Published online May 14, 2009. doi: 10.3748/wjg.15.2214
Revised: January 22, 2009
Accepted: January 29, 2009
Published online: May 14, 2009
AIM: To evaluate the feasibility and utility of confocal laser endomicroscopy (CLE) in the description of normal gastrointestinal (GI) mucosa and in the diagnosis of GI disorders in children, in comparison to histology.
METHODS: Forty-four patients (19 female) median age 10.9 years (range 0.7-16.6 years) with suspected or known GI pathology underwent esophago-gastro-duodenoscopy (OGD) (n = 36) and/or ileocolonoscopy (IC) (n = 31) with CLE using sodium fluorescein and acriflavine as contrast agents. Histological sections were compared with same site confocal images by two experienced pediatric and GI histopathologists and endoscopists, respectively.
RESULTS: Duodenum and ileum were intubated in all but one patient undergoing OGD and IC. The median procedure time was 16.4 min (range 7-25 min) for OGD and 27.9 min (range 15-45 min) for IC. A total of 4798 confocal images were compared with 153 biopsies from the upper GI tract from 36 procedures, and 4661 confocal images were compared with 188 biopsies from the ileocolon from 31 procedures. Confocal images were comparable to conventional histology both in normal and in pathological conditions such as esophagitis, Helicobacter pylori gastritis, celiac disease, inflammatory bowel disease, colonic heterotopia, and graft versus host disease.
CONCLUSION: CLE offers the prospect of targeting biopsies to abnormal mucosa, thereby increasing diagnostic yield, reducing the number of biopsies, decreasing the burden on the histopathological services, and reducing costs.
- Citation: Venkatesh K, Cohen M, Evans C, Delaney P, Thomas S, Taylor C, Abou-Taleb A, Kiesslich R, Thomson M. Feasibility of confocal endomicroscopy in the diagnosis of pediatric gastrointestinal disorders. World J Gastroenterol 2009; 15(18): 2214-2219
- URL: https://www.wjgnet.com/1007-9327/full/v15/i18/2214.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2214
Modern endoscopy has recently seen the development of technological advances with the aim of increasing and optimizing diagnostic yield from the procedure. These have included video and magnification endoscopes[1]. Greater surface definition has been achieved with chromo-endoscopy, and recently, narrow-band imaging has allowed greater definition of vascular architecture[2–4]. However in vivo sub-surface pathology remained obscure to the endoscopist until the advent of confocal endomicroscopy, which affords magnification up to 1000 ×, and with sequentially deeper images from the epithelial surface to approximately 250 &mgr;m below the surface. This allows histological assessment of the in vivo gastrointestinal (GI) mucosal structure at the cellular and subcellular level[56]. In addition, this technique avoids crush artefacts from the grasp biopsy forceps and changes from histopathological processing.
The diagnosis of upper GI disorders in children depends to a great extent on endoscopy and subsequent histology of biopsy specimens[7]. Pathology such as gastroesophageal reflux disease (GERD)[8–10], eosinophilic esophagitis (EE)[1112]; Helicobacter pylori (H pylori) gastritis[1314], and celiac disease (CD)[1516], in conjunction with various other investigative modalities have, as pivotal to their diagnosis, histological confirmation. Similarly, pediatric ileocolonic conditions such as inflammatory bowel disease (IBD)[1718], familial adenomatous polyposis (FAP), graft versus host disease (GVHD)[1920], and allergic colitis[2122] necessitate a tissue diagnosis.
The aims of this study were to evaluate the feasibility and utility of confocal laser endomicroscopy (CLE) in the description of confocal features of normal GI mucosa and in the diagnosis of GI disorders in children.
Forty-four patients with a potential diagnosis of GI pathology that required upper GI endoscopy and/or ileocolonoscopy as part of the clinical management were enrolled in the study. Written informed consent was obtained from parents and, where age and competency were appropriate, from each patient, before the examination. The study protocols were reviewed and approved by South Sheffield Regional Ethics Committee. Patient exclusion criteria were as follows: inability to give signed informed consent; age > 18 years; previous documented adverse reaction/allergy to sodium fluorescein or acriflavine hydrochloride; and non-correctable coagulopathy (PT > 14 s/platelet count < 90 000). The study was conducted between December 2005 and July 2007 at Sheffield Children’s Hospital NHS Foundation Trust.
