Published online Sep 7, 2009. doi: 10.3748/wjg.15.4196
Revised: July 13, 2009
Accepted: July 20, 2009
Published online: September 7, 2009
Treatment of hepatitis C, even when absolutely necessary, is almost impossible when interferon cannot be administered for any reason. We report a 65-year-old patient with chronic hepatitis C virus (HCV) infection and fibrosis, who was unable to receive interferon because of systemic hypersensitivity. The patient was desensitized successfully through gradual incremental exposure to interferon, and HCV infection was eradicated after a complete course of treatment, with no further allergic reactions. This case report that describes successful eradication of hepatitis C in a patient with advanced liver disease after desensitization to interferon revealed that desensitization may not necessarily damage the therapeutic efficacy of the drug.
- Citation: Taghavi SA, Eshraghian A. Successful interferon desensitization in a patient with chronic hepatitis C infection. World J Gastroenterol 2009; 15(33): 4196-4198
- URL: https://www.wjgnet.com/1007-9327/full/v15/i33/4196.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4196
| Dose number | Time from first dose (h) | Strength | Volume | Drug dosage (IU) |
| 1 | 0 | 1/100000 | 0.1 | 3 |
| 2 | 1 | 1/100000 | 0.1 | 3 |
| 3 | 2 | 1/10000 | 0.1 | 30 |
| 4 | 3 | 1/10000 | 0.1 | 30 |
| 5 | 4 | 1/1000 | 0.1 | 300 |
| 6 | 5 | 1/1000 | 0.1 | 300 |
| 7 | 6 | 1/100 | 0.1 | 3000 |
| 8 | 7 | 1/100 | 0.1 | 3000 |
| 9 | 8 | 1/100 | 0.1 | 3000 |
| 10 | 9 | 1/100 | 0.1 | 3000 |
| 11 | 10 | 1/100 | 0.1 | 3000 |
| 12 | 11 | 1/100 | 0.1 | 3000 |
| 13 | 12 | 1/100 | 0.1 | 3000 |
| 14 | 13 | 1/100 | 0.1 | 3000 |
| Cumulative dose | 24 666 |
| Dose number | Time from first dose (h) | Strength | Volume | Drug dosage (IU) |
| 1 | 0 | 1/100 | 0.1 | 3000 |
| 2 | 1 | 1/100 | 0.1 | 3000 |
| 3 | 2 | 1/10 | 0.1 | 30 000 |
| 4 | 3 | 1/10 | 0.1 | 30 000 |
| 5 | 4 | 1/1 | 0.1 | 300 000 |
| 6 | 5 | 1/1 | 0.1 | 300 000 |
| 7 | 6 | 1/1 | 0.1 | 300 000 |
| 8 | 7 | 1/1 | 0.1 | 300 000 |
| Cumulative dose | 1 266 000 | |||
Chronic hepatitis C virus (HCV) infection is one of the leading known causes of chronic liver diseases, including cirrhosis and hepatocellular carcinoma[1,2]. Risk factors associated with transmission of HCV include transfusion of infected blood products, injection drug use, employment in patient care or clinical laboratory work, exposure to an infected sex partner or household member, exposure to multiple sex partners, and low socioeconomic status[3].
HCV has a positive-sense, single-stranded RNA genome that has been classified into six different genotypes from 1 to 6[4,5]. The genotype determination is a relevant clinical practice, which not only helps predict the probability of sustained virological response (40%-45% for genotype 1 compared with 70%-80% for genotypes 2 and 3), but also is used routinely to determine duration of treatment (48 wk for genotypes 1 and 4 vs 24 wk for genotypes 2 and 3)[6,7]. Various genotypes have distinct geographical distributions around the world[8], and recent reports from Tehran and five cities from different locations in Iran, have shown that genotype 1a was predominant (47%), and 3a, 1b and 4 had a prevalence of 36%, 8% and 7%, respectively[9].
Interferon, in various forms or combinations, is the only proven effective treatment for hepatitis C. It has a fundamental, irreplaceable role in the treatment of patients with HCV infection. Treatment of hepatitis C, even when absolutely necessary, is almost impossible when interferon cannot be administered for any reason.
Here, we report a patient with chronic HCV infection and advanced fibrosis who was unable to receive interferon because of systemic hypersensitivity. The patient was desensitized successfully through gradual incremental exposure, and HCV infection was eradicated after a complete course of treatment, with no further allergic reactions.
The patient was a 65-year-old man who was referred to our liver clinic with a positive anti-HCV antibody ELISA. The patient’s main complaint was fatigue. Past medical history was positive for two coronary angioplasties for ischemic heart disease. No other medical diseases were present in his past history. General physical examination was normal except for mild hepatomegaly. Results of laboratory tests were within normal ranges except for a marginally low albumin level (3.4 g/dL) and mildly elevated aspartate aminotransferase (45-80 IU/mL) and alanine aminotransferase (46-78 IU/mL).
