Published online Jul 7, 2009. doi: 10.3748/wjg.15.3166
Revised: May 29, 2009
Accepted: June 5, 2009
Published online: July 7, 2009
AIM: To investigate microvascular injury quantitatively in the small bowel with respect to cardiopulmonary bypass (CPB) and related mechanisms.
METHODS: In 10 male SD rats, normothermic CPB was established and continued with a flow rate of 100-150 mL/kg per minute for 60 min, while another 10 sham-operated animals served as controls. An approximate 10-cm loop of the terminal ileum was exteriorized for observation by means of intravital fluorescence microscopy. The small bowel microcirculatory network including arterioles, capillaries, and collecting venules was observed prior to CPB, CPB 30 min, CPB 60 min, post-CPB 60 min and post-CPB 120 min. The intestinal capillary perfusion, microvascular permeability and leukocyte adherence were also measured.
RESULTS: The systemic hemodynamics remained stable throughout the experiment in both groups. In CPB animals, significant arteriolar vasoconstriction, blood velocity reduction and functional capillary density diminution were found. As concomitances, exaggerated albumin extravasation and increased leukocyte accumulation were also noted. These changes were more pronounced and there were no signs of restitution at the end of the observation period.
CONCLUSION: CPB induces significant microcirculatory injury of the small bowel in rats. The major underlying mechanisms are blood flow redistribution and generalized inflammatory response associated with CPB.
- Citation: Dong GH, Wang CT, Li Y, Xu B, Qian JJ, Wu HW, Jing H. Cardiopulmonary bypass induced microcirculatory injury of the small bowel in rats. World J Gastroenterol 2009; 15(25): 3166-3172
- URL: https://www.wjgnet.com/1007-9327/full/v15/i25/3166.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3166
Despite excellent improvements, there is increasing evidence to show that cardiopulmonary bypass (CPB) is related to a generalized inflammatory response and splanchnic edema formation in both clinical and experimental investigations[1–5]. Impairment of gastrointestinal perfusion during CPB may lead to loss of mucosal vascular integrity and increased permeability. The translocation of microorganisms and endotoxin into the systemic circulation can result in a continuing toxic insult and systemic tissue injury. This is thought to be one of the essential mechanisms in the development of sepsis, shock, post-perfusion complications, and multiple organ failure after CPB[5–7]. Identification of rational clinical therapeutic approaches requires a precise elucidation of the underlying pathophysiological processes of the intestinal microvasculature induced by CPB.
However, the exact regional hemodynamic and functional changes of the small intestine in terms of the response to CPB remain unclear. Indirect methods may not be sufficient to delineate the pathomechanisms of intestinal microcirculatory conditions within the different tissue compartments such as muscle, submucosa, and mucosa[8–10]. An appropriate experimental model is needed to investigate the variety of microvascular alterations including capillary perfusion injury, microvascular endothelial integrity loss, and leukocyte activation under both physiological and pathological conditions. In this study, using a rat model of CPB, we directly observed and quantitatively analyzed the microvascular alterations in small bowel by means of fluorescent intravital microscopy, aiming to demonstrate the influence of CPB in the different functional units of the microcirculatory network.
Adult male Sprague-Dawley rats (450-550 g) served as experimental animals. All animals received humane care in compliance with the principles of laboratory animal care formulated by the National Society of Medical Research and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Publication No. 85-23, revised 1996). Rats were anaesthetized with intraperitoneal administration of ketamine (50 mg/kg) and chlorpromazine (2 mg/kg). After tracheostomy and intubation, the animals were mechanically ventilated with about 10 mL/kg tidal volume, 60 breaths/min respiratory rate, and 100% inspiratory concentration of O2. Anesthesia was maintained throughout the experiment with additional doses of intravenous ketamine.
A rat CPB model previously developed by our group, with consistent excellent survival, was used in the current study[11]. Briefly, the right femoral artery was cannulated with a 24-gauge heparinized catheter for arterial pressure monitoring and blood gas analysis. The homolateral femoral vein was cannulated with a 20-gauge catheter for blood and fluid infusion. Following heparinization (500 U/kg), a 16-gauge catheter, modified to a multi-side-orifices cannula in the forepart, was inserted into the right jugular vein and advanced to the right atrium for blood drainage by gravity and siphon. A 22-gauge catheter was cannulated to the right carotid artery as the arterial infusion line for the CPB circuit. The membrane oxygenator was specially designed with a surface area for gas exchange of 0.05 m2 and the total assembly dynamic priming volume of 4 mL. A rolling pump was used to drive the blood through silicone arterial inflow tubing and then returned to the right carotid artery. Priming was composed of 8 mL heparinized fresh homologous blood obtained from a donor and 8 mL synthetic colloid (HAES-steril®). The CPB flow-rate was gradually adjusted, stabilized and performed at 100-150 mL/kg per minute throughout the experiment. To ensure the mean arterial pressure (MAP) above 60 mmHg and the hematocrit about 30%-40%, additional whole blood and Ringer’s solution was given during the experiment to supply the blood and fluid loss caused by surgery, sampling, leakage and evaporation. Body central temperature was monitored with a rectal probe and maintained at 36.5-38.0°C by a heating lamp placed above.
A modified intravital microscope (Shanghai 2XC4TV, China) with a 100 W mercury arc lamp was used to observe the intestinal microcirculation. With I2/3 and N2 filter blocks interposed into the light path, the green (530-560 nm) and the blue (450-490 nm) light was selected, respectively for epi-illumination. For in vivo microscopy, fluorescein isothiocyanate conjugated to bovine serum albumin (FITC-BSA), which fluoresces under blue light, allowed visualization of the intestinal microvessels. Acridine orange, which fluoresces under green light, stains leukocytes and allows their adhesion in the microvasculature to be monitored. The microscopic images were acquired by a charge-coupled device digital camera (Olympus C-5050, Japan), recorded by a digital video system (Cannon MVX-150i, Japan), and displayed on a high-resolution monitor (Patriot 798HD, China) for off-line analysis. The final magnification of the images can be approximately achieved by 250 × and 650 × with 10 × and 25 × long-distance objectives.
The animals were randomly divided into two groups. In the CPB group, 10 rats were subjected to the CPB circuit and received extracorporeal perfusion for 60 min. After the bypass was terminated, the remaining priming solution was infused gradually to maintain the main arterial pressure around 60 mmHg. Ten sham operated animals which were cannulated, heparinized and subjected to the CPB circuit but without CPB served as controls, and the CPB circuit was weaned 1 h later. The dosage of heparin for anticoagulation was the same in both groups.