Indications for upper GI endoscopy alone included: children with suspected GERD; Barrett’s esophagus; suspected peptic ulcer disease; suspected celiac disease based on raised anti-endomysial and tissue transglutaminase antibodies; and non-specific recurrent upper abdominal pain. Indications for ileocolonoscopy included: chronic diarrhea; presence of fecal blood; recurrent abdominal pain; weight loss; mutation of the APC gene; colonic heterotopia; and suspected GVHD.
Forty-four patients (19 female) with a median age of 10.9 years (range 0.7-16.6 years), and a median weight of 41.5 kg (range 8-97 kg) with suspected or known GI pathology were enrolled.
Patients undergoing ileocolonoscopy were admitted the previous day and had bowel preparation as for standard ileocolonoscopy. Patients undergoing upper GI endoscopy were admitted on the day of the procedure. All procedures occurred under general anesthesia, as is normal practice in our institution for pediatric GI endoscopy.
CLE involves the use of a highly miniaturized confocal microscope that has been incorporated into the distal tip of a flexible endoscope to allow in vivo microscopic examination of the gut mucosa. The confocal microscope uses a single optical fiber to deliver 488 nm laser light to the distal tip of the endoscope, where it is focused to a single diffraction-limited point within the tissue. The laser light excites fluorescent molecules within the tissue. Fluorescent light emanating from the specific point of focus is collected into the same optical fiber of the confocal microscope and delivered to the photodetector. Light emanating from outside the focally illuminated spot is not focused into the optical fiber and therefore, is geometrically rejected from detection. The focused point of laser light is scanned in a raster pattern across the field of view, and the intensity of the fluorescent signal returning to the detector from successive points is measured (12-bit digitization) to produce two-dimensional images that are en face to the tissue surface. By moving the microscope optics within the confocal microscope, the operator can dynamically adjust the imaging depth to allow microscopic imaging at and below the surface of the mucosa; hence each image is an optical section representing one focal plane within the specimen[523], and collection of multiple optical sections at successive depths results in true volumetric sampling of the tissue. As a three-dimensional volume is thus sampled, this can be thought of as a virtual biopsy.
The Pentax EC3870CILK endoscope has a 5-mm diameter miniaturized confocal microscope integrated into the distal tip of the endoscope. The diameter of the distal tip and insertion tube of the endoscope is 12.8 mm. In addition to the integrated confocal microscope, the distal tip also contains a color CCD camera which enables simultaneous confocal microscopy with standard video-endoscopy, air and water jet nozzles, two light guides, a 2.8-mm working channel, and an auxiliary water jet channel. During CLE, the laser delivers an excitation wavelength of 488 nm at a maximum laser output of 1 mW to the tissue (typically 300-700 &mgr;W). Confocal images can then be collected at either 1024 × 1024 pixels (0.8 frames/s) or 1024 × 512 pixels (1.6 frames/s). The optical sections have a 475 &mgr;m × 475 &mgr;m field of view, with a lateral resolution of 0.7 &mgr;m, axial resolution of 7.0 &mgr;m, and an imaging depth (z axis) range of 0-250 &mgr;m below the tissue surface, in 4-&mgr;m steps. The imaging depth below the tissue surface can be dynamically controlled by the operator. CLE magnifies images 1000-fold.
Fluorescein sodium (FS) 10% and acriflavine hydrochloride (AH) 0.05% were used as contrast agents. FS is highly water-soluble and, on intravenous administration, rapidly diffuses in seconds from the capillaries into the extra-vascular tissue. FS, when exposed to light of wavelength 465-490 nm (blue), emits light at longer wavelengths (520-650 nm, with the peak emission in the 520-530 nm green-yellow region)[24]. This enables visualization of microvessels, cells and connective tissue. However FS is not enriched in the nuclei of intestinal epithelial cells, and hence, the nuclei are not readily visible in the confocal images. To circumvent this limitation, AH (0.05%) is used topically to enrich the superficial nuclei and to a lesser extent the cytoplasm.
CLE was performed by a single experienced endoscopist (MT), who had completed the Mainz CLE training program prior to patient recruitment, using the confocal laser endomicroscope (EC3870CILK; Pentax, Tokyo, Japan). Ten to twenty milligrams Buscopan (hyoscine-N-butyl-bromide; Boehringer, Ingelheim, Germany) was given intravenously to limit peristaltic artefacts. Following duodenal or ileal intubation, 0.05-0.1 mL/kg of 10% FS was administered intravenously and flushed adequately with normal saline. AH (0.05%) was applied to the mucosa using a spray catheter at all sites undergoing confocal imaging.
