Published online Sep 7, 2009. doi: 10.3748/wjg.15.4170
Revised: July 1, 2009
Accepted: July 8, 2009
Published online: September 7, 2009
AIM: To identify prognostic factors of patients with hepatocellular carcinoma (HCC), who were treated by orthotopic liver transplantation (OLT).
METHODS: From January 2000 to October 2006, 165 patients with HCC underwent OLT. Various clinicopathological risk factors for actuarial and recurrence-free survival were identified using the Kaplan-Meier method with the log-rank test. The Cox proportional hazards model was used to identify independently predictive factors for actuarial and recurrence-free survival, which were used to propose new selection criteria. We compared the outcome of the subgroup patients meeting different criteria. Survival analysis was performed using the Kaplan-Meier method with the log-rank test.
RESULTS: The median follow-up was 13.0 mo (2.8-69.5 mo). Overall, 1-, 2-, 3- and 5-year actuarial survival was 73.3%, 45.6%, 35.4% and 32.1%, respectively. One-, 2-, 3- and 5-year overall recurrence-free survival was 67.0%, 44.3%, 34.5% and 34.5%, respectively. In univariate analysis, number of tumors, total tumor size, lobar distribution, differentiation, macrovascular invasion, microvascular invasion, capsulation of the tumor, and lymph node metastasis were found to be associated significantly with actuarial and tumor-free survival. By means of using the multivariate Cox proportional hazards model, total tumor size and macrovascular invasion were found to be independent predictors of actuarial and tumor-free survival. When the selection criteria were expanded into the proposed criteria, there was no significant difference in 1-, 2-, 3- and 5-year actuarial and tumor-free survival of the 49 patients who met the proposed criteria (97.6%, 82.8%, 82.8% and 82.8%, and 90.7%, 82.8%, 68.8% and 68.8%, respectively) compared with that of patients who met the Milan or University of California, San Francisco (UCSF) criteria.
CONCLUSION: Macrovascular invasion and total tumor diameter are the strongest prognostic factors. The proposed criteria do not adversely affect the outcome of liver transplantation for HCC, compared with the Milan or UCSF criteria.
- Citation: Li J, Yan LN, Yang J, Chen ZY, Li B, Zeng Y, Wen TF, Zhao JC, Wang WT, Yang JY, Xu MQ, Ma YK. Indicators of prognosis after liver transplantation in Chinese hepatocellular carcinoma patients. World J Gastroenterol 2009; 15(33): 4170-4176
- URL: https://www.wjgnet.com/1007-9327/full/v15/i33/4170.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4170
| n (%) | |
| Sex | |
| Male | 133 (89.9) |
| Female | 15 (10.1) |
| Age (yr) | Range 17-68 (median 45) |
| Etiology | |
| Hepatitis B | 137 (92.6) |
| Hepatitis B, C | 2 (1.4) |
| Alcoholic | 1 (0.7) |
| Idiopathic | 8 (5.4) |
| Pre-transplantation AFP (ng/mL) | |
| ≤ 400 | 60 (40.5) |
| > 400 | 88 (59.5) |
| Child–Pugh Class | |
| A | 76 (51.4) |
| B | 57 (38.5) |
| C | 15 (10.1) |
| Meld score | |
| < 14 | 107 (72.3) |
| ≥ 14 | 41 (27.2) |
| Pre-transplantation therapy | |
| Positive | 47 (31.7) |
| Negative | 101 (68.2) |
| Tumor stage | pTNM |
| I | 27 (18.2) |
| II | 40 (27.0) |
| IIIa | 66 (44.6) |
| IIIb | 10 (6.8) |
| IIIc | 5 (3.4) |
| Characteristics | n (%) |
| Tumor | |
| ≤ 3 | 101 (68.2) |
| > 3 | 47 (31.8) |
| Total tumor size (cm) | |
| ≤ 5 | 24 (16.2) |
| 5–9 | 30 (20.9) |
| > 9 | 94 (63.5) |