In all animals, via a small transverse abdominal incision, an approximate 10 cm loop of ileum was exteriorized and placed on a movable stage attached to the microscope. A 10 mm incision along the antimesenteric border was made with an electric microcautery device for visualization of the mucosal surface. The exposed segment was gently extended, held in place by stay sutures, and covered with a glass microscopical cover slide. The tissues were constantly superfused with warm Ringer’s lactate at 37°C to avoid drying and local hypothermia.
The intestinal microcirculation was visualized after intravenous administration of FITC-BSA (0.2 mL/100 g body weight, 5%) and acridine orange (0.1 mL/100 g body weight, 0.2%). To avoid damage to the microcirculation due to long periods of light exposure, the recording time varied 30-60 s. The microvasculature of smooth muscle and the submucosa was assessed from the serosal side in the oral closed part of the segment by adjusting the focus level of the microscope. The villous microcirculation was visualized from the mucosal surface through the longitudinal incision in the aboral part of the segment.
After stabilization for about 15 min, four non-overlapping regions of interest were defined and measured to obtain representative values for every parameter. The quantitative analysis of intestinal microcirculation was performed by means of the standard technique usually used in intravital microscopic studies in small animals[1213]. A computer-controlled image analysis system (ImageJ 1.30V, National Institutes of Health, USA) was applied in those processes. Microcirculatory parameters were recorded prior to CPB, CPB 30 min, CPB 60 min, post-CPB 60 min, and post-CPB 120 min. In addition, the systemic arterial pressure was monitored continually and the arterial blood gas analyses were performed repeatedly throughout the experiments.
SPSS for Windows 11.0.1 (SPSS Inc. 2001, USA) was used for statistics. All data were expressed as means ± SD. Following analysis of variance, the Student’s t test was used as a test for statistical significance of normal distributed values between the groups. To analyze differences within each group for time effects, paired Student’s t tests including a correction of the error according to Bonferroni probabilities for repeated measurements were used. P < 0.05 was considered statistically significant.