CLE image acquisition was performed by placing the tip of the colonoscope in direct contact with the target tissue site. Using gentle suction to stabilize the mucosa, image acquisition and focal plane z-axis scanning depth was then actuated using two discrete hand-piece control buttons. Confocal images were sequentially obtained from a third part of the duodenum, gastric antrum and body, and distal and proximal esophagus in the upper GI tract, and ileum, cecum, ascending, transverse, descending and sigmoid colon, and rectum in the ileocolon region. Confocal images were acquired simultaneously with ongoing video endoscopic imaging. Same site mucosal specimens were obtained using standard biopsy forceps. The biopsy specimens were fixed in buffered formalin solution, embedded in paraffin wax, and serial sections were obtained and stained with hematoxylin and eosin (HE). The histological specimens from each site were compared with same-site confocal images jointly by the endoscopists and two experienced pediatric and GI histopathologists (MC, CE).
Twenty-three patients underwent both upper GI and ileocolonoscopy, while 13 had upper GI endoscopy and eight had ileocolonoscopy alone. The youngest patient 8 mo of age, with suspected GVHD, had proctoscopy alone. The duodenum at upper GI endoscopy and the terminal ileum at ileocolonoscopy were intubated in all patients, except for one who weighed 10 kg, in whom the pylorus and ileocecal valve were both too narrow to accept the confocal endomicroscope. The youngest and smallest patient to have full successful examinations up to a third part of the duodenum and terminal ileum was 18 mo old and weighed 11 kg. The procedure time was 7-25 min (median 16.4 min) for upper GI endoscopy, and 15-45 min (median 27.9 min) for ileocolonoscopy. A total of 153 pinch biopsies were taken from the upper GI tract from 36 procedures and 188 from the ileocolon from 31 procedures.
No complications or adverse effects occurred, except on one occasion when precipitation was observed in the peripheral venous line when fluorescein was injected immediately after neostigmine.
Thirty-six patients underwent upper GI endoscopy. The duodenum was intubated in all patients except one. A total of 4798 confocal images were obtained, which included 2010 from the duodenum, 1616 from the stomach and 1172 from the esophagus, and were compared with 153 biopsies.
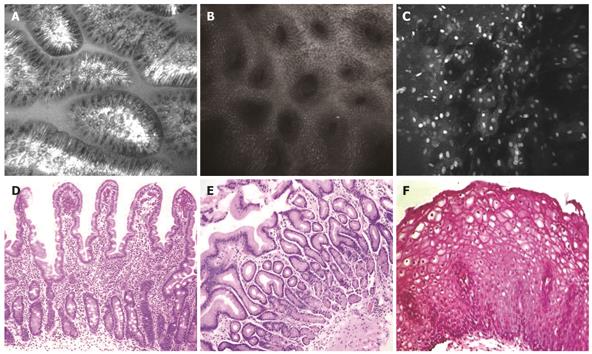
On confocal imaging, the duodenal villi had a long, slender and finger-like appearance (Figure 1A) similar to that in histological specimens (Figure 1C). The single layer of brush border columnar epithelial cells interspersed with intraepithelial lymphocytes and goblet cells was clearly visible. Crypts were not normally visible except in the presence of villous atrophy.
The gastric pits or foveolae appeared as invaginations on the surface epithelium (Figure 1B and D). Each confocal image showed several evenly spaced such pits lined by columnar epithelium. The center of the pits appeared dark.
The esophagus was lined by non-keratinized squamous epithelium with polygonal epithelial cells. The nuclei of the epithelial cells were highlighted clearly following topical administration of acriflavine (Figure 1C and F). Furthermore, the capillary loops in the papillae were visible in deeper planes following subsurface optical sectioning, and surface to capillary distance could be measured as each level was deeper by 4 &mgr;m. This allowed assessment of GERD-like histopathology, given that papillary height was increased and epithelial surface to papillary tip (i.e. where capillary loops appeared on confocal endomicroscopy) distance was thereby shorter.
A total of 31 patients underwent ileocolonoscopy. Two patients had only proctoscopy, while total ileocolonoscopy was performed in the rest, with the terminal ileum intubated in all but one patient, who weighed 10 kg. A total of 4661 confocal images, which included 945 from the terminal ileum, 2919 from the colon and 797 from the rectum, were compared with 184 biopsies.