| Lobar distribution | |
| Unilobar | 80 (54.1) |
| Bilobar | 68 (45.9) |
| Differentiation | |
| Well | 35 (23.6) |
| Moderate | 100 (67.6) |
| Poor | 13 (8.8) |
| Vascular invasion | |
| None | 47 (31.8) |
| Micro | 49 (33.1) |
| Macro | 52 (35.1) |
| Lymph node status | |
| Negative | 143 (96.6) |
| Positive | 5 (3.4) |
| Cirrhosis | |
| Negative | 13 (8.8) |
| Positive | 135 (91.2) |
| Predictors | Relative risk | 95% CI | P |
| Total tumor size ( ≤ 9 cm vs > 9 cm) | 2.291 | 1.654-3.173 | 0.000 |
| Tumor number ( ≤ 4 vs > 4) | 1.127 | 1.052-1.207 | 0.003 |
| Bilobar disease | 2.206 | 1.327-3.666 | 0.002 |
| Macrovascular invasion | 2.951 | 2.160-4.032 | 0.000 |
| Microvascular invasion | 4.479 | 2.126-9.436 | 0.000 |
| Positive lymph nodes | 12.472 | 4.457-34.898 | 0.000 |
| Differentiation | 1.641 | 1.014-2.654 | 0.044 |
| Capsule invasion | 1.462 | 1.092-1.957 | 0.011 |
In the 1980s, outcomes for orthotopic liver transplantation (OLT) for hepatocellular carcinoma (HCC) were discouraging. There were high recurrence rates and a low patient survival of 30% at 3 years[1]. After the Milan criteria (single tumor up to 5 cm or up to three tumors up to 3 cm) were introduced by Mazzaferro et al[2] in the early 1990s, recurrence rate fell to 8%, and tumor-free patient survival at 4 years was 83%. With worldwide adoption of these criteria, 5-year survival rates rose to 60%-80%[3-5].
Recently, there have been numerous proposals for expanding the Milan criteria. The University of California, San Francisco (UCSF) proposed an expansion of transplantation criteria[6]. The expanded criteria includes having a single tumor < 6.5 cm in diameter, or having no more than three tumors, the largest of which is < 4.5 cm in diameter, and with a total tumor diameter of < 8 cm. Using these criteria, 1- and 5-year recurrence-free survival rates were 90% and 75.2%, respectively. Marsh et al[7] have studied 407 cases who underwent OLT at the University of Pittsburgh between 1981 and 2002, and found that patients who exceeded the Milan criteria had a 49.7% recurrence-free survival rate at 5 years. Todo et al[8] have reported that patients with unresectable HCC who underwent living donor liver transplantation under the expanded indication criteria had 3-year survival and disease-free survival rates of 60.4% and 52.6%, respectively. In Kyoto[9], the only OLT exclusion criteria are for patients with extrahepatic metastasis or macroscopic vascular invasion, and there are no restrictions based upon on the number or size of tumors. There was no difference in the 4-year survival rate in the Kyoto study between patients with HCC who fit (66%) or do not fit (60%) the Milan criteria. Therefore, it has been suggested that there are differences in OLT criteria between the East and West, which may be correlated with race or combinations of underlying hepatic diseases.
Worldwide, 55% of HCC cases and deaths occur in China[10]. Over 90% of HCC patients in China are also infected with hepatitis B virus (HBV). These rates are different from those in Europe, America, and even Japan. To explore these issues, we evaluated the OLT outcomes for HCC patients at the Liver Transplantation Center of the West China Hospital, Chengdu, China. We aimed to analyze the effect of HCC prognostic factors on actuarial and recurrence-free survival after OLT, and to reevaluate the prediction criteria that are the basis for OLT selection.
