Published online Oct 21, 2009. doi: 10.3748/wjg.15.4938
Revised: August 24, 2009
Accepted: August 31, 2009
Published online: October 21, 2009
AIM: To identify risk factors predictive of intensive care unit (ICU) mortality in patients with ventilator-related pancreatitis. The clinical outcomes of patients with ventilator-related pancreatitis were compared with those of patients with pancreatitis-related respiratory failure as well as controls.
METHODS: One hundred and forty-eight patients with respiratory failure requiring mechanical ventilation and concomitant acute pancreatitis were identified from a prospectively collected dataset of 9108 consecutive patients admitted with respiratory failure over a period of five years. Sixty patients met the criteria for ventilator-related pancreatitis, and 88 (control patients), for pancreatitis-related respiratory failure.
RESULTS: Mortality rate in ventilator-related pancreatitis was comparable to that in ICU patients without pancreatitis by case-control methodology (P = 0.544). Multivariate logistic regression analysis identified low PaO2/FiO2 (OR: 1.032, 95% CI: 1.006-1.059, P = 0.016) as an independent risk factor for mortality in patients with ventilator-related pancreatitis. The mortality rate in patients with ventilator-related pancreatitis was lower than that in patients with acute pancreatitis-related respiratory failure (P < 0.001).
CONCLUSION: We found that low PaO2/FiO2 was an independent clinical parameter predictive of ICU mortality in patients with ventilator-related pancreatitis.
- Citation: Tseng CC, Fang WF, Chung YH, Wang YH, Douglas IS, Lin MC. Clinical outcomes in patients with ICU-related pancreatitis. World J Gastroenterol 2009; 15(39): 4938-4944
- URL: https://www.wjgnet.com/1007-9327/full/v15/i39/4938.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4938
| Characteristics | ICU survivorsn = 35 (58.3) | ICU non-survivorsn = 25 (41.7) | P |
| Age | 60.77 ± 20.71 | 66.72 ± 15.73 | 0.232 |
| Male gender | 19 (54.3) | 17 (80) | 0.004 |
| Apache II score | 24.69 ± 6.09 | 29.88 ± 5.15 | 0.001 |
| Lowest PaO2/FiO2 | 283.86 ± 64.13 | 175.32 ± 72.72 | < 0.001 |
| Lipase level | 830.93 ± 511.05 | 1374.88 ± 1391.60 | 0.072 |
| Amylase level | 227.34 ± 207.35 | 374.96 ± 368.87 | 0.079 |
| ARDS | 5 (14.3) | 13 (52) | 0.002 |
| SIRS | 5 (17.2) | 13 (52) | 0.002 |
| Vasopressor | 12 (34.3) | 9 (36) | 0.891 |
| Acute renal failure | 11 (31.4) | 17 (68) | 0.005 |
| RRT | 2 (5.7) | 9 (36) | 0.003 |
| CHF | 26 (74.3) | 11 (44) | 0.017 |
| CVA | 12 (34.3) | 10 (40) | 0.651 |
| Liver cirrhosis | 8 (22.9) | 7 (28) | 0.650 |
| COPD | 12 (34.3) | 8 (32) | 0.853 |
| Neoplastic disease | 3 (8.6) | 5 (20) | 0.199 |
| Diabetes mellitus | 17 (48.6) | 12 (48) | 0.965 |
| Hypertension | 19 (54.3) | 15 (60) | 0.660 |
| Ventilator-related pancreatitis (n = 60) | Controls (n = 180) | Pancreatitis related respiratory failure (n = 88) | |
| Age | 63.3 ± 18.9 | 65 ± 15 | 59.3 ± 18.7 |
| Male gender | 39 (65) | 110 (61.1) | 54 (61.4) |
| Serum lipase level | 1057.3 ± 1005.7 | - | 8274.4 ± 17018.6a |
| Serum amylase level | 288.9 ± 292.4 | - | 884.6 ± 1247.0a |
| Apache II score | 26.9 ± 6.2 | 26 ± 6 | 32.0 ± 6.8a |
| Lowest PaO2/FiO2 | 238.6 ± 86.2 | 224.9 ± 60.2 | 168.8 ± 66.5a |
| ARDS | 18 (30) | 62 (34.4) | 60 (68.1)a |
| SIRS | 18 (30) | 66 (36.7) | 47 (53.4)a |
| Vasopressor | 21 (35) | 64 (35.6) | 49 (55.7)a |
| RRT | 11 (18.3) | 31 (17.2) | 24 (27.2) |
| Coexisting illness | |||
| Congestive heart failure | 37 (61.7) | 105 (58.3) | 32 (36.4)a |
| Cerebrovascular disease | 22 (36.7) | 67 (37.8) | 22 (25) |
| Acute renal failure | 28 (46.7) | 68 (37.8) | 36 (40.9) |
| Liver cirrhosis | 15 (25) | 48 (26.7) | 41 (46.6)a |
| Obstructive lung disease | 20 (33.3) | 71 (39.4) | 20 (22.7) |
| Neoplastic disease | 8 (13.3) | 28 (15.6) | 18 (20.5) |
| Diabetes mellitus | 29 (48.3) | 97 (53.9) | 39 (44.3) |
| Hypertension | 34 (56.7) | 106 (58.9) | 31 (35.2) |
| ICU mortality | 25 (41.7) | 67 (37.2) | 65 (73.8)a |
| Predictors | Odd Ratio (95% CI) | P |
| Univariate analysis | ||
| Apache II score | 0.856 (0.772-0.948) | 0.003 |
| Lowest PaO2/FiO2 | 1.021 (1.011-1.032) | < 0.001 |
| ARDS | 6.500 (1.901-22.229) | 0.003 |