The MAP remained constant and the results of blood gas analysis were within the acceptable range in both groups using this experimental design and CPB technique (Table 1). However, during the entire bypass period, the heart kept beating and lung perfusion persisted. Due to the existence of the shunt, this could result in lower blood oxygenation in the CPB group vs the sham. As is the nature of CPB, hemodilution and a significant decrease in pH were observed in our model similar to that seen in clinical practice.
| Variables | Groups | Prior to CPB | CPB 30 min | CPB 60 min | Post CPB 60 min | Post CPB 120 min |
| MAP (mmHg) | CPB | 74 ± 8 | 77 ± 7 | 75 ± 6 | 75 ± 5 | 72 ± 5 |
| Sham | 73 ± 5 | 75 ± 6 | 77 ± 6 | 75 ± 7 | 77 ± 5 | |
| pH | CPB | 7.40 ± 0.02 | 7.36 ± 0.02ac | 7.31 ± 0.04bc | 7.27 ± 0.04bc | 7.22 ± 0.03bc |
| Sham | 7.40 ± 0.02 | 7.39 ± 0.01 | 7.40 ± 0.02 | 7.39 ± 0.02 | 7.39 ± 0.02 | |
| PaO2 (mmHg) | CPB | 414 ± 16 | 279 ± 13bc | 280 ± 14bc | 370 ± 21bc | 372 ± 21bc |
| Sham | 404 ± 16 | 401 ± 16 | 394 ± 15 | 406 ± 14 | 402 ± 19 | |
| PaCO2 (mmHg) | CPB | 38.8 ± 4.2 | 39.3 ± 2.8 | 38.9 ± 2.1 | 38.8 ± 3.8 | 40.2 ± 4.9 |
| Sham | 39.1 ± 2.2 | 40.9 ± 1.6 | 38.3 ± 2.8 | 38.6 ± 2.5 | 40.3 ± 3.9 | |
| Hematocrit (%) | CPB | 40.8 ± 2.2 | 33.0 ± 0.9bc | 33.4 ± 1.0bc | 32.3 ± 0.9bc | 32.8 ± 0.8bc |
| Sham | 39.7 ± 1.4 | 40.8 ± 2.1 | 40.2 ± 1.7 | 39.3 ± 1.9 | 38.5 ± 1.6 |
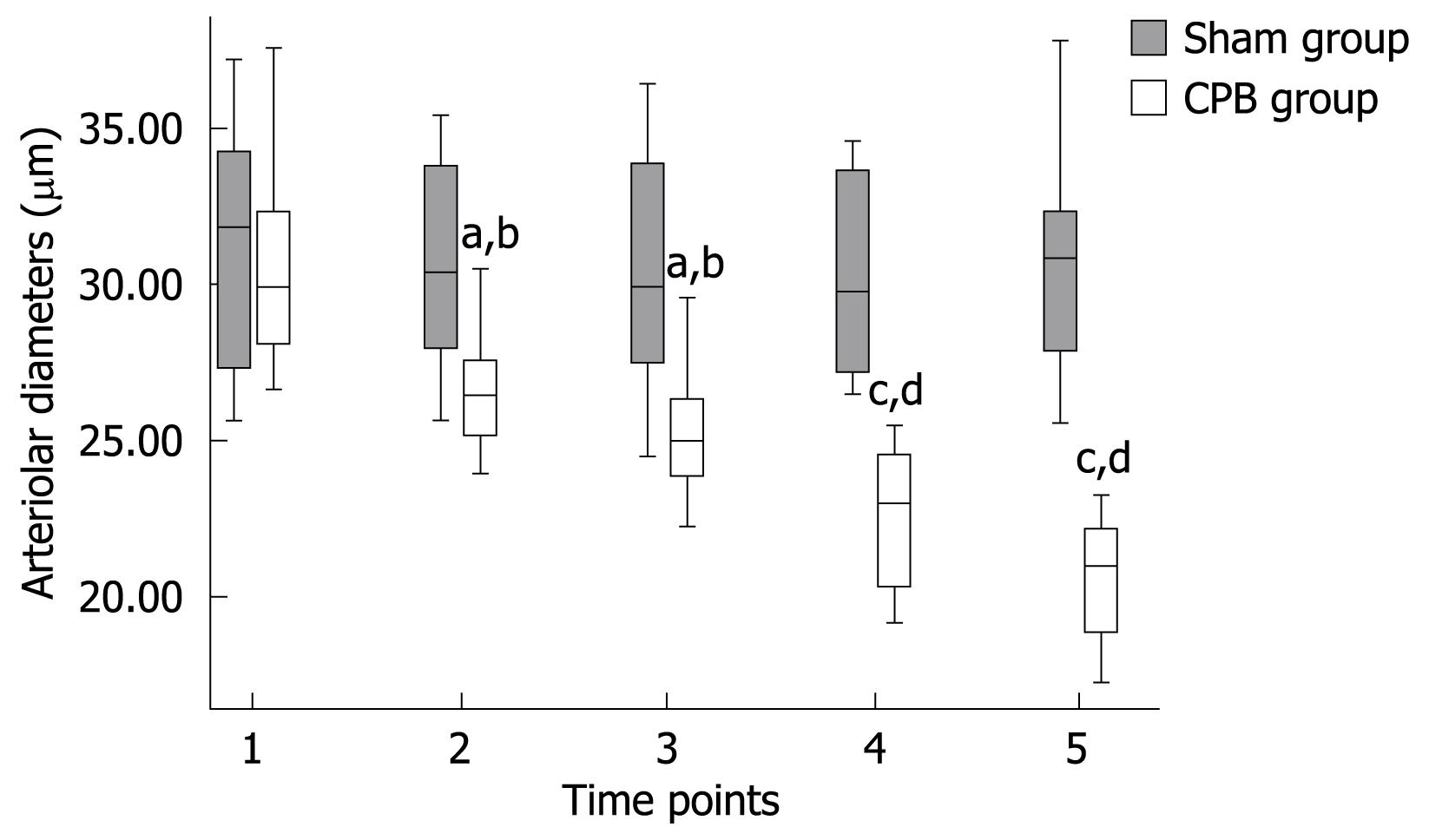
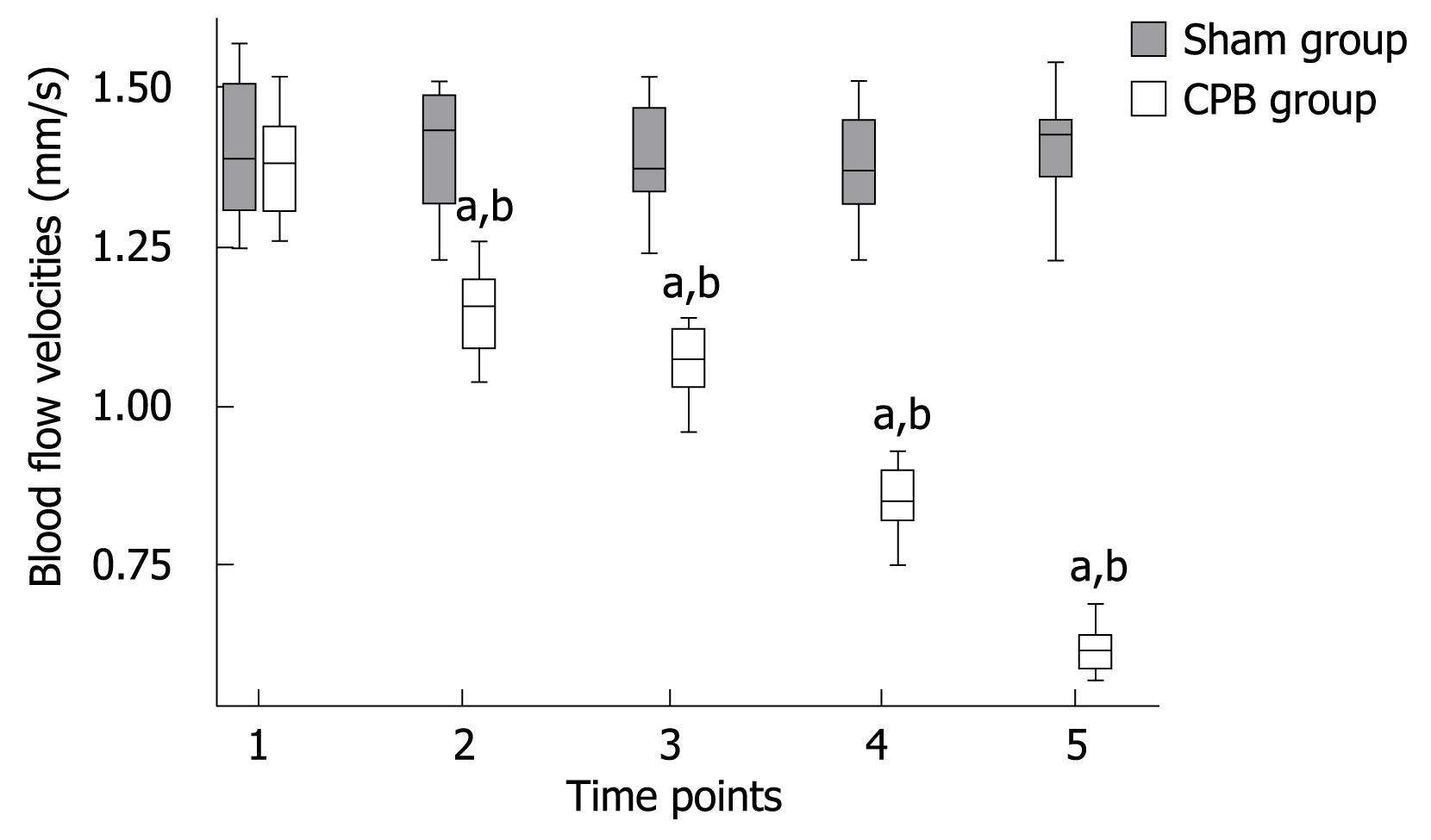
In the CPB group, a significant pathological segmental vasoconstriction not ascribed to undulating vasomotion was detected in small vessel segments. Mean arteriolar diameters of single arterioles in the mucosal layer were found to be diminished from 30.82 ± 3.57 prior to CPB to 20.59 ± 1.97 &mgr;m (P < 0.001) at the end of the experiment (Figure 1). Blood flow was apparently reduced in the collecting venules in the submucosa, with the red blood cell flow velocities reduced from 1.38 ± 0.08 mm/s prior to initiation of CPB to 0.62 ± 0.04 mm/s (P < 0.001) at 2 h after bypass (Figure 2). As a consequence, a concomitant hemoconcentration, typical sludge phenomena and cell aggregates within these vessels could be observed. In the control animals, the arterioles showed no changes in diameter and the blood cell velocities remained constant throughout the observation period. Functional capillary density (FCD) was expressed as the length of FITC-BAS perfused capillaries per observational area. Both the intestinal mucosa and the smooth muscle layer were analyzed. In the sham group, the perfusion was homogeneous in both compartment capillary networks (Table 2). However, in the CPB group, the FCD values decreased significantly during and after bypass and reached only 30% of initial values at the end of the observation period.
