9-12]. A previous study performed in vivo has shown that Astragalus-Polygonum Anti-Fibrosis Decoction, in which APS is one of the main components, significantly inhibited liver fibrosis induced by hepatitis B and suppressed hepatic inflammation[13], illustrating that APS may be helpful in eradicating HBV replication in vivo.
In this study, we investigated the antiviral effects of emodin and APS in HBV transgenic mice and found that the combination of emodin and ASP suppressed HBV replication in HBV transgenic mice. Although lamivudine had a stronger direct inhibitory effect on HBV replication, emodin and APS showed no HBV recurrence 7 d after the last treatment, suggesting a long-lasting effect and may prove to be a potential therapeutic modality for hepatitis B infections.
Emodin was purchased from Tianxingjian Bio. Co. (Xi’an, China), and was diluted to 5.80 mg/mL using dH2O just before administration. APS was purchased from Hongsheng Biotech. Co. (Xi’an, China) and was diluted to 28.80 mg/mL in H2O just before administration. Lamivudine was kindly provided by GlaxoSmithKline (GSK) China Co, and was diluted to 5 mg/mL in dH2O.
Sixty adult C57_TgN (HBVadr2.0) SMMU mice weighing between 18-24 g with an equal number of males and females, were provided by the Laboratory Animal Center and Department of Cell Biology of the Second Military Medical University. The mice were randomly divided into three groups with 20 mice in each group. Group A was the normal control, where the mice were administered physiological saline; group B was the positive control where the mice were administered lamivudine solution (100 mL/kg per day). Group C was the experimental group where the mice were administered physiological saline containing emodin and APS (57.59 and 287.95 mg/kg per day, respectively). The mice were treated daily for 3 wk followed by one week of recovery time without any treatment. The mice were then sacrificed. Blood was sampled from the abdominal aorta, centrifuged at 4°C, and plasma was stored at -20°C for assays; liver tissues were collected for histological examination.