Between January 2000 and October 2006, a total of 424 OLTs were performed at the Liver Transplantation Center of the West China Hospital. Among these, 165 (38.9%) patients were diagnosed with HCC, and 148 underwent follow-up. Follow-up excluded 17 patients who died of complications during the 3-mo period after the operation. Of the 148 HCC patients, 146 were diagnosed with HCC before liver transplantation. In two patients, HCC was found incidentally by pathological examination of the explanted liver after transplantation. Preoperative clinical data of the 148 HCC patients are shown in Table 1. Liver transplantation was considered for patients with HCC if the tumor was determined to be unresectable because of its location, or because of concomitant liver disease and HCC without extrahepatic spread.
Preoperative diagnosis of HCC was made using artery high-flow perfusion and imaging examinations, which showed intrahepatic tumors. Examinations included at least two of the following three methods: ultrasound, contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI). Macroscopic vascular invasion was observed using Doppler ultrasonography and contrast-enhanced CT before operation, and was validated through pathological examination of the impaired liver after operation. Diagnosis of HCC was confirmed by fine-needle aspiration cytology or biopsy of all known tumors. Pretransplant studies in patients with HCC included abdominal, thoracic, and head CT scans and bone scintigraphy to rule out extrahepatic tumor spread.
The patients were followed for tumor recurrence with CT scans and α-fetoprotein level (AFP) every 6 mo for 5 years. Annually thereafter, or as the clinical situation dictated, suspicious lesions in the liver or lung were biopsied. Bone lesions were not biopsied routinely but were observed for bone pain and progression of growth. A rising AFP level alone was not taken to be confirmatory of tumor recurrence, but the date of recurrence was taken as the time that the AFP level began to rise once tumor recurrence had been confirmed.
Immunosuppression consisted of cyclosporine or tacrolimus and corticosteroids, with or without azathioprine and mycophenolate. In cases in which acute rejection was suspected, a liver biopsy was performed, and steroid pulse therapy was conducted after the rejection diagnosis was made. The steroids were withdrawn 3-6 mo after surgery to minimize the risk of tumor recurrence[8,11]. Lamivudine was administrated to hepatitis-B-surface-antigen-positive patients before and after surgery, and hepatitis B immune globulin was administered to HBV-DNA-positive patients before, during and after surgery.
Cumulative survival time was calculated from the date of transplantation to the date of death, from the date of transplantation to the date of final follow-up, or from the date of transplantation to the date of loss to follow-up. The latter two conditions were tabulated from censored data. Tumor-free survival (TFS) time was calculated from the date of transplantation to the date when tumor recurrence and metastases were discovered, from the date of transplantation to the date of final follow-up, or from the date of transplantation to the date of loss to follow-up. The latter two conditions were also tabulated from censored data. Statistical analysis software (SPSS 13.0) was used for data processing and analysis. The Kaplan-Meier method was used to calculate the cumulative survival rate (CSR), TFS rate, and to present the corresponding survival curves in graphical form. The log-rank test was used to compare the differences between groups. The univariate Cox proportional hazard regression model was used to analyze each factor that might have influenced liver transplantation prognosis in HCC patients, and to identify factors with statistical significance. The multivariate Cox proportional hazard regression model was used to analyze and confirm the independent prognostic factors for OLT in HCC patients. Statistical significance was defined as P < 0.05.
The pathological data on HCC tumors and potential indicators are shown in Table 2. In general, OLT was offered when liver function was impaired, or when the HCC become unresectable. HCC was diagnosed before transplantation in 148 patients, and in two, we identified an incidental tumor during pathological examination of the explanted liver.
The median follow-up time for the 148 patients was 13.0 mo (2.8-69.5 mo). The follow-up time of 126 of these patients was ≥ 6 mo. Eighty-one patients survived, with a median follow-up of 15.5 mo (2.8-69.5 mo). Sixty-two patients died, with a median follow-up of 11.0 mo (3.0-38.0 mo). Five patients were lost to follow-up, with a median follow-up of 16.0 mo (14.0-23.0 mo).
The mean total cumulative survival time of the entire group was 33.6 mo (95% CI: 27.5-39.6 mo). The CSR was 73.3% at 12 mo, 45.6% at 24 mo, 35.4% at 36 mo, and 32.1% at 60 mo. The average TFS time of the entire group was 32.8 mo (95% CI: 26.4-39.1 mo). The TFS rate was 67.0% at 12 mo, 44.3% at 24 mo, 34.5% at 36 mo, and 34.5% at 60 mo.