| Variables | Groups | Prior to CPB | CPB 30 min | CPB 60 min | Post-CPB 60 min | Post-CPB 120 min |
| FCD in mucosa (cm-1) | CPB | 511 ± 17 | 406 ± 12ab | 328 ± 11ab | 219 ± 13ab | 150 ± 10ab |
| Sham | 504 ± 17 | 510 ± 20 | 499 ± 18 | 513 ± 14 | 507 ± 16 | |
| FCD in muscle (cm-1) | CPB | 221 ± 13 | 152 ± 10ab | 125 ± 9ab | 102 ± 8ab | 64 ± 7ab |
| Sham | 218 ± 14 | 221 ± 12 | 212 ± 17 | 217 ± 13 | 211 ± 14 |
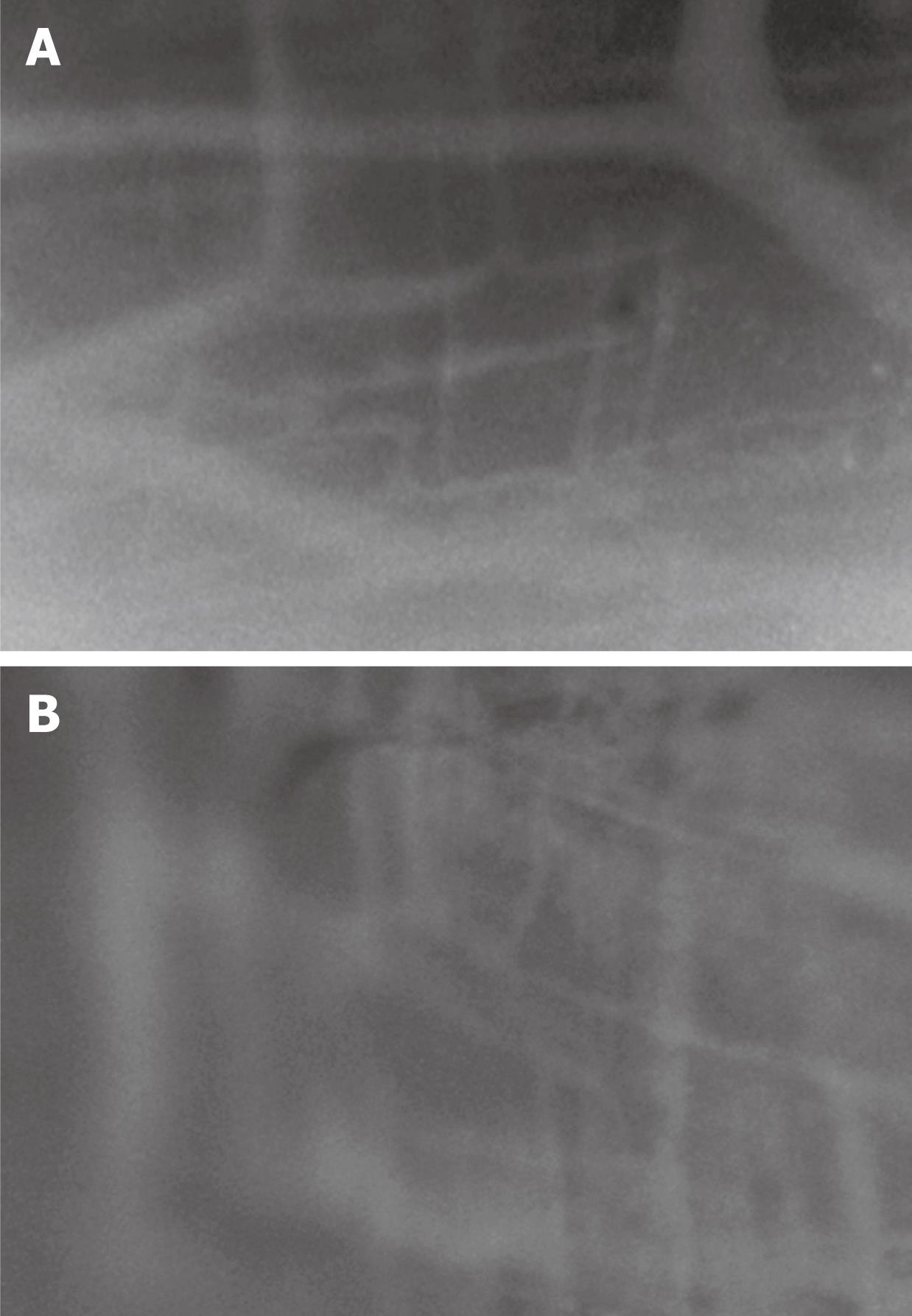
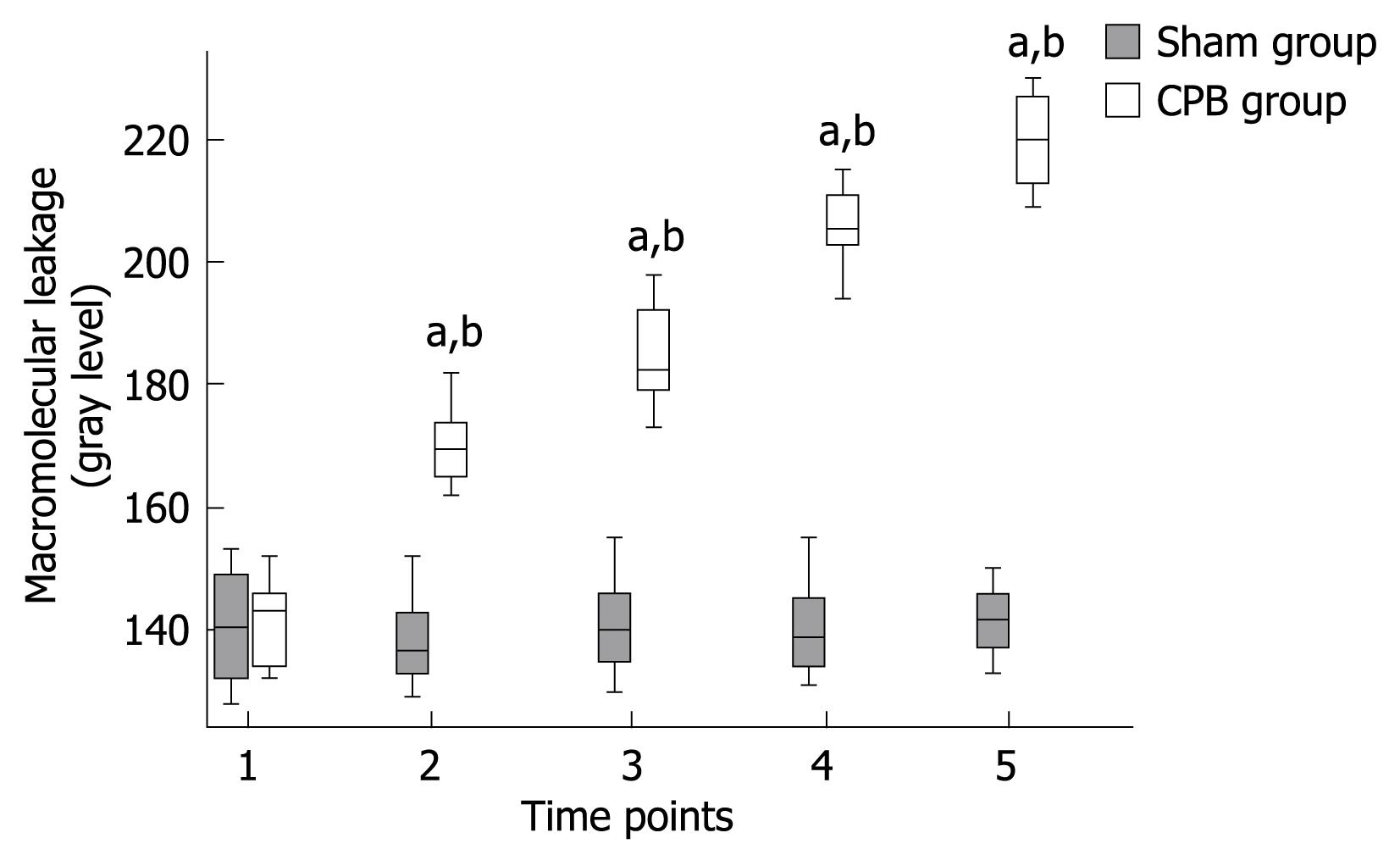
A significant increase in macromolecular leakage induced by CPB was observed in this study. Under circumstances resulting in increased microvascular permeability to macromolecules, FITC-BSA was seen to leak from the vasculature, appearing as a flare in the perivascular interstitium. The interstitial fluorescent intensity was proportional to the degree of FITC-BSA leakage from the vessels. In control animals, the vascular integrity was maintained with no observed increases in interstitial fluorescence during the experimental period (Figures 3 and 4). In the CPB group, the interstitial fluorescence increased at all time points from a gray level of 141.8 ± 7.3 at the beginning of CPB to 219.9 ± 7.1 at 2 h after weaning off bypass (P < 0.001).
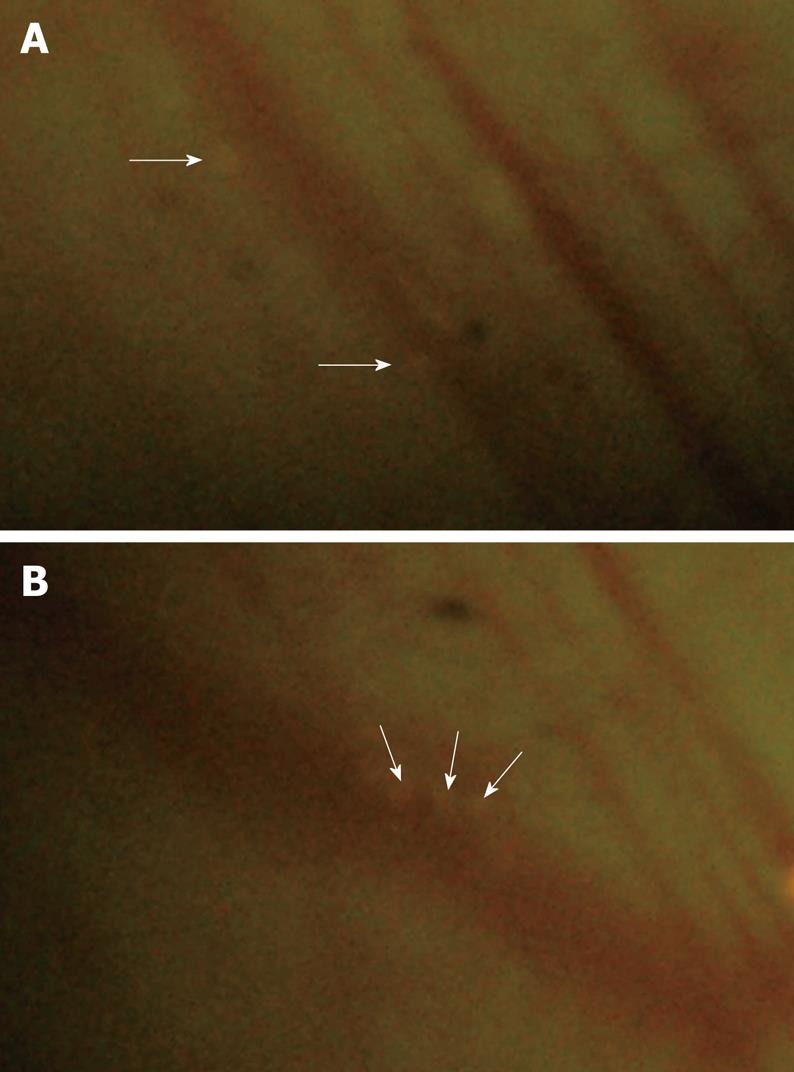
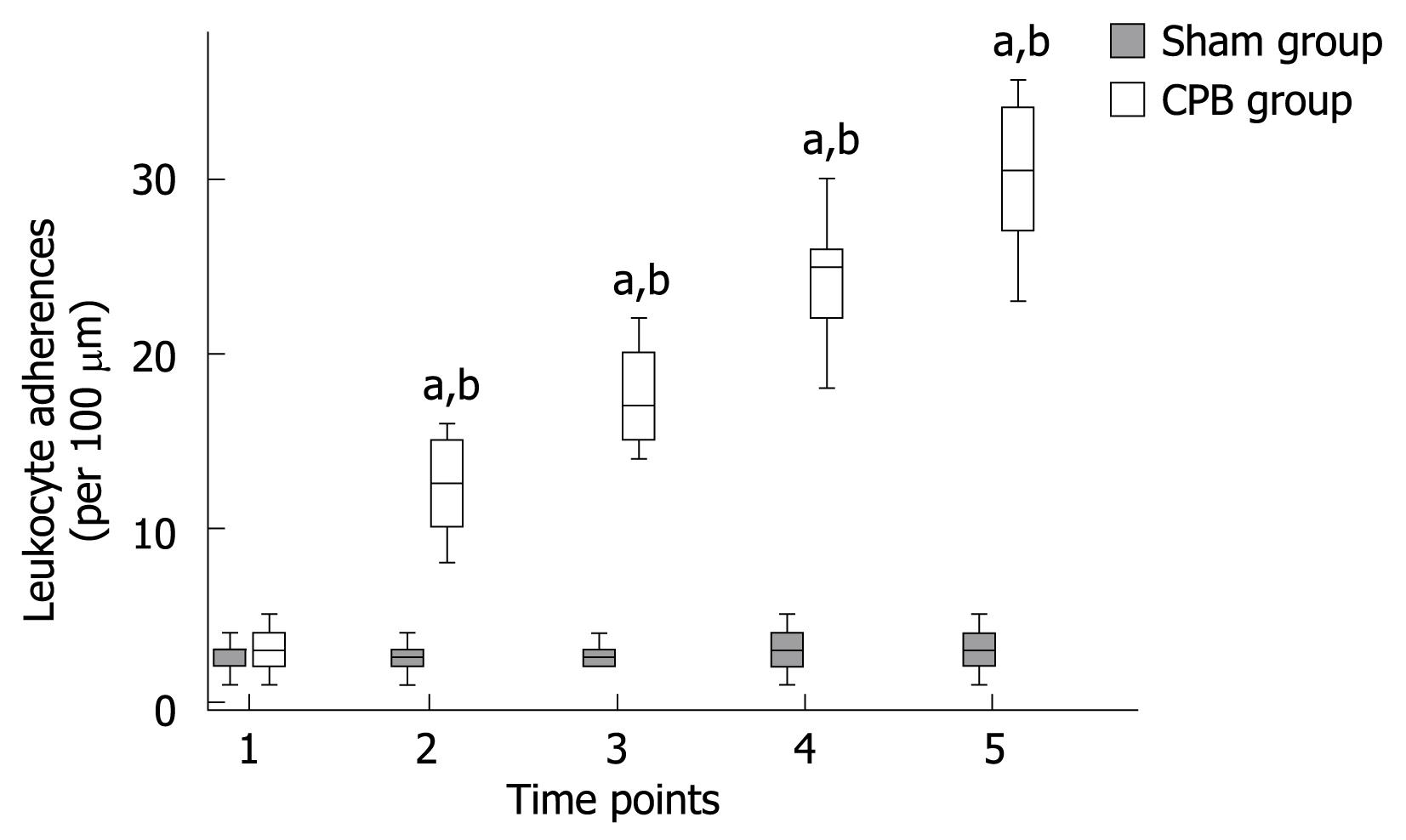