The weight of each animal was measured just before the first drug administration and after sacrifice. The alteration in animal weight was calculated from these two values.
Hepatic tissues were fixed using 4% paraformaldehyde phosphate-buffered saline (PBS, pH 7.4) and embedded in paraffin wax. After deparaffinization, 5 μm sections were stained with hematoxylin and eosin (HE), and liver condition was classified according to the standard formulae published by the China Medical Association in 1995.
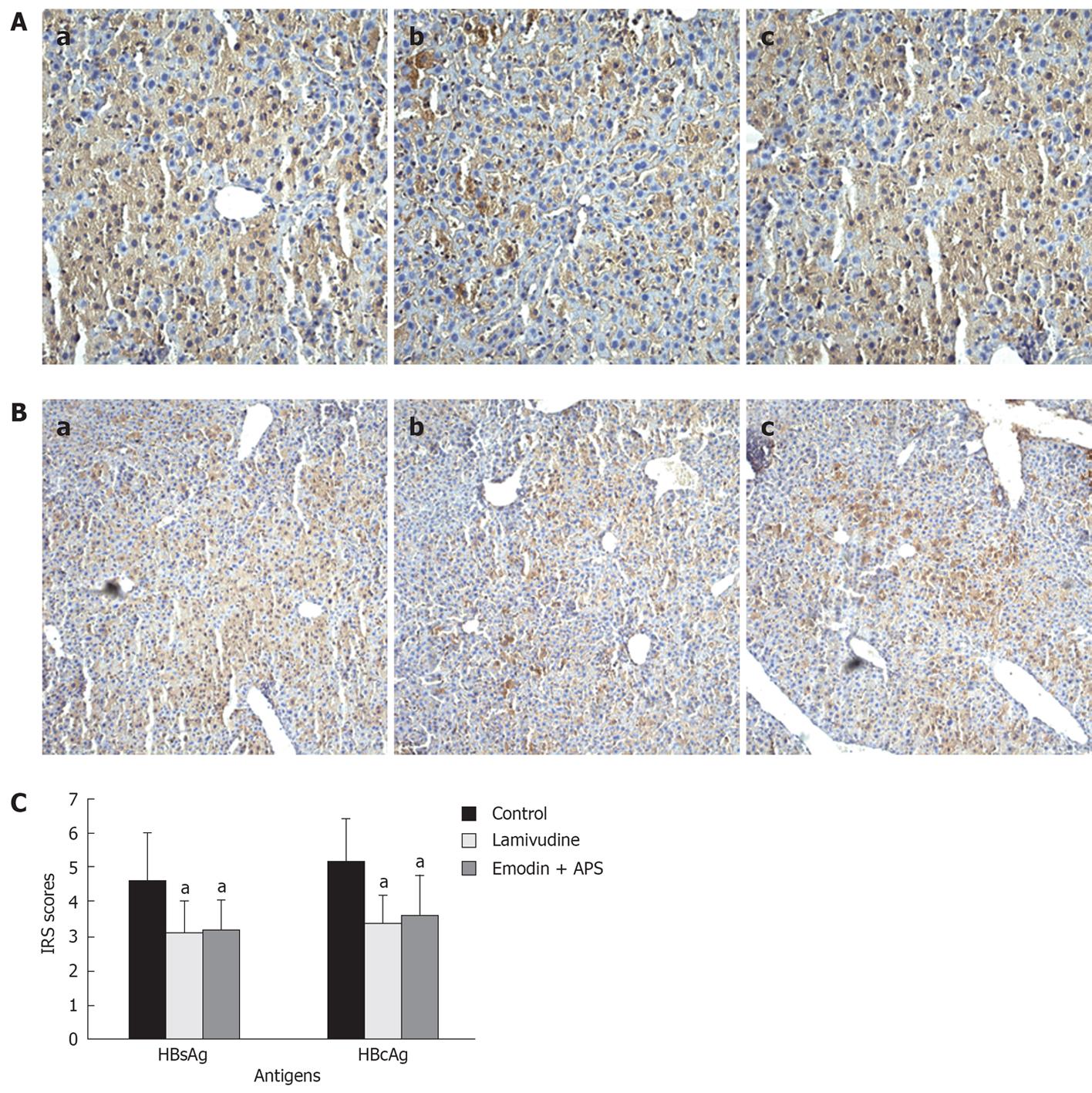
The surface antigen of the hepatitis-B-virus (HBsAg) and hepatitis B core antigen (HBcAg) were detected using immunohistochemical staining as previously described[13]. The stained slides were examined microscopically. Quantification of HBsAg and HBcAg positive cells was performed over several different areas of each section. The percentage of positive cells was evaluated by counting 100 cells (40 ×) in three consecutive tissue sections, and square scores were marked according to the following semi-quantitative criteria: 1: < 25% positive cells; 2: 26%-50% positive cells; 3: 51%-75% positive cells; 4: >75% positive cells. The intensity of the HBsAg staining within positive cells was evaluated, and the intensity scores were marked according to the following semi-quantitative criteria: 1: light yellow; 2: light brown; 3: chocolate brown. The final immunohistochemical reaction score (IRS) was calculated according to the formula IRS = positive staining scores × intensity scores.
HBsAg and HBeAg (hepatitis B e antigen) levels in serum were determined using Enzyme-Linked Immunosorbent Assay (ELISA) (Huatai Biotechnologies Co, Shanghai, China) according to the manufacturer’s instructions as previously described[14].
Blood was collected via the abdominal aorta after the mice were sacrificed. After centrifugation at 8000 r/min for 5 min, the serum was separated and stored at -20°C. HBV DNA content in serum was determined using real-time PCR (PG Biotech Co, Shenzhen, China) according to the manufacturer’s instructions.