Based on the results of the univariate Cox regression model analysis, the following 10 variables significantly affected CSR: (1) age; (2) largest tumor size; (3) total tumor size; (4) tumor number; (5) bilobar disease; (6) macrovascular invasion; (7) microvascular invasion; (8) lymph nodes positive; (9) differentiation; and (10) capsule invasion. The above 10 variables were analyzed using the multivariate Cox proportional hazard regression model and the stepwise regression method, to identify the independent factors that influenced total survival rate. The results showed that total tumor size and macrovascular invasion were the two risk factors that affected total survival rate.
According to the results of the univariate Cox regression model analysis (Table 3), the following eight variables significantly affected tumor-free survival rate: (1) total tumor size; (2) tumor number; (3) bilobar tumor; (4) tumor differentiation; (5) macrovascular invasion; (6) microvascular invasion; (7) tumor capsular invasion; and (8) lymph nodes positive. The above eight variables were analyzed using the multivariate Cox proportional hazard regression model and the stepwise regression method, to identify independent factors that influenced the TFS rate. The results showed that total tumor size, macrovascular invasion and lymph node status were the independent predictors of TFS.
Macrovascular invasion and total tumor size were prognostic factors that independently influenced CSR and TFS. Our proposed criteria for OLT selection included patients with total tumor size ≤ 9 cm and who were without macrovascular invasion or extrahepatic metastases, regardless of the number of tumor lesions.
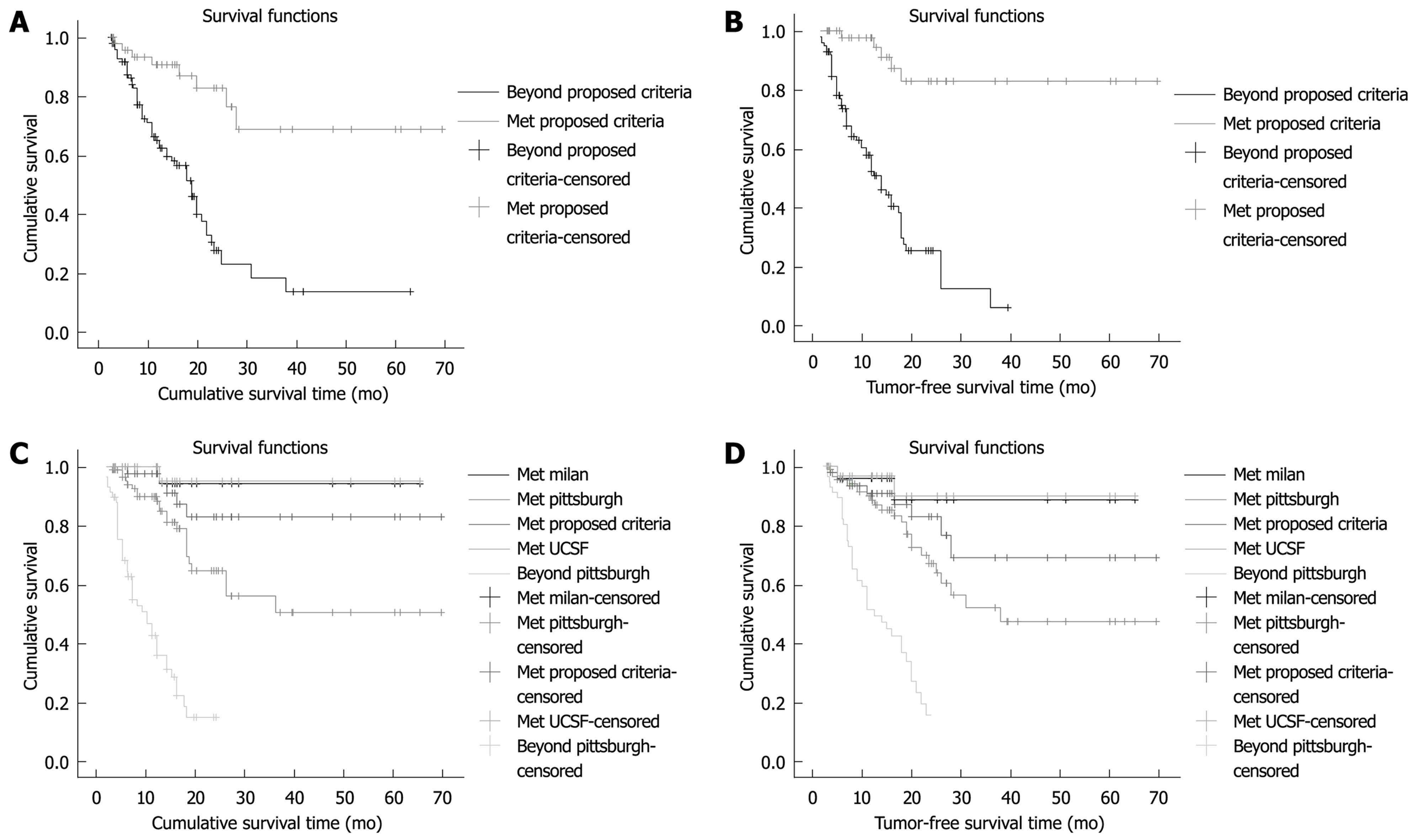
For the cases which conformed to the proposed criteria (n = 49), the total survival rate at 1, 2, 3 and 5 years was 97.6%, 82.8%, 82.8% and 82.8%, respectively (Figure 1A). The average survival time was 53.7 mo (43.8-63.6 mo). Among these 49 patients, 18 (16.3%) died by the end of follow-up, and seven (9.5%) died of recurrence. There were 41 cases of TFS (83.7%). The average TFS rate at 1, 2, 3 and 5 years was 90.7%, 82.8%, 68.8% and 68.8%, respectively (Figure 1B). The average TFS time was 60.0 mo (52.3-67.7 mo).