| SIRS | 6.500 (1.901-22.229) | 0.003 |
| Acute renal failure | 4.636 (1.540-13.963) | 0.006 |
| RRT | 9.281 (1.792-48.057) | 0.008 |
| Male gender | 3.368 (1.031-11.010) | 0.044 |
| Amylase level | 0.998 (0.996-1.000) | 0.080 |
| Lipase level | 0.999 (0.999-1.000) | 0.070 |
| Multivariate analysis | ||
| Lowest PaO2/FiO2 | 1.032 (1.006-1.059) | 0.016 |
Mechanical ventilation is an important method for maintaining gas exchange in patients with respiratory failure until the underlying disorders are corrected. However, it is also associated with numerous organ-system disorders that can significantly affect the outcome of critically ill patients[1]. Possible mechanisms include injurious ventilatory strategies, high pressure with hypovolemic status, and sympathetic stimulation. Injurious ventilatory strategies may induce the release of proinflammatory cytokines. High intrathoracic pressure with hypovolemic status can cause splanchnic hypoperfusion[2]. Sympathetic stimulation can promote the release of catecholamines[3] and result in organ ischemia. All of these mechanisms can result in a systemic inflammatory response and multiple organ dysfunction. Acute pancreatitis may also be induced during mechanical ventilation via these mechanisms. Moreover, ischemia or organ hypoperfusion alone is considered an important common mechanism for the induction of pancreatic injury[4,5]. Among the several mechanisms suggested to explain how mechanical ventilation can cause acute pancreatitis, splanchnic hypoperfusion appears to be of particular importance[1].
Acute pancreatitis is an inflammatory process that usually occurs in a previously normal pancreas and is diagnosed mainly by acute abdominal pain associated with a concomitant increase in serum amylase and lipase concentrations[6,7]. Alcoholism and gallstones are established as the most frequent causes of acute pancreatitis. Other risk factors - drugs, hypertriglyceridemia, hypercalcemia, viral infection, and connective tissue disease - are also common[8-10]. Many studies have examined the clinical spectrum of lung injury associated with acute pancreatitis. Pulmonary dysfunction ranging from hypoxemia to acute respiratory distress syndrome (ARDS) is one of the most important systemic manifestations of severe acute pancreatitis[11]. Patients with severe pancreatitis are frequently associated with acute respiratory failure that subsequently develops into ARDS[12,13]. The development of ARDS is associated with a high mortality and is highly correlated with hypoxemic status[14].
Acute lung injury and respiratory failure are frequent and potentially fatal complications of acute pancreatitis[15,16]. Importantly, the institution of positive pressure mechanical ventilation can itself induce acute pancreatitis by exacerbating splanchnic hypoperfusion[1,17]. Recent studies have reported that elevated serum lipase levels are frequently encountered in critically ill patients, and hypoperfusion and inflammatory processes associated with multiple-organ failure appear to result in pancreatitis. However, the incidence, natural history, and outcomes of ventilator-related pancreatitis (VRP) have not been characterized in humans.
The object of this study was to determine the risk factors predictive of clinical outcomes and intensive care unit (ICU) mortality in patients with VRP. We also compared the outcomes of patients who developed pancreatitis during mechanical ventilation (VRP) in patients admitted with equal physiological scoring severity without pancreatitis and those patients with a primary diagnosis of acute pancreatitis complicated by respiratory failure (PRRF) requiring mechanical ventilation. We sought to determine whether patients with VRP had a poorer prognosis than patients with PRRF.
Nine thousand one hundred and eight patients with acute respiratory failure admitted to the medical intensive care units (ICUs) at Chang Gung Memorial Hospital (CGMH), a tertiary care hospital in Kaohsiung, were identified from a prospectively collected dataset over a period of five years. This study was approved by our hospital’s institutional review board and was also in compliance with the Helsinki Declaration.