Data are presented as mean ± SE. Data was checked for normal distribution and equal variance. One-way ANOVA test analysis was carried out by SPSS 11.0 software. Differences were considered significant at P values of less than 0.05.
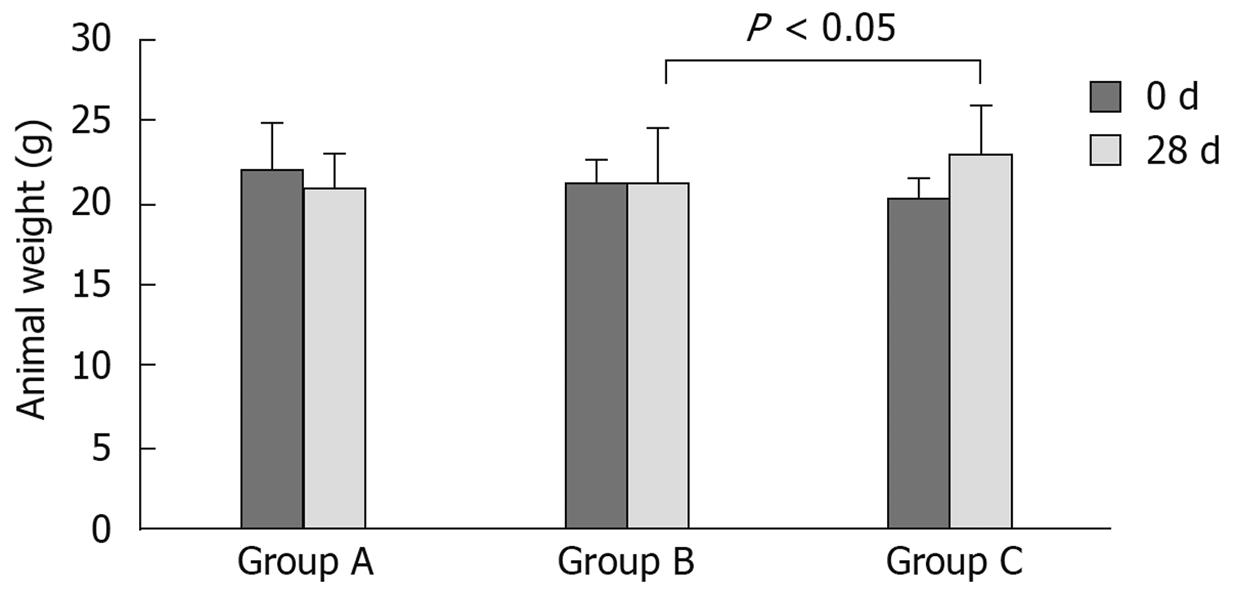
One mouse in group A died due to improper blood collection during the experiment at the third week. The other mice in all three groups were healthy during the experiments and their behaviour was normal. There was no significant weight alteration in the mice before or after the experiments. However, an increase in weight was observed in group C when compared with group B after 28 d of experimentation (Figure 1).