Comparisons of the CSR and the TFS at 1, 2, 3 and 5 years for different patient subgroups are shown in Figure 1C and D. CSR and TFS were similar in patients who met the proposed criteria and Milan criteria (P = 0.321, P = 0.331), and UCSF criteria (P = 0.229, P = 0.257). However, the patient number was increased more in the proposed criteria (n = 49), than the Milan (n = 24) or UCSF (n = 33) criteria. What’s more CSR was similar in patients who met the proposed criteria and the Pittsburgh criteria (P = 0.158). Although the patient number was greater in the Pittsburgh (n = 90) than the proposed criteria,the TFS for the Pittsburgh criteria was lower than that for the proposed criteria (P = 0.027).
Numerous studies have shown that tumor size is an important prognostic factor for liver transplantation in patients with HCC[8,12,13]. Bismuth et al[12] have found that HCC patients with two or fewer tumors, each with a diameter ≤ 3 cm, had an 83% 3-year CSR and TFS following liver transplantation and hepatectomy. Roayaie et al[14] have reported that HCC patients with tumor diameters > 7 cm (12 cases) and 5-7 cm (32 cases) had 5-year tumor-free rates of 34% and 55%, respectively, following transplantation. Tumor diameter > 7 cm and the presence of vascular invasion were correlated with HCC recurrence. In this study, total tumor size was shown to influence prognosis in the univariate Cox regression model analysis, and was shown to be an independent prognostic factor in the multivariate analysis. Compared to largest tumor size, tumor number can better predict the OLT prognosis in HCC patients. Univariate Cox regression analysis showed that the 2-year CSR and TFS rates were 73.5% and 75.6%, respectively. These rates were significantly different compared to those in cases where total tumor size was > 9 cm. Tumor number and size are believed to have a combined effect on HCC recurrence probability, and this effect is taken into consideration during selection of HCC OLT recipients in many institutions[2,6]. The total tumor size in the present study also reflects a combined effect of tumor number and size.
