Published online Sep 14, 2008. doi: 10.3748/wjg.14.5274
Revised: July 17, 2008
Accepted: July 24, 2008
Published online: September 14, 2008
AIM: To investigate the potential role of Active Chinese mistletoe lectin-55 (ACML-55) in tumor immune surveillance.
METHODS: In this study, an experimental model was established by hypodermic inoculating the colon cancer cell line CT26 (5 × 105 cells) into BALB/c mice. The experimental treatment was orally administered with ACML-55 or PBS, followed by the inoculation of colon cancer cell line CT26. Intracellular cytokine staining was used to detect IFN-γ production by tumor antigen specific CD8+ T cells. FACS analysis was employed to profile composition and activation of CD4+, CD8+, γδ T and NK cells.
RESULTS: Our results showed, compared to PBS treated mice, ACML-55 treatment significantly delayed colon cancer development in colon cancer -bearing Balb/c mice in vivo. Treatment with ACML-55 enhanced both Ag specific activation and proliferation of CD4+ and CD8+ T cells, and increased the number of tumor Ag specific CD8+ T cells. It was more important to increase the frequency of tumor Ag specific IFN-γ producing-CD8+ T cells. Interestingly, ACML-55 treatment also showed increased cell number of NK, and γδT cells, indicating the role of ACML-55 in activation of innate lymphocytes.
CONCLUSION: Our results demonstrate that ACML-55 therapy can enhance function in immune surveillance in colon cancer-bearing mice through regulating both innate and adaptive immune responses.
- Citation: Ma YH, Cheng WZ, Gong F, Ma AL, Yu QW, Zhang JY, Hu CY, Chen XH, Zhang DQ. Active chinese mistletoe lectin-55 enhances colon cancer surveillance through regulating innate and adaptive immune responses. World J Gastroenterol 2008; 14(34): 5274-5281
- URL: https://www.wjgnet.com/1007-9327/full/v14/i34/5274.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5274
Mistletoe (Viscum album) is a semiparasitic plant with many unusual properties. It was used as a kind of herbal remedy in the ancient Chinese Pharmacopoeia and has been used in traditional Chinese medicine for diseases, such as gonorrhea, syphilis, hypertension and rheumatism for thousands of years. The aqueous extract of European Mistletoe (EM) has been used in conventional cancer therapy for decades[1]. Therapeutic efficacy is mostly attributed to the mistletoe lectins (ML) ML-I, ML-II, ML-III, that belong to the “toxic lectin family” and represent ribosome deactivating proteins class II. They consist of one N-glycosidase (A chain) and one galactoside binding lectin (B chain) linked by a disulfide bridge. The lectins ML-I and ML-III preferentially bind to galactoside or N-acetylgalactosamine groups while ML-II can bind to both carbohydrates[2].
EM has recently been found to act through several distinct bioactivities as a potent immune modulator. First, EM exerts its broad immunostimulatory activity by activating different types of cells[3-5] in vivo and in vitro. Incubation of lymphocytes with EM could result in anti-tumoral cytotoxic T lymphocytes bearing phosphorylated mistletoe ligands[6,7]. Second, EM favors bridging of natural killer tumor cell conjugates, enhancing its efficiency of killing[8-10]. Third, it has been found that EM could activate immune responses by modulating the complex network of cytokines that regulate leukocyte functions. EM caused increased secretion of tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 from isolated human mononuclear cells in vitro[11,12]. Finally, EM has also been described as an inducer of cell apoptosis. In the presence of EM, human mononuclear cells and many cell lines underwent apoptosis[1,13].
While the EM has been studied intensively, less is known about the Chinese mistletoe as an anti-cancer drug. In our prior study, a mistletoe lectin was purified from Chinese mistletoe and the effect of the active Chinese mistletoe mectin-55 (ACML-55) on human γδ T cell cytotoxicity, apoptosis and modulation of the cytokine network was reported[14,15]. Although these investigations suggest that ACML-55 may modulate the immune response against tumor development, the precise mechanism by which ACML-55 regulates the immune function has not been studied systematically. In this study, we demonstrate that ACML-55 enhances tumor immune surveillance against colon cancer formation by regulating both innate and adaptive immune responses. Our results suggest that ACML-55 may be a useful complementary therapy for treating colon cancer.
BALB/c mice were purchased from Shanghai Experimental Animal Center, Chinese Academy of Science and were used at 6-8 wk of age in all experiments. All mice were maintained under specific pathogen-free conditions at Shanghai Jiao Tong University School of Medicine.
ACML-55 was dissolved in PBS at final concentration of 2 g/L. Mice were treated with ACML-55 or PBS orally (200 μL/mouse) once a day for 2 wk. Oral administration was achieved by gavage to ensure all mice received the entire dose.
Recombinant murine IL-2 was purchased from R&D Systems (Minneapolis, MN, USA). Anti-mouse antibodies (CD3, NK1.1, CD4, CD8, CD62L, CD44, anti-αβ, anti-γδ and IFN-γ) used for phenotypic and cytokine analysis were purchased from BD Biosciences (San Jose, CA, USA).