In this study, acute pancreatitis was diagnosed in patients meeting at least two of the following three criteria as previously described[18]: (1) acute abdominal pain and tenderness in the upper abdomen; (2) elevated levels of pancreatic enzyme (serum lipase and/or amylase) in the blood, urine, or ascitic fluid; and (3) abnormal imaging findings for the pancreas associated with acute pancreatitis. A search of medical records using a combination of the two diagnostic categories of acute respiratory failure and acute pancreatitis identified 163 patients. Among these, 75 patients were diagnosed with VRP. Fifteen patients with a history of alcoholism (alcohol consumption > 60 g daily for 10 years), gallstones (by hepatic sonography), hypertriglycemia (triglyceride > 500 U/L within 1 year), or the use of drugs that have been well documented to cause pancreatitis (such as propofol) were excluded to avoid confounding. We excluded other well-known etiologies of pancreatitis except for mechanical ventilation. Thus, 60 patients met the criteria for VRP and 88 patients were classified as having PRRF (Figure 1A).
In the patients with PRRF, the principal cause of acute respiratory failure requiring intubation was acute respiratory distress syndrome (ARDS), as a complication of acute pancreatitis.
Due to the wishes of the patients’ families and the medical culture in Taiwan, most patients were not receiving sedation. All patients with VRP complained of abdominal pain. The serum amylase and lipase levels were examined one day after the patients complained of abdominal pain following mechanical ventilation. Acute pancreatitis was confirmed by an elevated serum lipase level, three times the upper limit (> 570 U/L) with or without elevated serum amylase. Confirmatory imaging studies were not routinely used in this study. Among the VRP patients, only 28 (46.7%) underwent an imaging study for the diagnosis of pancreatitis. Fourteen patients (23.3%) underwent imaging studies but had indeterminate findings due to patient agitation or excessive bowel or respiratory distress. Eighteen patients (30%) underwent no imaging studies due to their critical condition.
Patient demographic characteristics, comorbidities, presence of ARDS, lowest PaO2/FiO2 ratio, acute renal failure, systemic inflammatory response syndrome (SIRS), vasopressor use, renal replacement therapy, serum amylase, lipase level, and ICU mortality were recorded. ARDS was diagnosed according to the criteria of the American-European Consensus Conference Committee[19]. Acute renal failure was defined as normal renal function prior to admission that was impaired along with disease progression occurring before or after acute respiratory failure, and defined with a cutoff value of creatinine ≥ 1.5 mg/dL. SIRS was defined as a temperature ≥ 38°C or ≤ 36°C, heart rate ≥ 90 beats/min, respiratory rate ≥ 20/min, and a white blood cell (WBC) count ≥ 12 000/mL or ≤ 4000/mL or > 10% immature neutrophils without a definite infection source and we noted this sign after pancreatitis had been diagnosed.
For the outcome analysis, each VRP subject was matched with 3 subjects from the cohort of 9108 patients with respiratory failure without pancreatitis by an investigator blinded to the outcomes. Matching was based on similarities in severity of illness as manifested by the Acute Physiology and Chronic Health Evaluation (APACHE) II score (± 2 points) at admission to ICU within 24 h, diagnostic category, status of lowest PaO2/FiO2, and diagnosis of ARDS. Among the matched cases,
all patients had no abdominal pain described in their chart during admission to the ICU or if abdominal pain was noted, there was no elevation in serum amylase and lipase level.
Continuous variables were summarized using means and standard deviations (mean ± SD), while categorical variables were summarized using counts and percentages.
Index cases and controls were compared using the Student’s t-test or the Mann-Whitney U test, as appropriate for continuous variables, or the χ2 test for categorical variables. Categorical variables were compared with the Pearson χ2 test and, if appropriate, Fisher’s exact test.
In patients with VRP, clinical parameters thought to be contributory to ICU mortality were analyzed by univariate regression analysis. Factors found to be statistically significant (P < 0.1) by univariate regression analysis were then retained for a multivariate logistic regression model to determine whether they remained predictive for ICU mortality.