Many other studies have shown that macrovascular invasion is a primary factor that influences prognosis after OLT in HCC patients[12,13,15-18]. Shetty et al[18] have concluded that macrovascular invasion and AFP levels were prognostic factors that influenced CSR and TFS. Moreover, microvascular invasion had no significant influence on prognosis. Bismuth et al[12] have reported that 10 of 60 (16.7%) HCC patients who underwent OLT had tumor thrombus in the main trunk of the portal vein, and had a 3-year survival rate of only 20%. Thus, they proposed that tumor thrombus in the main trunk of the portal vein was a major risk factor for OLT. Other studies have proposed that when HCC is accompanied by vascular invasion, tumor cells are more likely to be present in the circulation, rather than being restricted to the liver[19,20]. While circulating tumor cells may not develop into distant metastases, immunosuppressive treatment after OLT may increase the possibility of tumor recurrence. Currently, most transplantation centers advocate exclusion of HCC patients with tumor thrombus in the main trunk or right and left branches of the portal vein, for these reasons. In the present study, multivariate Cox regression analysis showed that macrovascular invasion was an independent risk factor that affected CSR and TFS. In the 52 cases with macrovascular invasion, the recurrence rate was as high as 71.2% during follow-up. The 1-year CSR was 53.5%, and TFS was 14.4%. The 2-year CSR and TFS were 34.2% and 12.9%, respectively. Therefore, OLT patients with macrovascular invasion had poor long-term prognosis.
Univariate analysis identified hepatic lobar distribution, differentiation, and capsule invasion as prognostic factors that influenced CSR and TFS in the present study. However, multivariate analysis showed that only total tumor size and macrovascular invasion were independent prognostic factors that influenced CSR and TFS. Therefore, hepatic lobar distribution, differentiation, and capsule invasion may have a relationship with total tumor size or macrovascular invasion. We believe that these three indicators may reflect malignant tumor invasion to a certain degree, since HCC patients who have bilobar distribution, low differentiation or capsular (or non-capsular) tumor invasion have a relatively high incidence of tumor recurrence and metastasis.
Our data showed that age was related significantly to CSR by univariate Cox regression analysis, with a relative risk of 0.963 (95% CI: 0.936-0.991). The younger the onset of HCC, the greater the possibility of tumor recurrence was after liver transplantation. An early age of HCC onset may lead to greater malignancy and faster disease development. An understanding of this mechanism requires further exploration.
Positive lymph nodes and distant metastasis are considered to be absolute contraindications for liver transplantation in HCC patients[21]. Marsh et al[22] have found that the average TFS time for 231 HCC liver transplantation cases with negative local lymph nodes was 140.6 ± 6.8 mo. The TFS time was significantly less, 5.3 ± 1.0 mo, for six cases with positive local lymph nodes. In the present study, five HCC cases (3.4%) had positive lymph nodes in the porta hepatis. Four of these patients died, and all five had tumor recurrence. The average survival time of these five patients was only 7.0 mo, and the average TFS time was 4.2 mo, in accordance with previous studies. Detection of lymph nodes can be observed in HCC patients with hepatitis, who have enlarged, inflammatory lymph nodes. Therefore, careful observations should be conducted during surgery, and all enlarged lymph nodes should be sent to the pathology department for intraoperative frozen-section examination. Liver transplantation should not be carried out in patients with confirmed positive lymph nodes.
China has the greatest incidence of liver cancer worldwide[10]. Each year, over half of liver cancer detections and deaths occur in China. Liver cancer (mainly HCC) has been the primary indication for liver transplantation. It is worth noting that the hepatic disease background and epidemiology of HCC is unique to China and is considerably different from that in Europe and America. Therefore, prognostic factors for liver transplantation in Chinese HCC patients are likely different from those in other countries. It is therefore necessary to explore the novel criteria for HCC liver transplantation in China.
