INTRODUCTION
Pneumocystis jiroveci (carinii) is a unicellular fungus that is found in the respiratory tracts of many mammals, including humans. The organism was first described in 1909 by Chagas[1] and later by Delanoë[2] who ultimately named the organism. Years later, Jirovec’s group isolated the organism from humans[3], and the organism was subsequently renamed after him. P. jiroveci (carinii) pneumonia (PCP) is the most common opportunistic infection in human immunodeficiency virus (HIV)-infected patients and, by definition, constitutes an acquired immunodeficiency syndrome (AIDS)-defining illness. In our hospital, which serves a population of 450 000, the incidence of PCP is approximately 12 per year. The majority of these are in patients following either solid organ, or bone marrow transplantation. Recently, there has been increased awareness and reporting of PCP and other opportunistic infections in patients with inflammatory bowel disease and other inflammatory conditions. This has been attributed to the increased use of anti-tumor necrosis factor (TNF)α therapy[4–8], but may reflect the increased surveillance for such infections in this group of patients. We report a case that highlights the importance of considering opportunistic infection in any immunosuppressed patients with inflammatory bowel disease, who present with unusual symptoms and signs, including those of worsening disease activity.
CASE REPORT
A 21-year-old Caucasian man presented in June 2005 age 18 years with 7 d of bloody diarrhea and abdominal discomfort. He reported a similar, but less severe, self-limiting episode 2 mo earlier. He had never smoked tobacco. On admission he had a temperature of 38°C and a pulse of 90 bpm. Admission laboratory tests included hemoglobin 7.5 g/dL (normal range 13.0-18.0 g/dL), mean corpuscular volume 69.4 fl (normal range 82-98 fl), C-reactive protein (CRP) 188 mg/L (normal range < 3 mg/L), and negative stool cultures. Flexible sigmoidoscopy demonstrated erythematous mucosa with a mucopurulent exudate and confluent ulceration, which continued beyond the extent of the examination. A diagnosis of acute severe ulcerative colitis was made and he was commenced on intravenous and rectal steroids. Histology subsequently confirmed distorted crypt architecture with cryptitis, micro-abscesses and increased inflammatory cells in the lamina propria. He responded well to intensive therapy and by day 3 his stool frequency had fallen to 3/d and CRP to 34 mg/L. He was discharged on day 7 with a reducing course of oral prednisolone and mesalazine (Asacol™ 800 mg bid). He was reluctant to use topical therapy. His symptoms relapsed once the prednisolone was reduced below 15 mg/d, and thus azathioprine was introduced in September 2005. He was commenced at 25 mg/d, increasing by 25 mg fortnightly to a maximum dose of 150 mg/d (2 mg/kg). Thiopurine methyl-transferase (TPMT) activity level was 51 nmol 6-MTG/g Hb per hour (normal range 25-55 nmoL 6-MTG/g Hb per hour). He had one minor further flare of his disease, but subsequently entered clinical remission using mesalazine 800 mg bid and azathioprine 150 mg/d. Twelve months later, a routine blood test demonstrated a low total white blood cell count of 2.2 × 109/L (normal range 3.8-10.6 × 109/L), with a neutrophil count of 1.34 × 109/L (normal range 1.8-6.5 × 109/L) and a lymphocyte count of 0.4 × 109/L (normal range 1.1-3.5 × 109/L). These results were confirmed on a repeat sample. He was advised to reduce azathioprine dose to 100 mg/d, and these cell counts recovered.
Our patient then travelled to Australia to participate in a cricket tour and for the next 3 mo he remained well and his blood monitoring was satisfactory. However, in February 2008, whilst on tour, he had an acute flare of his colitis, which required hospital admission. Admission blood tests demonstrated CRP 121 mg/L, hemoglobin 9.0 × 109/L and total white blood cell count 4.2 × 109/L. He underwent flexible sigmoidoscopy, which showed a large anal fissure and severe colitis, with extensive mucosal loss to the splenic flexure. A chest X-ray on admission demonstrated pneumomediastinum, but no cause was identified following a barium swallow and subsequent computed tomography (CT) scan of his thorax, abdomen and pelvis. He was treated with intravenous and rectal hydrocortisone and azathioprine was increased to 150 mg/d. His symptoms resolved and he was discharged, returning to the UK on a reducing course of steroids and azathioprine.