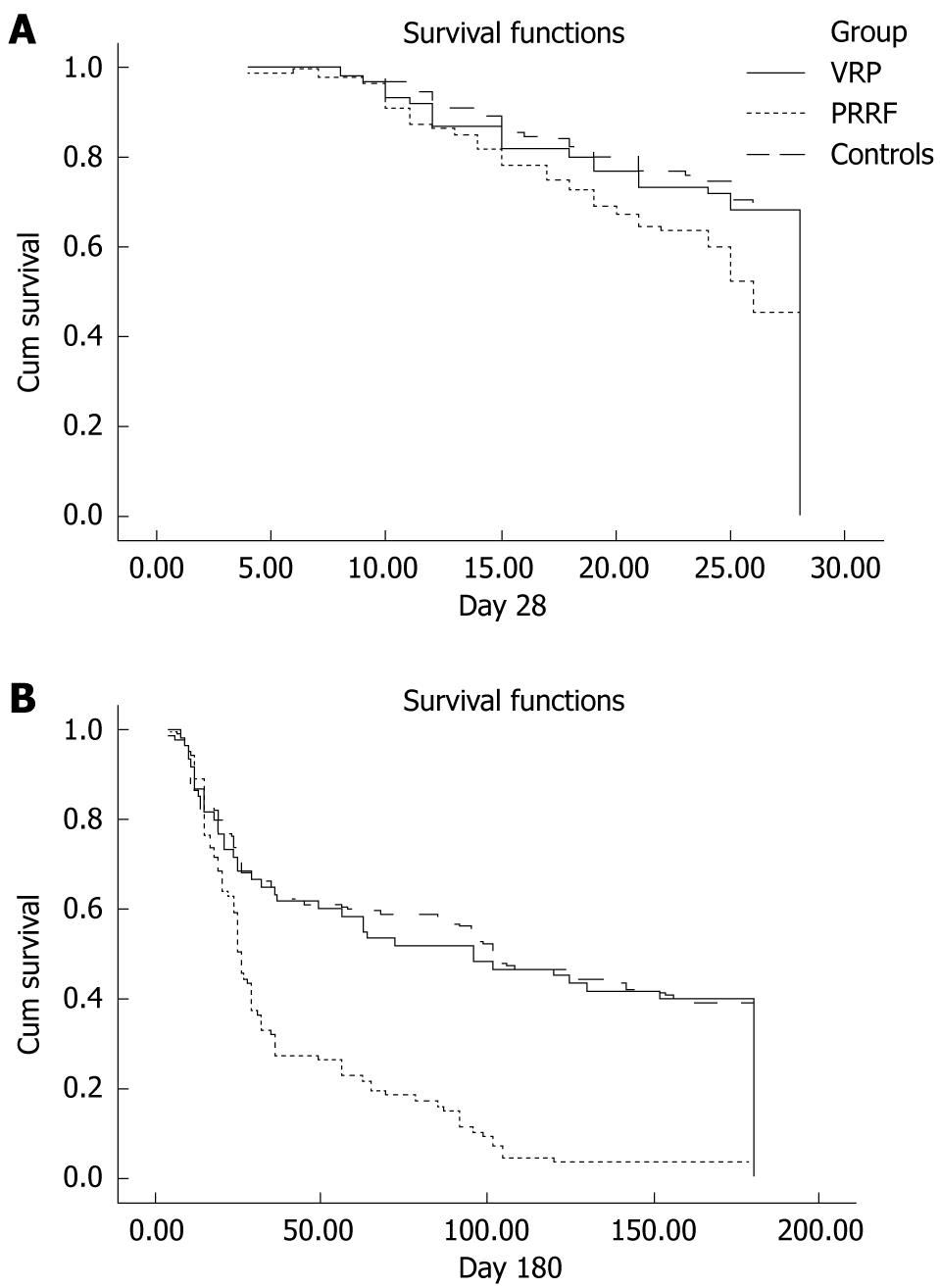
To assess the outcome between groups, survival time up to 6 mo post-admission was recorded, and Kaplan-Meier survival curves were constructed; comparison between the groups was by the log-rank test.
All testing was two-tailed and P < 0.05 were considered to be statistically significant.
Among 60 patients with VRP, 35 patients (58.3%) were ICU survivors and 25 were ICU non-survivors (41.7%). The mean number of days to pancreatitis after mechanical ventilation in patients with VRP was 11.90 ± 7.22 d (range from 3 to 31 d). Of the 60 patients with VRP, amylase and lipase levels normalized and abdominal pain subsided in 51. The other 9 patients died before further blood sampling for amylase and lipase could be performed. The mean number of days to recovery from pancreatitis (duration from the onset of abdominal pain to the date of normalized amylase and lipase levels) was 7.08 ± 3.48 d (range from 2 to 15 d). Among the VRP patients, only 28 (46.7%) underwent an imaging study for the diagnosis of pancreatitis. Of those patients, all underwent an abdominal echo study, and 3 received both an abdominal echo and a CT study. After reviewing the images, we found that all cases showed mild-to-moderate swelling of the pancreas, and there were no cases of pancreatic necrosis or fluid retention in the abdominal cavity. High APACHE II score (P = 0.001), low PaO2/FiO2 level (P < 0.001), ARDS status (P = 0.002), SIRS occurrence (P = 0.002), acute renal failure status (P = 0.005), requirement for renal replacement therapy (P = 0.003), and male gender (P = 0.040) were significantly more frequent in ICU non-survivors than in survivors. High serum amylase (P = 0.079) and lipase level (P = 0.072) were not correlated with a poor prognosis. Interestingly, an underlying history of congestive heart failure (P = 0.017) was more frequent in survivors (Table 1). On univariate regression analysis, acute renal failure (P = 0.006), APACHE II score (P = 0.003), lowest PaO2/FiO2 (P < 0.001), ARDS status (P = 0.003), SIRS occurrence (P = 0.003), renal replacement therapy (P = 0.008), and male gender (P = 0.044) were predictors of death (Table 2). On multivariate analysis, only the lowest PaO2/FiO2 (OR: 1.032, 95% CI: 1.006-1.059, P = 0.016) predicted death and was, therefore, an independent risk factor for mortality in patients with VRP (Table 3).