Here, we studied independent prognostic factors, and developed proposed criteria for liver transplantation in HCC patients. The proposed criteria are as follows: total tumor size ≤ 9 cm, and there should be no macrovascular invasion, positive lymph nodes or extrahepatic metastases, regardless of tumor number and distribution. According to the proposed criteria, we screened HCC patients with liver transplantation. The postoperative 2- and 5-year CSR was 82.8% and 68.8%, respectively, using our proposed criteria. There was no significant difference between the proposed and the Milan criteria (both 88.5%) (P = 0.321). Using the proposed criteria, the 2- and 5-year TFS rates were both 82.8%.
Compared to the Milan criteria (both rates 94.1%), there was no significant difference (P = 0.331). We compared the HCC cases that fitted the proposed criteria (49 cases) and those that exceeded the criteria (99 cases) (Figure 1A and B). The 2- and 5-year CSR of the cases that fitted the proposed criteria were 82.8% and 68.8%, respectively, while the CSR for cases exceeding the criteria were 27.9% and 14.0%, respectively (P < 0.001). The 1- and 2-year TFS of the cases that fitted the proposed criteria were 97.6% and 82.8%, respectively, while the TFS for cases exceeding the criteria were 52.5% and 25.7%, respectively (P < 0.001). Use of our proposed criteria included 16.9% more cases than the Milan criteria and 10.8% more cases than the UCSF criteria. In this study, we also analyzed cases that fitted the proposed criteria, but exceeded the Milan criteria (25 cases). Our results showed that the 1-, 2- and 5-year CSRs were 85.2%, 77.4% and 48.4%, respectively, and the corresponding TFS rates were 95.0%, 70.4% and 70.4%, respectively. It is agreed generally in the field of transplantation that HCC patients whose 5-year, post-transplantation, survival rate is ≥ 50% qualify as transplant candidates[23,24]. The present study showed that the 5-year CSR and TFS of the HCC patients were 68.8% and 82.8%, respectively. The 5-year CSR and TFS of the HCC patients that fitted our proposed criteria but exceeded the Milan criteria (25 cases) were 48.4% and 70.4%, respectively. The CSR and TFS rate curves of these patients were not significantly different compared to those of patients who fitted the Milan criteria (P = 0.105 and P = 0.115, respectively).
In summary, we suggest that the proposed criteria function to predict the prognosis of liver transplantation in patients with HCC. The proposed criteria allow us to increase the range of indicators for HCC patients in need of liver transplantation, and decrease the exclusion rate, and they allow more patients to receive therapeutic liver transplantation in China.
In the 1980s, the outcome of orthotopic liver transplantation (OLT) for hepatocellular carcinoma (HCC) was discouraging. After the Milan criteria (single tumor up to 5 cm, or up to three tumors up to 3 cm) were introduced by Mazzaferro et al in the early 1990s, recurrence rate fell to 8%, and tumor-free patient survival at 4 years was 83%. The Milan criteria have long been considered to be the classical criteria for selection of patients with HCC for liver transplantation. Recently, there have been numerous proposals for expanding the Milan criteria, such as the University of California, San Francisco (UCSF) criteria.
The Milan criteria have long been considered to be the classical criteria for selection of patients with HCC for liver transplantation. China has the greatest incidence of liver cancer worldwide. Each year, over half of liver cancer detections and deaths occur in China. A substantial proportion of adult Living donor liver transplantation patients not fulfilling the Milan or UCSF criteria have been found to survive longer than expected after transplantation. Therefore, it seems reasonable to attempt further reduction of unnecessary dropouts arising from the strict application of narrow selection criteria.
In the present study, the authors identified various clinicopathological risk factors for actuarial and recurrence-free survival of 165 patients with HCC who underwent OLT using the Kaplan-Meier method with the log-rank test. They found that macrovascular invasion and total tumor diameter, as assessed on explanted liver, were the strongest prognostic factors.