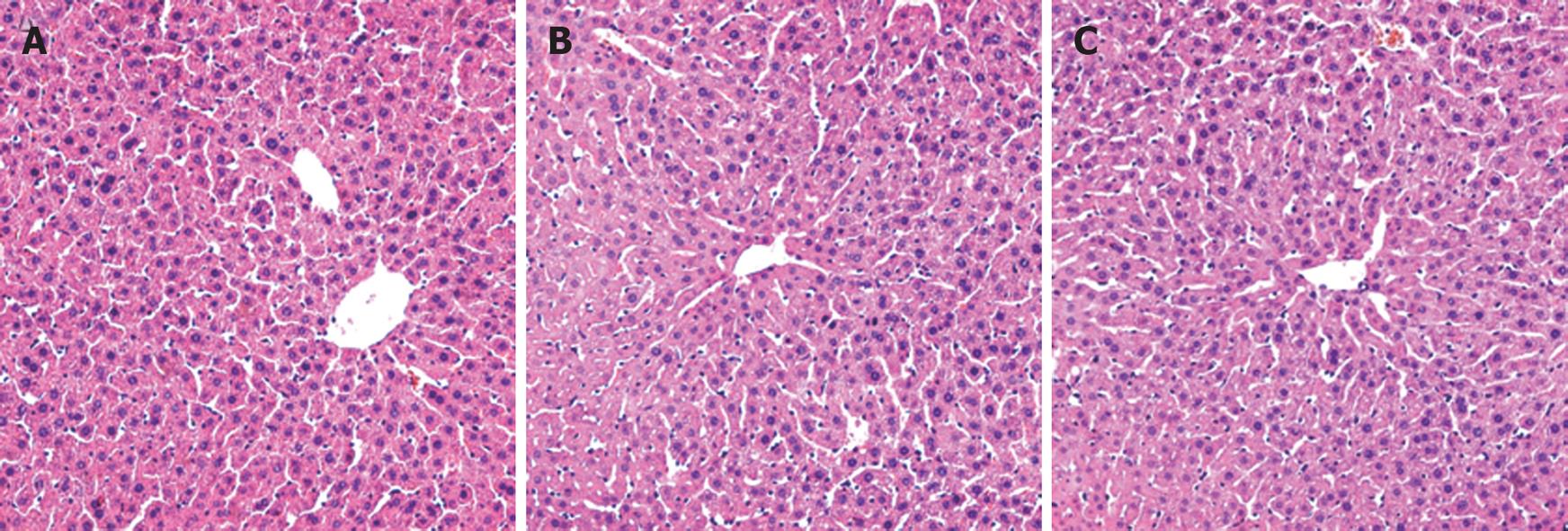
At the end of the experiment, there was no significant difference in the macro-appearance of the livers from mice in the three groups. The livers were pink, soft and their borders were even. Morphologically, the liver structure was intact, with few necrotic hepatocytes, limited inflammatory cell infiltration and fibrous tissue formation (Figure 2).
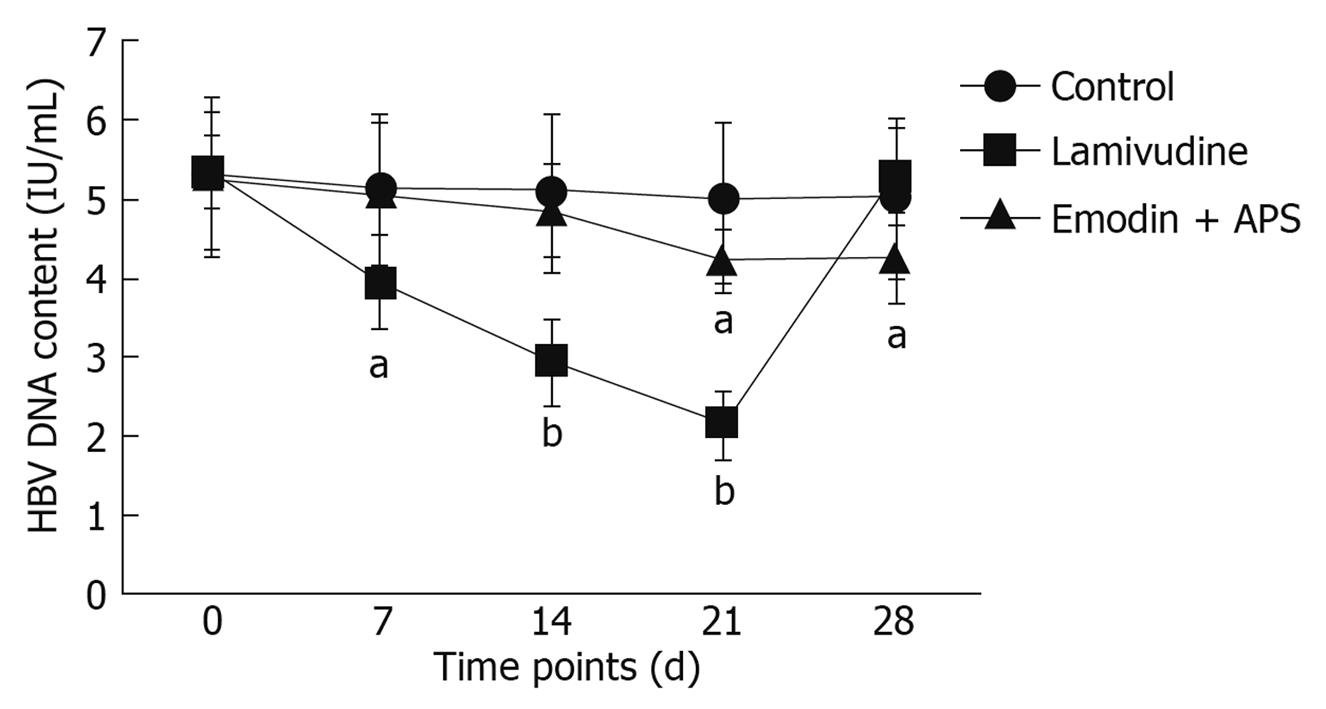
The use of real-time PCR showed that lamivudine significantly decreased serum HBV DNA content after one week of administration, and this inhibitory effect lasted up to 21 d. HBV DNA content increased to the original level when lamivudine administration was stopped for one week. However, emodin and APS did not decrease serum HBV DNA content after 7 or 14 d of administration (Figure 3), but reduced HBV DNA content after 21 d of administration, and this inhibitory effect lasted up to day 28, one week after administration ceased (Figure 3). There was no alteration in serum HBV DNA content in mice from the control group.
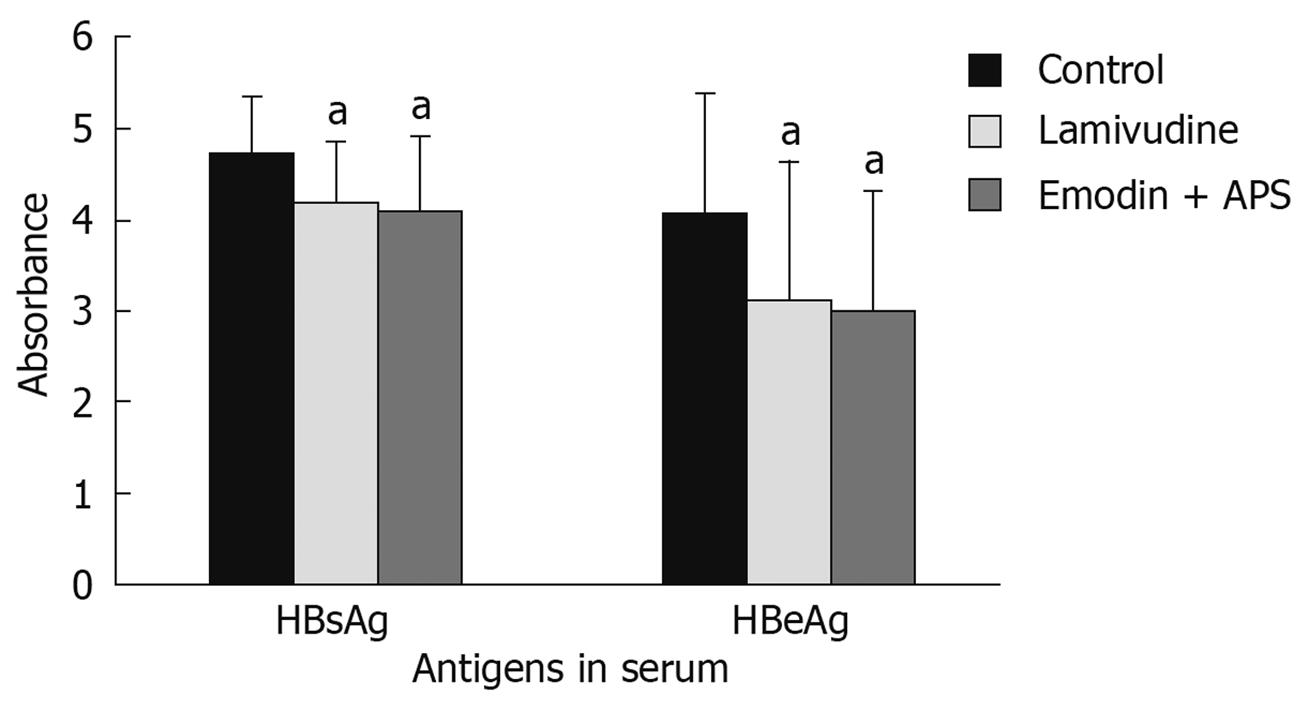
HBsAg and HBeAg levels in serum were determined using ELISA at day 28 of the experiment and showed that lamivudine and emodin + APS significantly decreased HBsAg and HBeAg levels in serum in the treated groups, compared with the control group (P < 0.05). However, there was no significant difference in HBsAg and HBeAg levels between the lamivudine group and the emodin + APS group (P > 0.05) (Figure 4).