Table 2 details the baseline characteristics of the 60 cases with VRP, 88 cases with PRRF, and 180 controls without pancreatitis. There were no statistically significant differences between the cases with VRP and the controls in any of the analyzed parameters. Between the patients with VRP and PRRF, there were no statistically significant differences in age, gender, or requirement for renal replacement therapy. More subjects with VRP had congestive heart failure and liver cirrhosis than those with PRRF. PRRF patients were significantly sicker, with higher serum lipase (P = 0.001), serum amylase levels (P < 0.001), and APACHE II scores (P < 0.001), as well as ARDS status (P < 0.001), SIRS occurrence (P < 0.001), and vasopressor requirements (P = 0.019). PRRF was also associated with higher mortality than VRP (P < 0.001). However, there was no statistical difference in mortality rates between VRP patients and the control group (P = 0.544).
Short- and long-term outcomes were significantly better in patients with VRP. Patients with VRP were more likely to be alive at day 28 than patients with PRRF (68.3% vs 45.5%, P = 0.007), to be discharged from ICU (58.3% vs 26.2%, P < 0.001), and to have survived during the 6-mo follow-up period (40% vs 3.4%, P < 0.001). However, the 28 d survival (P = 0.567), ICU survival (P = 0.544), and 6 mo survival (P = 0.498) rates were comparable between patients with VRP and controls. Survival curves were constructed using the Kaplan-Meier method to explain the survival differences between the groups (Figure 1).
This retrospective analysis yielded three main findings. First, patients with respiratory failure needing ventilator support may develop acute pancreatitis. When patients were diagnosed with VRP, clinical parameters such as a high APACHE II score, low PaO2/FiO2, SIRS occurrence, ARDS status, acute renal failure, renal replacement therapy, and male gender predicted mortality. Multivariate logistic regression showed that low PaO2/FiO2 was an independent risk factor for mortality. Secondly, the short- and long-term outcomes in patients with VRP were not worse than those in non-pancreatitis patients with an equal severity score, and were better than those in patients with PRRF, although both groups had respiratory failure and acute pancreatitis. Thirdly, patients with PRRF had higher APACHE II scores, more frequent ARDS, lower PaO2/FiO2 levels, greater frequency of SIRS, and pressor-requiring shock, as well as higher serum lipase and amylase levels than VRP patients.
Using these diagnostic criteria, the incidence of acute pancreatitis in patients requiring mechanical ventilation was lower than we had anticipated. A possible reason is that intubated and sedated patients are frequently unable to indicate and localize serious abdominal pain and this may be overlooked by clinical staff. We speculate that the actual incidence of acute pancreatitis in the ICU is higher than appreciated and this entity would be more readily detected if comprehensive physical examination was conducted and laboratory testing initiated in patients with abdominal pain.
Numerous potential mechanisms could account for VRP. Mechanical ventilation, frequently with high levels of positive end-expiratory pressure (PEEP), can increase intrathoracic pressure and result in decreased venous return[20]. Reduced preload in the return could result in decreased cardiac output and hypotension. Splanchnic blood flow is decreased in these settings in parallel with PEEP-induced reductions in cardiac output[21]. Mechanical ventilation with PEEP is also associated with increased renin-angiotensin-aldosterone activity and elevated catecholamine levels because of sympathetic activation[3,22]. Elevation of serum catecholamines can contribute to splanchnic hypoperfusion due to vasoconstriction and redistribution of blood away from the splanchnic vascular bed[23]. The adverse effects of mechanical ventilation under injurious ventilatory strategies suggest an important role of cytokines in the pathogenesis of multiple organ complications. Pro-inflammatory cytokines can affect many organs and induce a variety of physiological and biochemical responses to critical illness[24]. They can lead to a series of intracellular signaling events via highly specific cell surface receptors that typically result in elaboration of other cytokines within the target cell. If these processes are not attenuated, excessive amplification of the inflammatory cascade and overproduction of pro-inflammatory mediators can occur with the uncontrolled activation of the immune system and cause target organ damage. All of these mechanisms may result in organ ischemia and failure. If ischemic injury to the pancreas or pancreatic inflammation related to systemic inflammatory mediators occurs, it could account for the increased serum lipase and amylase levels observed in critically ill patients[4,5]. Among several mechanisms suggested to explain how mechanical ventilation unfavorably results in acute pancreatitis, splanchnic hypoperfusion appears to be particularly important[1].