Leukocyte adherence was analyzed within postcapillary venules of the intestinal submucosa. A leukocyte was considered to be adherent in each vessel segment if it did not move or detach from the endothelial lining for at least 30 s. Stationary leukocytes that had migrated to the interstitium but remained in close proximity to the capillaries were also regarded as adherent. Data was expressed as the number of adherent leukocytes per 100 &mgr;m length of venular endothelial surface. In the sham group, only occasional adherent leukocytes (2.7 ± 0.9 cells/100 &mgr;m) were found firmly adhered to the microvascular endothelium throughout the experimental period (Figures 5 and 6). In the CPB group, a rapid, sustained, and significant increase in leukocyte adherence (about 10-fold at the end of experiments) was induced within the submucosal capillaries (P < 0.001) during and after CPB.
Nowadays, due to various improvements, CPB has made cardiovascular surgery more and more practical and safe even in high-risk patients. However, there are still many complications which are thought to result from a systemic inflammatory response and non-occlusive visceral ischemia[571415]. Recent research has suggested that the intestinal mucosa is extremely sensitive to ischemia-reperfusion (I/R) injury and the gut is both the source and the target of inflammatory mediators[1617]. These changes might contribute to a severe loss of intestinal function with significant complications or even multiorgan failure and systemic shock as a pathological consequence. In clinical practice, the incidence of gastrointestinal complications is low (0.58%-2.0%), however, they are associated with an unacceptable high mortality (15%-63%), especially in patients with pre-existing impairment of bowel perfusion[151819]. With the aim of identifying effective preventive and therapeutic approaches, studies on the underlying pathomechanisms of CPB in small bowel capillary perfusion seem to be imperative.
Methods such as laser Doppler flow measurements and tonometry have been described in this area, but they are both indirect measures which do not allow quantitative analysis in different segments of the microvascular bed[92021]. In contrast, intravital microscopy allows direct observation and quantitative analysis of the tissues and organs at the level of microcirculation and acquires more information on the pathologic role of CPB. The mesentery has been used frequently in studies of the intestinal microvasculature, but it has very little in common with intestinal microvascular networks[2223]. In our experiments, the use of intravital microscopy employing different fluorescent dyes, allowed the simultaneous quantitative investigation of the microcirculation within all layers of the small intestine, i.e. subserosa, smooth muscle, submucosa and mucosa. For the first time, we directly investigated the intestinal microcirculatory changes within different layers relating to CPB by intravital microscopy.