HBsAg and HBcAg expression in mouse liver tissue was also investigated using immunohistochemistry. HBsAg and HBcAg positive staining was brown or dark brown, and mainly localised within the cytoplasm. Both HBsAg and HBcAg were distributed throughout the liver tissue, especially in the portal area and around the central vein area (Figure 5A and B). The positive ratios of HBsAg staining in hepatocytes were 80% (control group), 75% (lamivudine group) and 80% (emodin + APS group), respectively, and the positive ratios of HBcAg staining were 55% (control group), 45% (lamivudine group) and 50% (emodin + APS group), respectively. There was no significant difference in the positive ratios of HBsAg and HBcAg staining in hepatocytes between these three groups (P > 0.05). However, lamivudine and emodin + APS decreased the positive staining of HBsAg and HBcAg in hepatocytes after analysis according to the IRS (P < 0.05) (Figure 5C).
The transgenic mouse model used in this study was established by integration of HBV genome into mouse genome using a microinjection method, and it has been confirmed that HBV genes can be stably expressed, replicated and packaged in the mouse[15]. The present study showed that administration of lamivudine for 3 wk significantly reduced serum HBV DNA content. However, after ceasing administration for 1 wk, HBV DNA returned to the original level. Similar results were obtained following administration of lamivudine to hepatitis B patients, suggesting that this transgenic mouse model can mimic HBV infection in man.