A mortality comparison demonstrated a lower survival rate in PRRF than in VRP. Acute lung injury and ARDS, which have high mortality rates, are the most common manifestations of extra-abdominal organ dysfunction in patients with severe acute pancreatitis. The pathophysiology of ARDS is described as increased pulmonary vasculature leaking protein-rich transudate into the alveolar space and decreased lung compliance clinically manifested as refractory hypoxemia, and radiologically as diffuse infiltration in the lungs. In the pathogenesis of systemic complications of pancreatitis, the role of active enzymes in circulation, the liberation of proinflammatory cytokines, decreased normal defense mechanisms, and the increased production of nitric oxide have been studied[25,26]. The mortality and severity of the disease appear to be influenced by events occurring subsequent to the pancreatic injury as a result of the release of cytokines and other mediators. Hypoxemia is the most common sign presenting in patients with respiratory insufficiency resulting from severe acute pancreatitis; however, its presentation was not related to the development of atelectasis, pleural effusion, or pulmonary consolidation during the course of the disease. Severe hypoxemia is also a factor that predicts a poor prognosis. A recent study has shown that a baseline hypoxemia of less than 60 mmHg was a significant risk factor for pulmonary consolidation and ARDS, and can be used as a marker of poor outcome[14]. Sustained systemic inflammatory states with multiple organ failure are frequently encountered and have a high attributable mortality rate[27]. Progression to a shock state requiring vasopressor use or acute renal failure requiring renal replacement therapy is particularly ominous[28,29].
Our study demonstrated that a high APACHE II score, low PaO2/FiO2, ARDS status, presence of SIRS criteria, acute renal failure, and the need for renal replacement therapy were predictors of outcome in patients with a diagnosis of ventilator-related pancreatitis; only a low PaO2/FiO2 level was an independent predictive factor as determined by multivariate logistic regression analysis. Thus, as mentioned above, hypoxemia or disease progression to ARDS are poor signs not only in PRRF patients but also in VRP patients.
To date, no specific management strategy has been proposed for acute pancreatitis with multiple organ failure other than intensive supportive treatment. The evidence available indicates that patients with severe acute pancreatitis do not benefit from therapy with available antisecretory drugs or protease inhibitors. Supportive therapy, such as vigorous hydration, analgesia, correction of electrolytes and glycemic disorders, and pharmacological or mechanical support targeted at specific organs, are still the mainstay of therapy[30]. However, severe acute pancreatitis is still characterized by rapidly progressive multiple organ failure and high mortality, and both surgical and conservative therapies yield poor outcomes[31]. Thus, most emphasis is placed on preventing the progression to multiple organ failure[32,33].
Congestive heart failure was more common in VRP patients than in PRRF patients and was a good prognostic predictor for ICU mortality in patients with VRP. Acute pulmonary edema related to congestive heart failure was a frequent cause of respiratory failure in VRP patients; in contrast, the most common indication for intubation in patients with PRRF was ARDS. Heart failure-related pulmonary edema can often be reversed with diuretics and renal replacement therapy if there is concomitant renal failure. In contrast, there are no effective pharmacologic treatments for non-cardiogenic pulmonary edema associated with ARDS other than the treatment of the underlying disease. Conservative treatments with protective ventilatory strategies and fluid management may help to improve the hypoxemic status of patients with ARDS, however, these strategies had no benefit on mortality[34,35]. This may explain, in part, why VRP patients in our study had better outcomes than PRRF patients and VRP patients with congestive heart failure had a better prognosis than those without congestive heart failure.
There are some limitations in this study. First, as this was a retrospective study, not every patient in the ICUs with abdominal pain had blood sampling for amylase and lipase levels. This ascertainment bias may have resulted in an overestimation of the incidence of VRP. Secondly, the Ranson score is widely used in predicting outcomes from severe acute pancreatitis[36-38]. However, Ranson’s criteria were not systematically collected in our cohort. Thus, we did not include Ranson’s criteria as part of our analysis, therefore potentially limiting external validity. Third, although radiological imaging, particularly computed tomography, is valuable for diagnosis, risk stratification, and outcome prediction of acute pancreatitis[36,39], not every patient had an imaging study to confirm acute pancreatitis in our study.
In conclusion, our findings suggested that low PaO2/FiO2 was an independent clinical parameter predictive of ICU mortality in patients with VRP. We also demonstrated that VRP was not associated with a higher mortality rate when compared with ICU patients with comparable disease severity but without pancreatitis and was associated with better outcomes than PRRF.