The majority of studies on CPB relating to pathophysiological processes have been performed in large animal models[24]. These models have substantial limitations, mainly in that their costs are extremely high, instruments are very complex and they are too labor intensive. Rats are one of the most standard models for intravital microscopy and have been extensively used for the experimental investigation of multiple organ injury in various areas[121322]. In our experiments, a rat CPB model with full flow perfusion was established. This model also reduced the cost of animals and equipment.
In the CPB group, the blood pressures were kept constant at a physiological level throughout the experiments without vasoactive drugs and the systemic temperatures were stable at around 37°C. Despite the consistent condition of systemic hemodynamics, microvascular perfusion impairments of the small bowel were clearly demonstrated. Arteriolar vasoconstriction, the reduction in blood cell velocities, together with the consequence of intravascular hemoconcentration and sludge formation, reflected hypoperfusion at the microvascular level. The reduction in the FCD values at the end of the observation period to approximately 30% of the pre-bypass values revealed that the capillary bed had been severely injured. The extravasation of FITC-BSA showed a distinct increase in the permeability of the vascular wall which might result in a loss of the natural barrier function against toxins and microorganisms. This might aggravate the injury to capillary perfusion further. Enhanced leukocyte adherence to the venular wall within submucosal vessels was also demonstrated in this study, which indicated that activated leukocytes may be the primary mediators and may have a causative role in the following pathophysiological sequelae.
The potential underlying pathogenesis of the intestinal injury induced by CPB includes several pathophysiological mechanisms[1141525], such as a transient reduction in systemic blood pressure and low cardiac outflow (LCO), hypotemperature in the bypass process, redistribution of local organ blood flow, reactions of inflammatory mediators or vasoconstrictive agents, and obstruction of arteriolae with fragments of cells or tissues. With our model, due to the fact that the flow rate of the bypass circuit was kept constant and the arterial blood pressure remained unchanged, the influences of macrohemodynamics could be avoided. This allowed the conclusion that the observed changes in microvascular perfusion in the small bowel were not only a result of systemic hypoperfusion or LCO syndrome. By keeping the systemic and tissue temperatures in the normal range, we could exclude the effects of temperature on the microvascular perfusion in our study. Redistribution of blood flow and induction of inflammatory response seemed to be the major mechanisms responsible for the observed results[226]. Non-physiological patterns of perfusion originating from the rolling pump results in maldistribution of blood flow away from visceral organs as a reaction to CPB which serves as the primary contributor to microvascular impairments. It is important to note that the degree of damage to the microcirculation reached a maximum even after CPB removal for 2 h, suggesting that the ensuing inflammatory process as a consequence of CPB could be the dominant mechanism. The contact of blood and blood cells with the bypass circuit surfaces, the trauma induced by mechanical stress from the pump, together with the I/R injury of the gastrointestinal tract resulting from the transient decrease in local blood flow can induce the release of inflammatory mediators (e.g. cytokines, thromboxane, and histamine, etc), secretion of proteases (e.g. collagenase and elastase), activation of complements, extravasation of leukocytes, and the generation of oxygen free radicals[61223]. These substances may lead to vasoconstriction, hemoconcentration and sludge formation, endothelial cell swelling and the consequent occlusion of vessels, and an increase in vascular permeability.
In conclusion, using intravital microscopy, progressive microvascular injury in the small bowel was demonstrated in a rat CPB model. The ensuing injury response was associated with microvascular perfusion failure, vascular permeability increase, and promotion of circulating leukocyte adherence. These changes in microvessels were even more prominent after the bypass period and no signs of restitution could be observed. The observed phenomena may have resulted from the redistribution of blood flow in different organs and the generation of inflammatory metabolites with potential negative effects on microvascular perfusion during and after CPB. The information obtained from our experiments will facilitate further investigations of different therapeutic regimens aimed at ameliorating microvascular damage due to CPB.
Impairment of intestinal microvascular perfusion induced by cardiopulmonary bypass (CPB) is thought to be related to mucosal edema formation and microvascular barrier injury, which may result in postoperative gastrointestinal complications and multiple organ failure. A better understanding of the microvascular alterations during and after CPB will facilitate the identification of therapeutic strategies.
In general, experimental investigations on the intestinal injury induced by CPB are scarce. The identification of rational clinical therapeutic approaches requires a precise elucidation of the underlying pathophysiological processes of intestinal microvasculature induced by CPB.
With a novel rat model of CPB, the authors quantitatively investigated the microvascular alterations post CPB in small bowel by means of fluorescent intravital microscopy, which allows the simultaneous quantitative investigation of the microcirculation of the small intestine.
By understanding pathomechanisms of CPB in small bowel capillary perfusion, this study may help to identify effective preventive and therapeutic approaches.
Functional capillary density (FCD) is one of the parameters obtained by intravital microscopy using epi-illumination of the tissue surface or trans-illumination of thin tissue layers. FCD, defined as the length of red cell-perfused capillaries per observation area (cm-1), has been used as an indicator of the quality of tissue perfusion in various animal models. Quantitative analysis of FCD in randomly selected regions of the tissue is performed by means of a computer-assisted video analysis system which allows calculation of the length of RBC-perfused capillaries.
In this paper, the authors used intravital microscopy for assessment of intestinal microcirculation in rats during and after CPB. The authors confirmed arteriolar vasoconstriction, blood velocity reduction and FCD diminution, exaggerated albumin extravasation and increased leukocyte accumulation in intestine in rats during and after CPB.