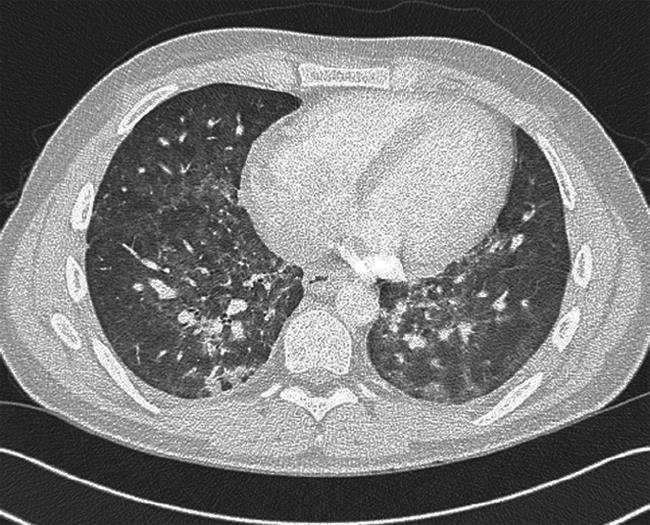
On arrival back in the UK, his colitis rapidly relapsed and he was re-admitted to hospital. On admission, he was passing six bloody liquid stools per day. Blood tests demonstrated hemoglobin 9.9 g/dL, total white blood cell count 2.0 × 109/L, neutrophil count 1.60 × 109/L, lymphocyte count 0.22 × 109/L and CRP 61 mg/L. An abdominal X-ray did not show evidence of colonic dilatation. He was recommenced on intravenous hydrocortisone and azathioprine was stopped in view of the leukopenia. After 48 h, his stool frequency and urgency had much improved, his CRP fell to 11 mg/L and total white cell count increased to 2.8 × 109/L (lymphocyte count 0.39 × 109/L, neutrophil count 2.2 × 109/L). However, on converting to oral steroids on day 3, CRP rose to 146 mg/L and he became febrile. He was noted to have a dry cough, and reported breathlessness on exertion, which on direct questioning he admitted had been present immediately prior to leaving Australia. No abnormal chest signs were elicited, but his oxygen saturation was noted to be 95% at rest (on room air), falling to 90% on exertion. A chest X-ray was normal, but he was clearly at risk of opportunistic infection because of his recent leukopenia and long history of immunosuppression, and was also at risk of thromboembolic disease in view of his recent long-haul flight and active colitis. A CT pulmonary angiogram with full lung views did not show pulmonary emboli, but demonstrated bilateral, small-volume hilar lymphadenopathy (8 mm) and widespread patchy ground glass changes in both lungs, with more confluent consolidation within the basal segments (Figure 1). Bronchoalveolar lavage washings were positive for P. jiroveci (carinii) on immunofluorescence. An HIV test was negative. The patient was commenced on a 2-wk course of intravenous co-trimoxazole (2.4 g qds) and was maintained on a reducing course of oral steroids (prednisolone 40 mg/d) and high dose oral mesalazine (Asacol™ 2.4 g bid). His respiratory and gastrointestinal symptoms improved and his inflammatory markers and white cell count normalized within 5 d. He was discharged at 14 d with a further week of oral co-trimoxazole. Three days later he was re-admitted with a severe blanching rash on his trunk and limbs and worsening diarrhea. A drug reaction to co-trimoxazole was suspected and he was switched to oral clindamycin (600 mg tds) and primaquine (30 mg/d), with slow resolution of the rash and improvement in his stool frequency. Blood tests for cytomegalovirus serology and polymerase chain reaction were negative. He was discharged and remains under close follow up. He had no recurrence of his respiratory symptoms and his colitis remains in clinical remission on high dose oral and rectal mesalazine.
Figure 1 CT scan demonstrating parenchymal abnormalities caused by PCP.
DISCUSSION
We report the case of a 21-year-old Caucasian man, naïve to anti-TNFα therapy, who presented with PCP following treatment with azathioprine and steroids for ulcerative colitis. Azathioprine is a commonly used immunosuppressive agent. It acts by inhibiting purine synthesis by disrupting normal purine incorporation into ribonucleic acids. It is a pro-drug, which is converted in the body to the active metabolites 6-mercaptopurine and 6-thioinosinic acid. Its recognized side effects include hepatitis, pancreatitis and bone marrow suppression. The incidence of these side effects relates in part to the activity of a genetically moderated enzyme involved in the metabolism of thiopurine compounds including 6-mercaptopurine. This enzyme is thiopurine methyltransferase (TPMT). TPMT activity assays are thus used to identify patients with deficient and low activity (approximate 8.5% population), who are at increased risk of bone marrow toxicity from thiopurine drugs, enabling dose adjustments or avoidance. In 2007, in the Royal Devon and Exeter Hospital, 104 patients with inflammatory bowel disease were commenced on azathioprine. In our department, this is the first case of PCP in a patient with inflammatory bowel disease who was naïve of anti-TNFα therapy. In this case, the patient’s TPMT was normal (51 nmoL 6-MTG/g Hb per hour). From relatively early in the disease course, he was noted to have steroid-dependent disease, and 9 mo following diagnosis, was commenced on 2 mg/kg azathioprine. This dose was tolerated well initially with no evidence of agranulocytosis, pancreatitis or abnormal liver function on regular blood tests. However, after 1 year of receiving this dose, a routine blood test showed leukopenia, which was confirmed on a repeat sample. The dose was, therefore, reduced to 100 mg/d and the leukopenia resolved. Three months later, he had a further acute flare of his ulcerative colitis whilst in Australia and his azathioprine dose was increased to 150 mg/d (2 mg/kg per day). On returning to the UK, he was subsequently admitted because of symptom recurrence and was noted to be leukopenic, at which point his azathioprine was stopped. In total, this patient received azathioprine for 2 years and 6 mo, with a maximum dose of 2 mg/kg. He became leukopenic on two occasions, the second corresponding with the onset of his respiratory symptoms.