Mechanical ventilation is an important method in rescuing patients with respiratory failure, but it is also associated with numerous organ-system disorders. Acute pancreatitis may also be caused by mechanical ventilation, for which splanchnic hypoperfusion is considered the most important mechanism. However, the risk factors predictive of clinical outcomes and intensive care unit (ICU) mortality in patients with ventilator-related pancreatitis are still unclear. Thus, we conducted this study to clarify the clinical outcomes in patients with ventilator-related pancreatitis.
Patients with severe acute pancreatitis may develop acute respiratory failure resulting in poor clinical outcomes. Such a concept is well documented. However, the notion of ventilator-related pancreatitis is not well understood, even though pancreatitis truly occurs after mechanical ventilation. We conducted this study to illustrate the different clinical outcome in patients with ventilator-related pancreatitis, pancreatitis related respiratory failure and equal severity ICU patients without pancreatitis.
The authors clarify the risk factors for predicting mortality in patients with ventilator-related pancreatitis. Short- and long-term outcomes in patients with ventilator-related pancreatitis are also illustrated by a comparison with patients who had pancreatitis-related respiratory failure and ICU patients admitted with equal physiological severity scoring without pancreatitis.
By understanding the nature of ventilator-related pancreatitis, the authors found that low PaO2/FiO2 was an independent clinical parameter predictive of ICU mortality in patients with ventilator-related pancreatitis. Despite the simultaneous diagnosis of acute respiratory failure and acute pancreatitis, patients with ventilator-related pancreatitis had better outcomes than patients with pancreatitis-related respiratory failure.
Ventilator-related pancreatitis is a disease in which pancreatitis occurs after mechanical ventilation by numerous mechanisms. Possible mechanisms include injurious ventilatory strategies, high pressure with hypovolemic status, and sympathetic stimulation mechanisms resulting in conditions such as pancreatic ischemia or pancreatic hypoperfusion are thought to be the cause of pancreatic injury.
This interesting paper looks at a group of patients with a condition about which we know very little. They have coined the terms “ICU-related pancreatitis” and “ventilator-related pancreatitis”, which are not terms currently in use but are reasonable. The findings of this study make sense.
Peer reviewer: Chris E Forsmark, Professor, Division of Gastroenterology and Hepatology, University of Florida, FL, 32610-0214, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Lin YP
| 1. | Mutlu GM, Mutlu EA, Factor P. GI complications in patients receiving mechanical ventilation. Chest. 2001;119:1222-1241. [Cited in This Article: ] |
| 2. | Luecke T, Pelosi P, Quintel M. [Haemodynamic effects of mechanical ventilation]. Anaesthesist. 2007;56:1242-1251. [Cited in This Article: ] |
| 3. | Selldén H, Sjövall H, Ricksten SE, Ricksten SE. Sympathetic nerve activity and central haemodynamics during mechanical ventilation with positive end-expiratory pressure in rats. Acta Physiol Scand. 1986;127:51-60. [Cited in This Article: ] |
| 4. | Manjuck J, Zein J, Carpati C, Astiz M. Clinical significance of increased lipase levels on admission to the ICU. Chest. 2005;127:246-250. [Cited in This Article: ] |
| 5. | Serrano N. Increased lipase plasma levels in ICU patients: when are they critical? Chest. 2005;127:7-10. [Cited in This Article: ] |
| 6. | Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586-590. [Cited in This Article: ] |
| 7. | Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198-1210. [Cited in This Article: ] |
| 8. | Lee JK, Enns R. Review of idiopathic pancreatitis. World J Gastroenterol. 2007;13:6296-6313. [Cited in This Article: ] |
| 9. | Acosta JM, Ledesma CL. Gallstone migration as a cause of acute pancreatitis. N Engl J Med. 1974;290:484-487. [Cited in This Article: ] |
| 10. | Steer ML. Classification and pathogenesis of pancreatitis. Surg Clin North Am. 1989;69:467-480. [Cited in This Article: ] |
| 11. | Napolitano LM. Pulmonary consequences of acute pancreatitis: critical role of the neutrophil. Crit Care Med. 2002;30:2158-2159. [Cited in This Article: ] |
| 12. | Ranson JH, Roses DF, Fink SD. Early respiratory insufficiency in acute pancreatitis. Ann Surg. 1973;178:75-79. [Cited in This Article: ] |
| 13. | Pastor CM, Matthay MA, Frossard JL. Pancreatitis-associated acute lung injury: new insights. Chest. 2003;124:2341-2351. [Cited in This Article: ] |