| 1. | Kumle B, Boldt J, Suttner SW, Piper SN, Lehmann A, Blome M. Influence of prolonged cardiopulmonary bypass times on splanchnic perfusion and markers of splanchnic organ function. Ann Thorac Surg. 2003;75:1558-1564. [Cited in This Article: ] |
| 2. | Tsunooka N, Hamada Y, Imagawa H, Nakamura Y, Shiozaki T, Suzuki H, Kikkawa H, Miyauchi K, Watanabe Y, Kawachi K. Ischemia of the intestinal mucosa during cardiopulmonary bypass. J Artif Organs. 2003;6:149-151. [Cited in This Article: ] |
| 3. | Velissaris T, Tang A, Murray M, El-Minshawy A, Hett D, Ohri S. A prospective randomized study to evaluate splanchnic hypoxia during beating-heart and conventional coronary revascularization. Eur J Cardiothorac Surg. 2003;23:917-924; discussion 924. [Cited in This Article: ] |
| 4. | Tsunooka N, Maeyama K, Hamada Y, Imagawa H, Takano S, Watanabe Y, Kawachi K. Bacterial translocation secondary to small intestinal mucosal ischemia during cardiopulmonary bypass. Measurement by diamine oxidase and peptidoglycan. Eur J Cardiothorac Surg. 2004;25:275-280. [Cited in This Article: ] |
| 5. | Ohri SK, Velissaris T. Gastrointestinal dysfunction following cardiac surgery. Perfusion. 2006;21:215-223. [Cited in This Article: ] |
| 6. | Rossi M, Sganga G, Mazzone M, Valenza V, Guarneri S, Portale G, Carbone L, Gatta L, Pioli C, Sanguinetti M. Cardiopulmonary bypass in man: role of the intestine in a self-limiting inflammatory response with demonstrable bacterial translocation. Ann Thorac Surg. 2004;77:612-618. [Cited in This Article: ] |
| 7. | Fitzgerald T, Kim D, Karakozis S, Alam H, Provido H, Kirkpatrick J. Visceral ischemia after cardiopulmonary bypass. Am Surg. 2000;66:623-626. [Cited in This Article: ] |
| 8. | Cox CS Jr, Allen SJ, Brennan M. Analysis of intestinal microvascular permeability associated with cardiopulmonary bypass. J Surg Res. 1999;83:19-26. [Cited in This Article: ] |
| 9. | Booker PD, Pozzi M. A placebo-controlled study of the effects of dopexamine on gastric mucosal perfusion in infants undergoing hypothermic cardiopulmonary bypass. Br J Anaesth. 2000;84:23-27. [Cited in This Article: ] |
| 10. | Haisjackl M, Germann R, Hasibeder W, Schwarz B, Salak N, Pajk W, Bonatti J, Nussbaumer W, Klima G, Kox W. Mucosal tissue oxygenation of the porcine jejunum during normothermic cardiopulmonary bypass. Br J Anaesth. 1999;82:738-745. [Cited in This Article: ] |
| 11. | Dong GH, Xu B, Wang CT, Qian JJ, Liu H, Huang G, Jing H. A rat model of cardiopulmonary bypass with excellent survival. J Surg Res. 2005;123:171-175. [Cited in This Article: ] |
| 12. | Kalia N, Pockley AG, Wood RF, Brown NJ. Effects of hypothermia and rewarming on the mucosal villus microcirculation and survival after rat intestinal ischemia-reperfusion injury. Ann Surg. 2002;236:67-74. [Cited in This Article: ] |
| 13. | Massberg S, Gonzalez AP, Leiderer R, Menger MD, Messmer K. In vivo assessment of the influence of cold preservation time on microvascular reperfusion injury after experimental small bowel transplantation. Br J Surg. 1998;85:127-133. [Cited in This Article: ] |
| 14. | Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715-S720. [Cited in This Article: ] |
| 15. | Byhahn C, Strouhal U, Martens S, Mierdl S, Kessler P, Westphal K. Incidence of gastrointestinal complications in cardiopulmonary bypass patients. World J Surg. 2001;25:1140-1144. [Cited in This Article: ] |
| 16. | Baue AE. Sepsis, systemic inflammatory response syndrome, multiple organ dysfunction syndrome, and multiple organ failure: are trauma surgeons lumpers or splitters? J Trauma. 2003;55:997-998. [Cited in This Article: ] |
| 17. | Pimenta MB, Aguilar-Nascimento JE, Martins DC, Silva DR, Bacelo KL, Bocchese IC, Zaffani S, Zaffani E, Silveira EA, Carmo AV. The intestinal tract as the major source of interleukin 6 production during abdominal aortic clamping and hind limb ischaemia-reperfusion injury. Acta Cir Bras. 2007;22 Suppl 1:34-39. [Cited in This Article: ] |
| 18. | Geissler HJ, Fischer UM, Grunert S, Kuhn-Régnier F, Hoelscher A, Schwinger RH, Mehlhorn U, Hekmat K. Incidence and outcome of gastrointestinal complications after cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 2006;5:239-242. [Cited in This Article: ] |
| 19. | Filsoufi F, Rahmanian PB, Castillo JG, Scurlock C, Legnani PE, Adams DH. Predictors and outcome of gastrointestinal complications in patients undergoing cardiac surgery. Ann Surg. 2007;246:323-329. [Cited in This Article: ] |
| 20. | Bastien O, Piriou V, Aouifi A, Flamens C, Evans R, Lehot JJ. Relative importance of flow versus pressure in splanchnic perfusion during cardiopulmonary bypass in rabbits. Anesthesiology. 2000;92:457-464. [Cited in This Article: ] |
| 21. | Bastien O, Piriou V, Aouifi A, Evans R, Lehot JJ. Effects of dopexamine on blood flow in multiple splanchnic sites measured by laser Doppler velocimetry in rabbits undergoing cardiopulmonary bypass. Br J Anaesth. 1999;82:104-109. [Cited in This Article: ] |
| 22. | Anthoni C, Rijcken EJ, Laukoetter MG, Spiegel HU, Senninger N, Schurmann G, Krieglstein CF. Submucosal collecting venules: a reliable site for intestinal intravital microscopy in rats. J Invest Surg. 2002;15:259-267. [Cited in This Article: ] |
| 23. | Kurose I, Anderson DC, Miyasaka M, Tamatani T, Paulson JC, Todd RF, Rusche JR, Granger DN. Molecular determinants of reperfusion-induced leukocyte adhesion and vascular protein leakage. Circ Res. 1994;74:336-343. [Cited in This Article: ] |
| 24. | Modine T, Azzaoui R, Fayad G, Lacroix D, Bordet R, Warembourg H, Gourlay T. A recovery model of minimally invasive cardiopulmonary bypass in the rat. Perfusion. 2006;21:87-92. [Cited in This Article: ] |
| 25. | Ohri SK. Systemic inflammatory response and the splanchnic bed in cardiopulmonary bypass. Perfusion. 1996;11:200-212. [Cited in This Article: ] |
| 26. | Tao W, Zwischenberger JB, Nguyen TT, Vertrees RA, McDaniel LB, Nutt LK, Herndon DN, Kramer GC. Gut mucosal ischemia during normothermic cardiopulmonary bypass results from blood flow redistribution and increased oxygen demand. J Thorac Cardiovasc Surg. 1995;110:819-828. [Cited in This Article: ] |