Using real-time PCR, we found that emodin + APS significantly reduced serum HBV DNA content, although this effect was weaker than that observed with lamivudine. Interestingly, the reduction in serum HBV DNA content in the emodin + APS group lasted longer, compared with the lamivudine group. This suggests that emodin + APS had a weaker but long-lasting antiviral effect on HBV.
HBV antigens including HBsAg, HBcAg and HBeAg are important markers for hepatitis B development in patients[16]. Our results indicate that emodin + APS significantly decreased the expression of these antigens in both serum and liver tissue and lamivudine had a stronger inhibitory effect. The mechanism of this inhibition is still largely unknown. Previous studies have shown that emodin can inhibit several different viruses such as herpes simplex virus (type I and II), parainfluenza virus and Coxsackie virus[17-20]. More importantly, our previous study demonstrated that emodin can inhibit HBV replication in vitro[8]. Therefore it is not surprising that emodin can inhibit HBV in vivo. APS has been used for chronic liver disease in China for thousands of years, and is known to increase CD3+ and CD4+ T-cells and the ratio of CD4+/CD8+ T-cells in mice, suggesting an immunoregulative effect[10]. Therefore, the combination of emodin and APS not only resulted in inhibition of HBV replication, but also regulation of the immunologic system to eradicate HBV in vivo. This may explain the weaker and long-lasting effects of emodin + APS.