Steroids have been the cornerstone of the treatment of acute ulcerative colitis since Truelove and Witts first reported their pioneering trial results in 1955[9]. Steroid use has, however, been associated with PCP in HIV-negative patients[10]. Since being diagnosed with ulcerative colitis, this patient received two courses of oral steroids, which were prolonged (12 mo and 8 mo) because of symptom recurrence on weaning the dose. However, he had not received any steroids for 9 mo prior to his admission in Australia. During this admission, he received 5 d of intravenous steroids and he received a further 2 d on returning to the UK. These were the first courses of intravenous steroids that he had received since the initial course that was used at diagnosis. Interestingly, adjunctive steroid use in the treatment of PCP has been shown to reduce the incidence of death and respiratory failure associated with severe infection[1112]. This patient developed respiratory symptoms immediately prior to his return to the UK and we hypothesize that his initial improvement with high dose intravenous steroids and subsequent deterioration on lower dose oral steroids, was to the result of partial treatment of PCP by steroids. It is also noteworthy that his colitis symptoms and elevated inflammatory markers improved significantly once his PCP was treated with intravenous co-trimoxazole, without any need to increase his steroid dose. We, therefore, conclude that both the symptoms and blood test abnormalities were likely to have been caused by PCP, rather than active colitis per se. This case demonstrates the potential of steroid-responsive opportunistic infections to mimic worsening colitis symptoms in patients with ulcerative colitis. It is, therefore, important to consider opportunistic infection in immunosuppressed patients with inflammatory bowel disease, whose inflammatory markers appear disproportionate to the severity of the disease activity.
It is difficult to be sure exactly when this patient contracted PCP. It is noteworthy that pneumomediastinum was recognised on a chest X-ray shortly after he was admitted in Australia, before the introduction of intravenous steroids. Pneumomediastinum is the presence of extra-alveolar air in the mediastinum, which is thought to arise from free air leaking from ruptured alveoli. Historically, the incidence of spontaneous pneumomediastinum has been reported to be 1 in 32 896[13]. However, in HIV infection, spontaneous pneumomediastinum has been well described in association with PCP[14–17]. Indeed, in one series, the incidence was reported to be as high as 9.5% of cases[17]. If our patient had contracted PCP prior to his admission in Australia, it is likely to have occurred at a time when he was taking 100 mg/d azathioprine without supplementary steroids.
The advent of anti-TNFα therapy has resulted in improved disease control in many patients with inflammatory bowel disease. Unfortunately, it has also resulted in an increase in the reported number of patients with inflammatory bowel disease who present with opportunistic infections[4–6]. This has resulted in increased vigilance for such infections in this group of patients. In the case described, the patient is likely to have developed PCP either as a result of treatment with 100 mg azathioprine (1.25 mg/kg per day) or following a 5-d course of intravenous steroids and 2 mg/kg azathioprine. The presence of pneumomediastinum, a recognised complication of PCP, before he was exposed to intravenous steroids would appear to make the former possibility more likely. He had not received steroids for 9 mo prior to this admission, and at no stage did he receive rescue therapy. During the 6 mo that preceded this admission, he was maintained on a stable dose of azathioprine (100 mg/d) and was not leukopenic. He never received anti-TNFα therapy. With the trend towards increasing use of early immunomodulation, this degree of immunosuppression would not, we believe, be considered excessive nor particularly likely to cause an otherwise fit 21-year-old man to develop PCP. However, this case highlights the importance of considering such diagnoses in any patients with inflammatory bowel disease who are immunosuppressed, irrespective of which agents they may have received.