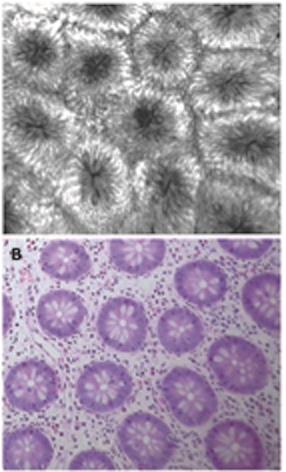
The confocal appearance of the normal ileum and colon in adults has been described previously[25]. The villi in the terminal ileum appeared similar to those in the duodenum. Colonic architecture on confocal imaging showed numerous evenly distributed crypts lined by columnar-shaped enterocytes (Figure 2A and B). The luminal openings of the crypts appeared as black holes in the horizontal axis. The mucin-containing goblet cells were readily identified and appeared dark. At deeper planes, the vessel architecture had a hexagonal, honeycomb pattern, which represented a network of capillaries that outlined the stroma surrounding the luminal openings of the crypts. Individual red cells were also visible as black dots in the lumen of the capillaries.
Upper GI pathology: Two patients had histologically proven esophagitis. At CLE, capillary loops were visible at about 24 and 44 &mgr;m below the surface epithelial layer, which indicated the presence of papillae. In comparison, capillary loops were seen at a median of 72 &mgr;m (range 48-100 &mgr;m) from the surface of the esophageal mucosa in those without histologically proven esophagitis.
One patient with suspected H pylori upon endomicroscopy of the gastric antrum showed multiple focal lesions that resembled focal accumulation of H pylori, which was subsequently confirmed by Campylobacter-like organism test and histology. These lesions were demonstrated both on the surface of the epithelium and deeper in the crypt lumen.
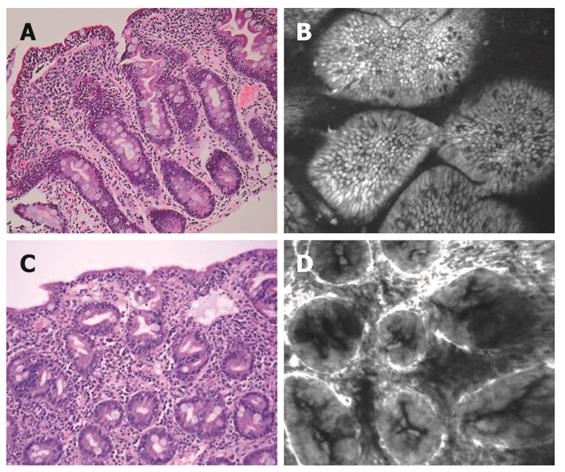
Four patients had a histological diagnosis of celiac disease (Figure 3A and C). Three patients had Marsh type 3b with marked villous atrophy, increased intra-epithelial lymphocytes and crypt hyperplasia. CLE features (Figure 3B) in these patients were as follows: (1) increased basal width of villi; (2) gross distortion of the cellular architecture of the villous epithelium with loss of the honeycomb pattern; (3) damaged villous border; (4) “sticky” villi with inter-villous bridging; and (5) in-folding of villi. One patient had total villous atrophy. Confocal imaging revealed absence of villi with crypt hyperplasia (Figure 3D).
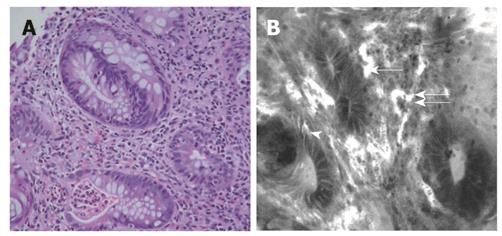
Lower GI pathology: Seven patients had a histological diagnosis of IBD, including three patients each with ulcerative colitis and Crohn’s disease, and one with indeterminate colitis. Features of IBD seen on confocal imaging included bifid crypts, crypt distortion and destruction, crypt abscess/cryptitis goblet cell depletion and inflammatory cell infiltration, enlarged tortuous vessel architecture (Figure 4B), and comparable to histology (Figure 4A).
Other lower GI pathologies: Two patients with suspected GVHD following bone marrow transplantation underwent proctoscopy. Apoptotic nuclei were visualized during confocal imaging. This was confirmed on histology of biopsy specimens. A rare case of colonic heterotopia that presented with persistent diarrhea and had large tracts of abnormal looking mucosa on endoscopy, showed squamous, gastric and small-intestinal mucosal features on confocal imaging. Histology confirmed the presence of aberrant mucosa.