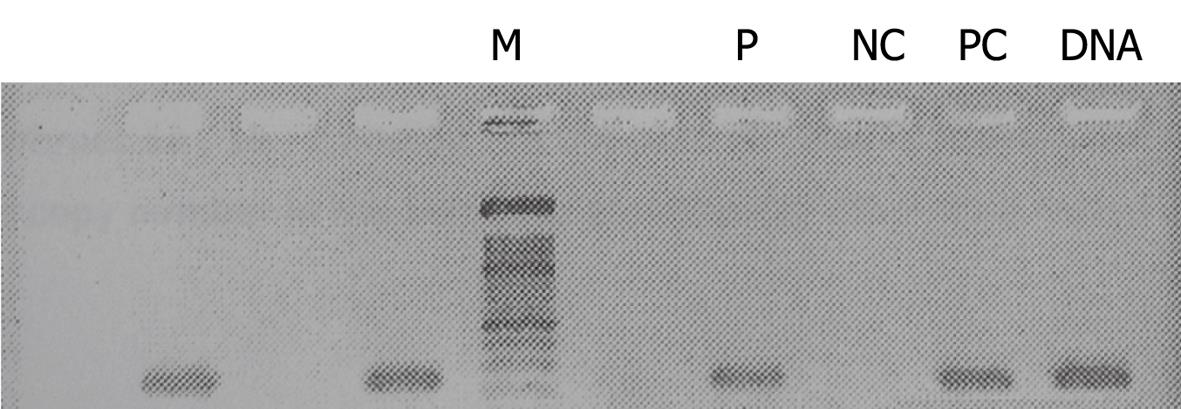
Reverse transcription polymerase chain reaction (RT-PCR) revealed a cDNA band that indicated the presence of HCV (Figure 1). Genotype-specific primers for HCV genotyping showed the virus to be of genotype 1b. Liver needle biopsy showed chronic hepatitis with moderate activity and advanced fibrosis (grade 9/18, stage 5/6).
The patient was selected for treatment with interferon plus ribavirin. The dosage of ribavirin was kept constant at 1200 mg/d throughout treatment. Since he was not able to afford the cost of pegylated interferon therapy, PDferon®, a brand of interferon α-2b produced in Iran (Pooyeshdarou Pharmaceutical Co., Tehran, Iran), was used.
After administration of the first dose of interferon α2b, the patient developed generalized maculopapular rash with severe itching and low-grade fever. As a result, treatment was stopped, with a diagnosis of hypersensitivity to interferon.
Since there was no other choice for treatment of hepatitis C, after careful discussion with the patient about possible benefits and side effects, a decision was made to proceed with a course of desensitization. The patient was kept in hospital during the first and second day of the treatment, with close observation of his vital signs. Resuscitation equipment was kept at the bedside. A routine blood count and chemistry were performed on days 1 and 2 and revealed normal results. A 1-mL vial of PDferon® that contained 3 000 000 IU was diluted to 1/100 000 concentration, and 0.1 mL of the resulting solution was injected subcutaneously.
Desensitization started from 8 am on the first day and dose escalation continued until the sixth dose (14.00 h). The dose was kept the same during the afternoon and injections stopped at 21.00 h to allow for patient rest (Table 1). Desensitization restarted on the next day at the same time, with a 1/100 concentration (Table 2). From day 3 onwards, until the end of the first week, the drug was given at a dose of 1 500 000 IU/d in the morning, with a 1-h period of observation after the injections. From the second week until the end of the treatment (54 wk), interferon was given at a dosage of 3 000 000 IU every other day.