The authors reviewed their single-center experience with 148 OLTs for HCC and proposed a new prognostic score.
| 1. | Pichlmayr R, Weimann A, Ringe B. Indications for liver transplantation in hepatobiliary malignancy. Hepatology. 1994;20:33S-40S. [Cited in This Article: ] |
| 2. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [Cited in This Article: ] |
| 3. | Figueras J, Jaurrieta E, Valls C, Benasco C, Rafecas A, Xiol X, Fabregat J, Casanovas T, Torras J, Baliellas C. Survival after liver transplantation in cirrhotic patients with and without hepatocellular carcinoma: a comparative study. Hepatology. 1997;25:1485-1489. [Cited in This Article: ] |
| 4. | American Liver Tumor study group. A randomized prospective multi- institutional trial of orthotopic liver transplantation or partial hepatic resection with or without adjuvant chemotherapy for hepatocellular carcinoma. Investigator Booklet and protocol. 1998;. [Cited in This Article: ] |
| 5. | Sobin LH, Wittekind HC; International Union Against Cancer (UICC). TNM classification of tumors. New York: Wiley-Liss 1997; 74-77. [Cited in This Article: ] |
| 6. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [Cited in This Article: ] |
| 7. | Marsh JW, Dvorchik I. Liver organ allocation for hepatocellular carcinoma: are we sure? Liver Transpl. 2003;9:693-696. [Cited in This Article: ] |
| 8. | Todo S, Furukawa H. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451-459; discussion 459-461. [Cited in This Article: ] |
| 9. | Takada Y, Ito T, Ueda M, Sakamoto S, Haga H, Maetani Y, Ogawa K, Ogura Y, Oike F, Egawa H. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteria. Dig Dis. 2007;25:299-302. [Cited in This Article: ] |
| 10. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [Cited in This Article: ] |
| 11. | Mazzaferro V, Rondinara GF, Rossi G, Regalia E, De Carlis L, Caccamo L, Doci R, Sansalone CV, Belli LS, Armiraglio E. Milan multicenter experience in liver transplantation for hepatocellular carcinoma. Transplant Proc. 1994;26:3557-3560. [Cited in This Article: ] |
| 12. | Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145-151. [Cited in This Article: ] |
| 13. | Roayaie S, Haim MB, Emre S, Fishbein TM, Sheiner PA, Miller CM, Schwartz ME. Comparison of surgical outcomes for hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a western experience. Ann Surg Oncol. 2000;7:764-770. [Cited in This Article: ] |
| 14. | Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533-539. [Cited in This Article: ] |
| 15. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [Cited in This Article: ] |
| 16. | Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221-228; discussion 228-229. [Cited in This Article: ] |
| 17. | Hemming AW, Nelson DR, Reed AI. Liver transplantation for hepatocellular carcinoma. Minerva Chir. 2002;57:575-585. [Cited in This Article: ] |
| 18. | Shetty K, Timmins K, Brensinger C, Furth EE, Rattan S, Sun W, Rosen M, Soulen M, Shaked A, Reddy KR. Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl. 2004;10:911-918. [Cited in This Article: ] |
| 19. | Kar S, Carr BI. Detection of liver cells in peripheral blood of patients with advanced-stage hepatocellular carcinoma. Hepatology. 1995;21:403-407. [Cited in This Article: ] |
| 20. | Kienle P, Weitz J, Klaes R, Koch M, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M. Detection of isolated disseminated tumor cells in bone marrow and blood samples of patients with hepatocellular carcinoma. Arch Surg. 2000;135:213-218. [Cited in This Article: ] |
| 21. | Herrero JI, Sangro B, Quiroga J, Pardo F, Herraiz M, Cienfuegos JA, Prieto J. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl. 2001;7:631-636. [Cited in This Article: ] |
| 22. | Marsh JW, Dvorchik I, Bonham CA, Iwatsuki S. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538-543. [Cited in This Article: ] |
| 23. | Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [Cited in This Article: ] |
| 24. | Roayaie S, Llovet JM. Liver transplantation for hepatocellular carcinoma: is expansion of criteria justified? Clin Liver Dis. 2005;9:315-328. [Cited in This Article: ] |