A definitive diagnosis of GI disorders in children usually requires GI endoscopy and histology of biopsy tissue. Technological innovations have led to the development of chromo-endoscopy, for which dyes such as methylene blue and indigo carmine are used to aid localization of lesions, and magnifying endoscopy has enabled visualization of surface structures at approximately × 100 magnification. In adults, several studies have validated these techniques in differentiating neoplastic from non-neoplastic lesions[26–29], diagnosis of neoplastic lesions in flat and depressed lesions in the colorectum, and in cancer surveillance in patients with long-standing ulcerative colitis[3031]. Confocal endomicroscopy is a newly developed tool that enables surface and subsurface imaging of living cells in the mucosa during ongoing endoscopy. It offers the combination of video endoscopy and confocal endomicroscopy, which uniquely provides in vivo histology and what might be termed a virtual biopsy[6]. The confocal endomicroscopy images obtained are in a single optical plane parallel to the surface of the tissue. Collection of multiple optical sections at successive depths allows detailed visualization of successive tissue layers, and allows sampling of a three-dimensional volume of tissue. This is in contrast to conventional histology in which the tissue is sectioned vertically, making it possible to see all the tissue layers in one view using a bench top light microscope. Hence, it is pertinent that confocal images require comparison with similarly sectioned histological images. The endoscopist also requires training in normal and abnormal microscopic anatomy, which takes time. Also a certain amount of training in using the endomicroscope and interpreting the image data is necessary.
In this study, the feasibility of CLE in the diagnosis of GI disorders was determined in children as young as 8 mo of age and as light as 10 kg, for the first time. Confocal findings of normal GI mucosa are described. In addition, confocal features in conditions such as pediatric manifestations of GERD, H pylori gastropathy, celiac disease, IBD, GVHD, and colonic heterotopia were illustrated.
The tantalizing prospect of targeted biopsies or even a biopsy-free endoscopic procedure in the diagnosis of childhood GI disorders arises, with obvious potential benefits in terms of avoidance of biopsy-associated complications, and diminution of the considerable histological burden that this patient cohort places on already over-stretched histopathological services, along with the prospect of considerable associated cost savings.
Histology of biopsy specimens has a major role in the definitive diagnosis of pediatric gastrointestinal (GI) disorders, but is subject to changes from crush artefacts and processing, in addition to an inherent delay in diagnosis. Confocal laser endomicroscopy (CLE) is a recent development that enables surface and subsurface imaging of living cells in vivo at × 1000 magnification.
The relatively new tool of CLE has been used in the assessment and in vivo diagnosis of various GI disorders in adults, such as Barrett’s esophagus, esophageal, gastric and colorectal cancers, and inflammatory bowel disease and other forms of colitis.
This is believed to be the first study to assess this innovation in the diagnosis of pediatric GI disorders. This study confirmed the feasibility and diagnostic reliability of CLE for the in vivo diagnosis of a wide range of GI disorders in childhood.
This study provides a basis for future studies on the use of this advanced diagnostic technique in providing a real-time diagnosis during endoscopy in children.
This was a well-conducted study, which shows that it is feasible to use confocal endomicroscopy in the diagnosis of childhood GI disorders.
| 1. | Bosco JJ, Barkun AN, Isenberg GA, Nguyen CC, Petersen BT, Silverman WB, Slivka A, Taitelbaum G, Ginsberg GG. Gastrointestinal endoscopes: May 2003. Gastrointest Endosc. 2003;58:822-830. [Cited in This Article: ] |
| 2. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [Cited in This Article: ] |
| 3. | Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568-577. [Cited in This Article: ] |
| 4. | Jung M, Kiesslich R. Chromoendoscopy and intravital staining techniques. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:11-19. [Cited in This Article: ] |
| 5. | Delaney PM, Harris MR. Fiberoptics in confocal microscopy. Handbook of biological confocal microscopy. New York: Springer 2006; 501-515. [Cited in This Article: ] |