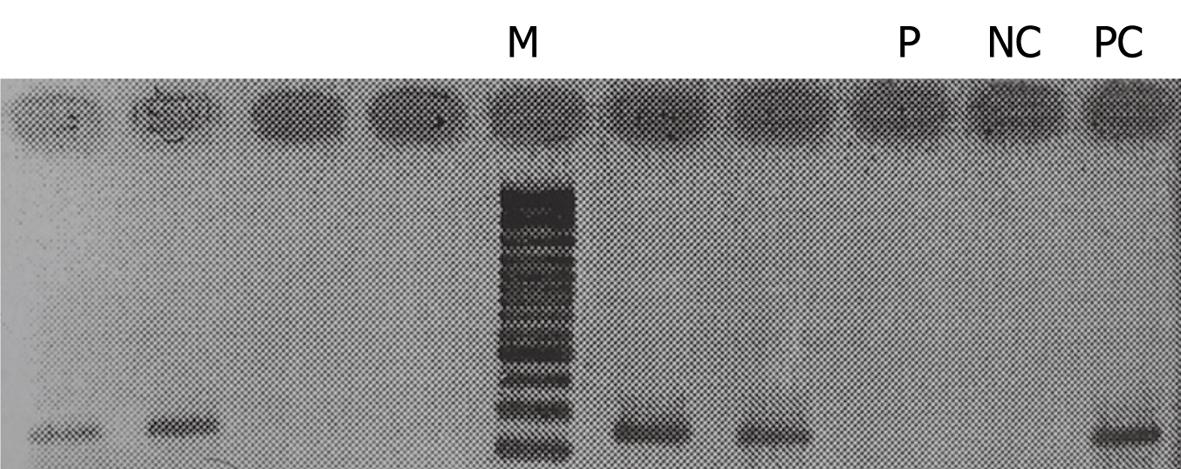
The only observed reaction during the treatment was a mild generalized pruritus at the start of day 2, with no accompanying rash or change in vital signs. These symptoms responded to intramuscular injection of diphenhydramine. After completing the treatment course, the patient recovered from HCV and nested RT-PCR revealed no cDNA band, which indicated eradication of HCV (Figure 2). RT-PCR was repeated another two times at 6-wk intervals, with the last one at 18 mo after the end of treatment. All turned out to be negative.
Since the patient had advanced liver fibrosis and there was no alternative treatment for hepatitis C, after extensive discussion with the patient about the possible side effects, as well as alternatives, a decision was made to proceed with desensitization. The protocol was based on previous experience with desensitization to penicillin. This decision was reviewed by another independent gastroenterologist and the patient gave written informed consent.
Interferon products are used worldwide as an effective treatment for chronic hepatitis C. Although several potential antiviral drugs are in the pipeline, interferon is still the only proven effective treatment for this viral disease.
Considering the above facts, when a patient is in great need of antiviral therapy for hepatitis C (advanced fibrosis for instance) and has an allergic reaction to interferon, there are very limited, if any, treatment options available. Although desensitization through gradual exposure to incremental doses is an established option for treatment of allergic reaction to protein and non-protein drugs[10], there are no reports of its use for desensitization to interferon. The main theoretical risk (beside the proven risk of anaphylaxis) with desensitization to protein drugs such as interferon is that it induces neutralizing antibodies, which potentially are directed towards the effective sites of the drug, which renders it ineffective, or at least reduces its therapeutic potential.
This case report describes successful eradication of hepatitis C in a patient with advanced liver disease, and is an indication that desensitization may not necessarily damage the therapeutic efficacy of the drug. Although in this case a regular (non-pegylated) interferon product was used, it may be expected that the same procedure can be applied to pegylated interferon products, since it is the protein component towards which hypersensitivity is usually directed.
Further studies including measurement and characterization of possible contributing antibodies are needed before this method can be suggested as a standard protocol for interferon desensitization.
| 1. | Willems M, Metselaar HJ, Tilanus HW, Schalm SW, de Man RA. Liver transplantation and hepatitis C. Transpl Int. 2002;15:61-72. [Cited in This Article: ] |
| 2. | Alberti A, Benvegnù L. Management of hepatitis C. J Hepatol. 2003;38 Suppl 1:S104-S118. [Cited in This Article: ] |
| 3. | Donahue JG, Muñoz A, Ness PM, Brown DE Jr, Yawn DH, McAllister HA Jr, Reitz BA, Nelson KE. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992;327:369-373. [Cited in This Article: ] |
| 4. | Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S-65S. [Cited in This Article: ] |
| 5. | Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, Shiffman ML, Zeuzem S, Craxi A, Ling MH. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. International Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1493-1499. [Cited in This Article: ] |
| 6. | Saracco G, Ciancio A, Olivero A, Smedile A, Roffi L, Croce G, Colletta C, Cariti G, Andreoni M, Biglino A. A randomized 4-arm multicenter study of interferon alfa-2b plus ribavirin in the treatment of patients with chronic hepatitis C not responding to interferon alone. Hepatology. 2001;34:133-138. [Cited in This Article: ] |
| 7. | Alavian SM, Einollahi B, Hajarizadeh B, Bakhtiari S, Nafar M, Ahrabi S. Prevalence of hepatitis C virus infection and related risk factors among Iranian haemodialysis patients. Nephrology (Carlton). 2003;8:256-260. [Cited in This Article: ] |
| 8. | Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148-162. [Cited in This Article: ] |
| 9. | Samimi-Rad K, Nategh R, Malekzadeh R, Norder H, Magnius L. Molecular epidemiology of hepatitis C virus in Iran as reflected by phylogenetic analysis of the NS5B region. J Med Virol. 2004;74:246-252. [Cited in This Article: ] |
| 10. | Gammon D, Bhargava P, McCormick MJ. Hypersensitivity reactions to oxaliplatin and the application of a desensitization protocol. Oncologist. 2004;9:546-549. [Cited in This Article: ] |