In conclusion, for the first time, we demonstrated that emodin and APS had a weak but long-lasting inhibitory effect on HBV replication in vivo, which may provide a new therapeutic option for hepatitis B infection.
The current treatment strategy for hepatitis B is to eradicate the replication and infection of hepatitis B virus (HBV) using anti-virus drugs such as interferon and nucleoside analogs such as lamivudine. However, the actual effects of these treatments are neither ideal nor adequate. The aim of this study was to evaluate the anti-viral effect of emodin plus Astragalus polysaccharide (APS) in HBV transgenic mice.
In this study, the authors found that the combination of emodin and ASP suppressed HBV replication in HBV transgenic mice, which might be a potential alternative treatment method for HBV patients.
This is the first time that the combination of emodin and ASP was found to suppress HBV replication in vivo, which is valuable for anti-HBV drug screening in the future.
This study provides valuable experimental evidence for future anti-HBV drug studies. It may provide an alternative strategy for therapeutic intervention in the treatment of patients with HBV.
Emodin (1,3,8-tri-hydroxy-6-methylanthraquinone) is an active component of herbal medicine derived from the genera Rheum, Polygonum, Rhamnus and Senna. APS is a bioactive chemical in Astragalus membranaceus (AM) which has been used in medicine for centuries in China.
This is a well performed experimental study showing for the first time a moderate effect on in-vivo HBV replication of two molecules derived from Chinese Traditional Medicine drugs.
Peer reviewer: Juan-Ramón Larrubia, PhD, Gastroenterology Unit and Liver Research Unit, Guadalajara University Hospital, Donante de Sangre s/n, 19002 Guadalajara, Spain
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP
| 1. | Zoulim F, Perrillo R. Hepatitis B: reflections on the current approach to antiviral therapy. J Hepatol. 2008;48 Suppl 1:S2-S19. [Cited in This Article: ] |
| 2. | But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008;14:1652-1656. [Cited in This Article: ] |
| 3. | Palumbo E. New drugs for chronic hepatitis B: a review. Am J Ther. 2008;15:167-172. [Cited in This Article: ] |
| 4. | Keeffe EB, Dieterich DT, Pawlotsky JM, Benhamou Y. Chronic hepatitis B: preventing, detecting, and managing viral resistance. Clin Gastroenterol Hepatol. 2008;6:268-274. [Cited in This Article: ] |
| 5. | Liu JP, McIntosh H, Lin H. Chinese medicinal herbs for chronic hepatitis B. Cochrane Database Syst Rev. 2001;CD001940. [Cited in This Article: ] |
| 6. | Dang SS, Zhang X, Jia XL, Cheng YA, Song P, Liu EQ, He Q, Li ZF. Protective effects of emodin and astragalus polysaccharides on chronic hepatic injury in rats. Chin Med J (Engl). 2008;121:1010-1014. [Cited in This Article: ] |
| 7. | Huang Q, Lu G, Shen HM, Chung MC, Ong CN. Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 2007;27:609-630. [Cited in This Article: ] |
| 8. | Shuangsuo D, Zhengguo Z, Yunru C, Xin Z, Baofeng W, Lichao Y, Yan'an C. Inhibition of the replication of hepatitis B virus in vitro by emodin. Med Sci Monit. 2006;12:BR302-BR306. [Cited in This Article: ] |