| 6. | Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706-713. [Cited in This Article: ] |
| 7. | Thomson M. The pediatric esophagus comes of age. J Pediatr Gastroenterol Nutr. 2002;34 Suppl 1:S40-S45. [Cited in This Article: ] |
| 8. | DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190-200. [Cited in This Article: ] |
| 9. | Tolia V, Wuerth A, Thomas R. Gastroesophageal reflux disease: review of presenting symptoms, evaluation, management, and outcome in infants. Dig Dis Sci. 2003;48:1723-1729. [Cited in This Article: ] |
| 10. | Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet. 2006;367:2086-2100. [Cited in This Article: ] |
| 11. | Fox VL, Nurko S, Furuta GT. Eosinophilic esophagitis: it's not just kid's stuff. Gastrointest Endosc. 2002;56:260-270. [Cited in This Article: ] |
| 12. | Straumann A, Simon HU. The physiological and pathophysiological roles of eosinophils in the gastrointestinal tract. Allergy. 2004;59:15-25. [Cited in This Article: ] |
| 13. | Ricci C, Holton J, Vaira D. Diagnosis of Helicobacter pylori: invasive and non-invasive tests. Best Pract Res Clin Gastroenterol. 2007;21:299-313. [Cited in This Article: ] |
| 14. | Graham DY, Kato M, Asaka M. Gastric endoscopy in the 21st century: appropriate use of an invasive procedure in the era of non-invasive testing. Dig Liver Dis. 2008;40:497-503. [Cited in This Article: ] |
| 15. | Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990;65:909-911. [Cited in This Article: ] |
| 16. | Farrell RJ, Kelly CP. Diagnosis of celiac sprue. Am J Gastroenterol. 2001;96:3237-3246. [Cited in This Article: ] |
| 17. | Fefferman DS, Farrell RJ. Endoscopy in inflammatory bowel disease: indications, surveillance, and use in clinical practice. Clin Gastroenterol Hepatol. 2005;3:11-24. [Cited in This Article: ] |
| 18. | Carvalho R, Hyams JS. Diagnosis and management of inflammatory bowel disease in children. Semin Pediatr Surg. 2007;16:164-171. [Cited in This Article: ] |
| 19. | Xu CF, Zhu LX, Xu XM, Chen WC, Wu DP. Endoscopic diagnosis of gastrointestinal graft-versus-host disease. World J Gastroenterol. 2008;14:2262-2267. [Cited in This Article: ] |
| 20. | Ross WA, Ghosh S, Dekovich AA, Liu S, Ayers GD, Cleary KR, Lee JH, Couriel D. Endoscopic biopsy diagnosis of acute gastrointestinal graft-versus-host disease: rectosigmoid biopsies are more sensitive than upper gastrointestinal biopsies. Am J Gastroenterol. 2008;103:982-989. [Cited in This Article: ] |
| 21. | Goldman H, Proujansky R. Allergic proctitis and gastroenteritis in children. Clinical and mucosal biopsy features in 53 cases. Am J Surg Pathol. 1986;10:75-86. [Cited in This Article: ] |
| 22. | Xanthakos SA, Schwimmer JB, Melin-Aldana H, Rothenberg ME, Witte DP, Cohen MB. Prevalence and outcome of allergic colitis in healthy infants with rectal bleeding: a prospective cohort study. J Pediatr Gastroenterol Nutr. 2005;41:16-22. [Cited in This Article: ] |
| 23. | Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686-695. [Cited in This Article: ] |
| 24. | Lipson BK, Yannuzzi LA. Complications of intravenous fluorescein injections. Int Ophthalmol Clin. 1989;29:200-205. [Cited in This Article: ] |
| 25. | Hoffman A, Goetz M, Vieth M, Galle PR, Neurath MF, Kiesslich R. Confocal laser endomicroscopy: technical status and current indications. Endoscopy. 2006;38:1275-1283. [Cited in This Article: ] |
| 26. | Tung SY, Wu CS, Su MY. Magnifying colonoscopy in differentiating neoplastic from nonneoplastic colorectal lesions. Am J Gastroenterol. 2001;96:2628-2632. [Cited in This Article: ] |
| 27. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [Cited in This Article: ] |
| 28. | Emura F, Saito Y, Taniguchi M, Fujii T, Tagawa K, Yamakado M. Further validation of magnifying chromocolonoscopy for differentiating colorectal neoplastic polyps in a health screening center. J Gastroenterol Hepatol. 2007;22:1722-1727. [Cited in This Article: ] |
| 29. | Kato S, Fu KI, Sano Y, Fujii T, Saito Y, Matsuda T, Koba I, Yoshida S, Fujimori T. Magnifying colonoscopy as a non-biopsy technique for differential diagnosis of non-neoplastic and neoplastic lesions. World J Gastroenterol. 2006;12:1416-1420. [Cited in This Article: ] |
| 30. | Matsumoto T, Kudo T, Jo Y, Esaki M, Yao T, Iida M. Magnifying colonoscopy with narrow band imaging system for the diagnosis of dysplasia in ulcerative colitis: a pilot study. Gastrointest Endosc. 2007;66:957-965. [Cited in This Article: ] |
| 31. | Thorlacius H, Toth E. Role of chromoendoscopy in colon cancer surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:911-917. [Cited in This Article: ] |