| 14. | Polyzogopoulou E, Bikas C, Danikas D, Koutras A, Kalfarentzos F, Gogos CA. Baseline hypoxemia as a prognostic marker for pulmonary complications and outcome in patients with acute pancreatitis. Dig Dis Sci. 2004;49:150-154. [Cited in This Article: ] |
| 15. | Raghu MG, Wig JD, Kochhar R, Gupta D, Gupta R, Yadav TD, Agarwal R, Kudari AK, Doley RP, Javed A. Lung complications in acute pancreatitis. JOP. 2007;8:177-185. [Cited in This Article: ] |
| 16. | Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340-1344. [Cited in This Article: ] |
| 17. | Kahle M, Lippert J, Willemer S, Pabst W, Martin P. Effects of positive end-expiratory pressure (PEEP) ventilation on the exocrine pancreas in minipigs. Res Exp Med (Berl). 1991;191:309-325. [Cited in This Article: ] |
| 18. | Koizumi M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Sekimoto M, Hirota M, Kimura Y, Takeda K. JPN Guidelines for the management of acute pancreatitis: diagnostic criteria for acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:25-32. [Cited in This Article: ] |
| 19. | Vitale GC, Larson GM, Davidson PR, Bouwman DL, Weaver DW. Analysis of hyperamylasemia in patients with severe head injury. J Surg Res. 1987;43:226-233. [Cited in This Article: ] |
| 20. | Steingrub JS, Tidswell M, Higgins TL. Hemodynamic consequences of heart-lung interactions. J Intensive Care Med. 2003;18:92-99. [Cited in This Article: ] |
| 21. | Love R, Choe E, Lippton H, Flint L, Steinberg S. Positive end-expiratory pressure decreases mesenteric blood flow despite normalization of cardiac output. J Trauma. 1995;39:195-199. [Cited in This Article: ] |
| 22. | Tanaka S, Sagawa S, Miki K, Claybaugh JR, Shiraki K. Changes in muscle sympathetic nerve activity and renal function during positive-pressure breathing in humans. Am J Physiol. 1994;266:R1220-R1228. [Cited in This Article: ] |
| 23. | Aneman A, Pontén J, Fändriks L, Eisenhofer G, Friberg P, Biber B. Hemodynamic, sympathetic and angiotensin II responses to PEEP ventilation before and during administration of isoflurane. Acta Anaesthesiol Scand. 1997;41:41-48. [Cited in This Article: ] |
| 24. | Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol. 1998;64:135-146. [Cited in This Article: ] |
| 25. | Leindler L, Morschl E, László F, Mándi Y, Takács T, Jármai K, Farkas G. Importance of cytokines, nitric oxide, and apoptosis in the pathological process of necrotizing pancreatitis in rats. Pancreas. 2004;29:157-161. [Cited in This Article: ] |
| 26. | Browne GW, Pitchumoni CS. Pathophysiology of pulmonary complications of acute pancreatitis. World J Gastroenterol. 2006;12:7087-7096. [Cited in This Article: ] |
| 27. | Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132-144. [Cited in This Article: ] |
| 28. | Tran DD, Oe PL, de Fijter CW, van der Meulen J, Cuesta MA. Acute renal failure in patients with acute pancreatitis: prevalence, risk factors, and outcome. Nephrol Dial Transplant. 1993;8:1079-1084. [Cited in This Article: ] |
| 29. | García-Fernández N, Lavilla FJ, Rocha E, Purroy A. Assessment of haemostatic risk factors in patients with acute renal failure associated with severe systemic inflammatory response syndrome. Development of a prognostic index. Nephron. 2002;92:97-104. [Cited in This Article: ] |
| 30. | Wilmer A. ICU management of severe acute pancreatitis. Eur J Intern Med. 2004;15:274-280. [Cited in This Article: ] |
| 31. | Gerlach H. Risk management in patients with severe acute pancreatitis. Crit Care. 2004;8:430-432. [Cited in This Article: ] |
| 32. | Agarwal N, Pitchumoni CS. Acute pancreatitis: a multisystem disease. Gastroenterologist. 1993;1:115-128. [Cited in This Article: ] |
| 33. | Tao HQ, Zhang JX, Zou SC. Clinical characteristics and management of patients with early acute severe pancreatitis: experience from a medical center in China. World J Gastroenterol. 2004;10:919-921. [Cited in This Article: ] |
| 34. | Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564-2575. [Cited in This Article: ] |
| 35. | Acute lung injury and the acute respiratory distress syndrome in Ireland: a prospective audit of epidemiology and management. Crit Care. 2008;12:R30. [Cited in This Article: ] |
| 36. | Hagiwara A, Miyauchi H, Shimazaki S. Predictors of vascular and gastrointestinal complications in severe acute pancreatitis. Pancreatology. 2008;8:211-218. [Cited in This Article: ] |
| 37. | Kaya E, Dervisoglu A, Polat C. Evaluation of diagnostic findings and scoring systems in outcome prediction in acute pancreatitis. World J Gastroenterol. 2007;13:3090-3094. [Cited in This Article: ] |
| 38. | Williams M, Simms HH. Prognostic usefulness of scoring systems in critically ill patients with severe acute pancreatitis. Crit Care Med. 1999;27:901-907. [Cited in This Article: ] |