Mistletoe lectins were isolated from extract of Chinese mistletoe, a subspecies of V. album according to previously described methods[14] with our own modifications. Briefly, the air-dried mistletoe (3 kg), collected from Sichuan province, China, was crushed and purified twice with 20 L methanol/water (1:1, V/V). The homogenate was filtered through a nylon cloth. After filtration, with its volume reduced to 2 liters, the aqueous phase was successively partitioned with cyclohexane, dichloromethane and ethyl acetate. Ethanol was added to the concentrated aqueous phase to a final concentration of 85% (V/V). A precipitate was obtained and separated from the supernatant by centrifugation (8000 g, 20 min). The supernatant was concentrated and ethanol was added to 85% (V/V). After centrifugation, the precipitate was collected and combined with the former precipitate. The final yield of ML extract was 100 g from 3 kg mistletoe. All the precipitate was dissolved in 100 mL phosphate buffer (10 mmol/L, pH 6.5) and the stock solution of mistletoe extract was stored at -80°C.
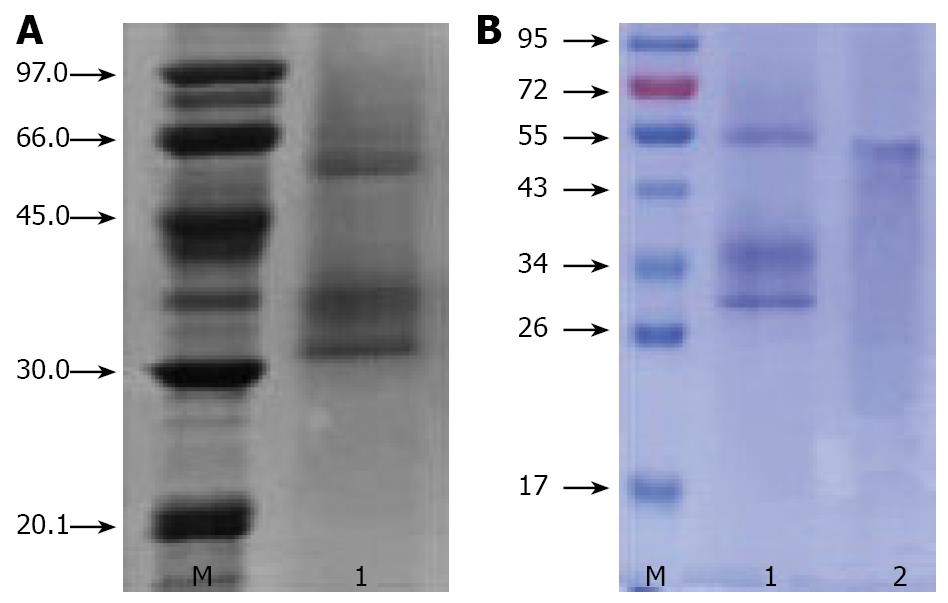
To obtain the pure Chinese mistletoe protein, extract was further purified by CM-Sepharose column chromatography[14]. The aqueous layer (1 mL) was applied to a column of CM-Sepharose (1.5 cm × 20 cm) equilibrated with 10 mmol/L phosphate buffer (pH 6.5). After washing with 10 mmol/L phosphate buffer (pH 6.5) and 100 mmol/L NaCl in the same buffer at a rate of 0.5 mL/min, a peak eluted with 500 mmol/L NaCl in the same buffer was dialyzed with PBS (pH 7.4). The fractions containing hemagglutinating protein were collected and then applied to a column of Con A column (1.5 cm × 20 cm) equilibrated with 10 mmol/L PBS (pH 7.4). The column was washed with PBS (pH 7.4) and eluted with 300 mmol/L glucose in the same buffer. Fractions were subject to sodium dodecyl sulphate (SDS)-electrophoresis and fractions containing 55 kDa protein were pooled, dialyzed against water and freeze-dried.
The molecular mass and purity of ACML-55 was determined by SDS-PAGE. Twelve percent polyacrylamide gel was used as resolving gel and 5% was used as stacking gel. To further denature the proteins by reducing disulfide linkages, the samples were heated at 100ºC for 3 min in the presence of a reducing agent, then electrophoresed using electrophoresis system at 200 V for 75 min and lastly the gel was stained with Coomassie brilliant R-250 to show bands.
Colon cancer cell line CT26 and OVA-expressing EG7 cell line were purchased from ATCC (Manassas, VA, USA). For tumor induction, colon cancer cell line CT26 cells (5 × 105 cells/mouse) were injected subcutaneously, and tumor growth was monitored and recorded daily for over 3 wk as described in our previous studies[15]. For some experiments, EG7 tumor cells were also administered intraperitoneally (1.0 × 106 tumor cells/mouse).
Mice were treated with ACML-55 or PBS for 2 wk as described above. These treated mice (n = 5 for each group) were immunized with 200 μg of CT26 tumor lysate emulsified in CFA in the hind footpad, as described in previous studies[16]. On day 7 post-immunization, draining lymph node cells were harvested, lymphocytes were cultured with comptumor air ratio RPMI-1640 containing 200 μg/mL CT26 tumor lysate for 24 h, with brefeldin A added for the last 3 h. Cells were then used for intracellular cytokine staining as described below.