| 9. | Dang SS, Jia XL, Cheng YA, Chen YR, Liu EQ, Li ZF. Inhibitory effect of Huangqi Zhechong decoction on liver fibrosis in rat. World J Gastroenterol. 2004;10:2295-2298. [Cited in This Article: ] |
| 10. | Yuan C, Pan X, Gong Y, Xia A, Wu G, Tang J, Han X. Effects of Astragalus polysaccharides (APS) on the expression of immune response genes in head kidney, gill and spleen of the common carp, Cyprinus carpio L. Int Immunopharmacol. 2008;8:51-58. [Cited in This Article: ] |
| 11. | Li J, Bao YX, Zhu X, Liu J, Wang HP. [Immunoregulatory activity of polysaccharopeptide and Astragalus polysaccharides on EAC tumor-bearing mice]. Zhongguo Zhongyao Zazhi. 2008;33:924-927. [Cited in This Article: ] |
| 12. | He FL, Wang L, Zhang XK, Zeng JZ. [Emodin induces apoptosis of cancer cells and inhibits retinoid X receptor transcriptional activity]. Yaoxue Xuebao. 2008;43:350-355. [Cited in This Article: ] |
| 13. | Ding Y, Zhao L, Mei H, Zhang SL, Huang ZH, Duan YY, Ye P. Exploration of Emodin to treat alpha-naphthylisothiocyanate-induced cholestatic hepatitis via anti-inflammatory pathway. Eur J Pharmacol. 2008;590:377-386. [Cited in This Article: ] |
| 14. | Dang SS, Wang BF, Cheng YA, Song P, Liu ZG, Li ZF. Inhibitory effects of saikosaponin-d on CCl4-induced hepatic fibrogenesis in rats. World J Gastroenterol. 2007;13:557-563. [Cited in This Article: ] |
| 15. | Chen H, Weng L. [Comparison on efficacy in treating liver fibrosis of chronic hepatitis B between Astragalus Polygonum anti-fibrosis decoction and jinshuibao capsule]. Zhongguo Zhongxiyi Jiehe Zazhi. 2000;20:255-257. [Cited in This Article: ] |
| 16. | Xu R, Gan X, Fang Y, Zheng S, Dong Q. A simple, rapid, and sensitive integrated protein microarray for simultaneous detection of multiple antigens and antibodies of five human hepatitis viruses (HBV, HCV, HDV, HEV, and HGV). Anal Biochem. 2007;362:69-75. [Cited in This Article: ] |
| 17. | Hu YP, Hu WJ, Zheng WC, Li JX, Dai DS, Wang XM, Zhang SZ, Yu HY, Sun W, Hao GR. Establishment of transgenic mouse harboring hepatitis B virus (adr subtype) genomes. World J Gastroenterol. 2001;7:111-114. [Cited in This Article: ] |
| 18. | Li ZF, Zhang S, Lv GB, Huang Y, Zhang W, Ren S, Yang J, Dang SS. Changes in count and function of splenic lymphocytes from patients with portal hypertension. World J Gastroenterol. 2008;14:2377-2382. [Cited in This Article: ] |
| 19. | Baumert TF, Barth H, Blum HE. Genetic variants of hepatitis B virus and their clinical relevance. Minerva Gastroenterol Dietol. 2005;51:95-108. [Cited in This Article: ] |
| 20. | Hsiang CY, Ho TY. Emodin is a novel alkaline nuclease inhibitor that suppresses herpes simplex virus type 1 yields in cell cultures. Br J Pharmacol. 2008;155:227-235. [Cited in This Article: ] |
| 21. | Andersen DO, Weber ND, Wood SG, Hughes BG, Murray BK, North JA. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antiviral Res. 1991;16:185-196. [Cited in This Article: ] |
| 22. | Lin CW, Wu CF, Hsiao NW, Chang CY, Li SW, Wan L, Lin YJ, Lin WY. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. Int J Antimicrob Agents. 2008;32:355-359. [Cited in This Article: ] |
| 23. | Semple SJ, Pyke SM, Reynolds GD, Flower RL. In vitro antiviral activity of the anthraquinone chrysophanic acid against poliovirus. Antiviral Res. 2001;49:169-178. [Cited in This Article: ] |