Cultured draining lymph node cells were stained with FITC-anti-CD8 antibody followed by fixation with 2% formaldehyde and permeabilization with 0.5% saponin (w/v) for intracellular IFN-γ staining, using PE-anti-IFN-γ as described previously[17]. PE-conjugated rat IgG2a (BD Pharmingen) was used as an isotype control. Gating was performed on CD8+ T cells and the percentage of IFN-γ+ cells was reported.
Both ACML-55 treated mice (n = 4) and control mice (n = 4) were inoculated intraperitoneally with 1.0 × 106 EG7 tumor cells. On day 10, splenocytes from these mice were isolated and stained with FITC-anti-CD8 and PE-tetramer antibodies for OVA. Percentage of tetramer positive CD8+ T cells was shown by FACS analysis.
Both ACML-55 treated mice (n = 4) and control mice (n = 4) were inoculated intraperitoneally with 5 × 105 CT26 cells. On day 10, splenocytes from these mice were isolated and stained with one of the following antibody combinations: FITC-anit-CD3 and PE-anti-NK1.1; FITC-anti-γδ and PE-anti-αβ; PE-anti-CD62L, CyChrome- anti-CD44, FITC-anti-CD8a and APC-anti-CD4. Percentages of different cell subpopulations were shown by FACS analysis.
Statistical significance was evaluated by two tailed unpaired Student’s test or non-parametric analysis if SDs were significantly different between two compared groups using InStat 2.03 for Macintosh software (Graph Pad Software). The incidence of tumor development was compared and analyzed using the log rank test, performed by Graph Pad Prism Version 3.0a for Macintosh (Graph Pad Software). P < 0.05 was used to denote statistical significance.
Chinese ML extractions were analyzed by SDS-PAGE. In the presence of the reducing agent, it showed an estimated 55 kDa band consisting of two bands of a 30 kDa A chain and a 34 kDa B Chain (Figure 1).
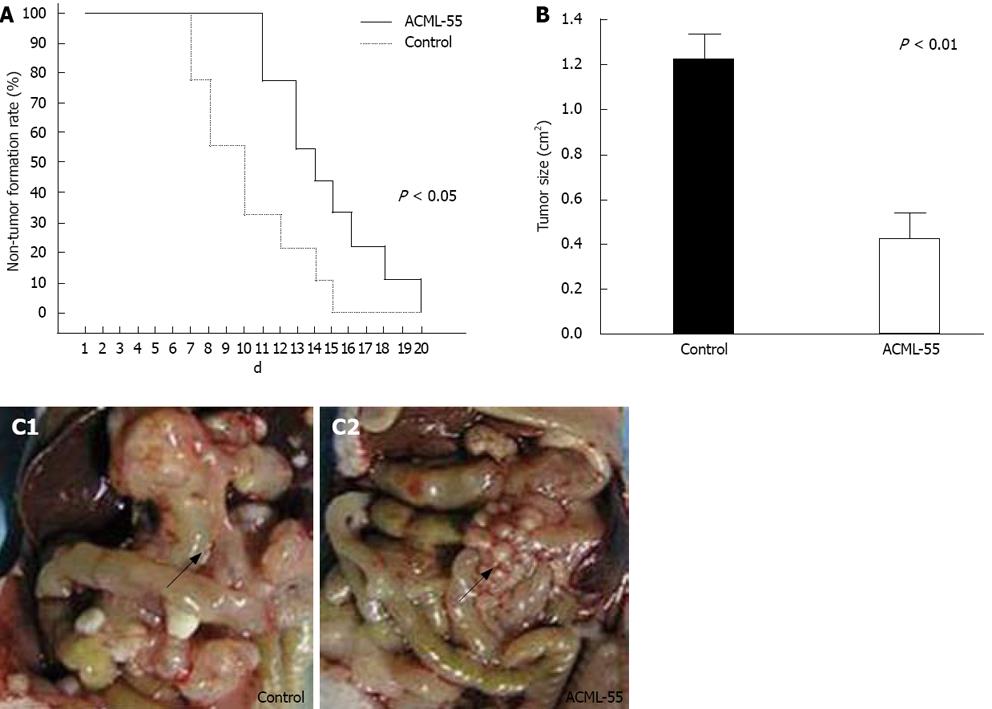
Based on the findings that EM reduces the metastasis of rat mammary adenocarcinomas and its ability to modulate immune functions[18,12], we hypothesized that ACML-55 might enhance tumor immune surveillance. To assess the effect of ACML-55 on tumor development, ACML-55 or PBS was administered to sex- and age- matched BALB/c mice by gavage daily for 2 wk, followed by subcutaneous inoculation of CT26 cells (5 × 105 cells/mouse). Tumor growth was observed and recorded daily as previously described[16,19]. Compared to control group (PBS treated), ACML-55 treated mice showed delayed tumor development (Figure 2A) as well as reduced tumor size (Figrue 2B). ACML-55 treated mice were much more resistant to tumor cell growth upon subcutaneous tumor inoculation (Figrue 2C). The results indicate that ACML-55 significantly enhances tumor surveillance.
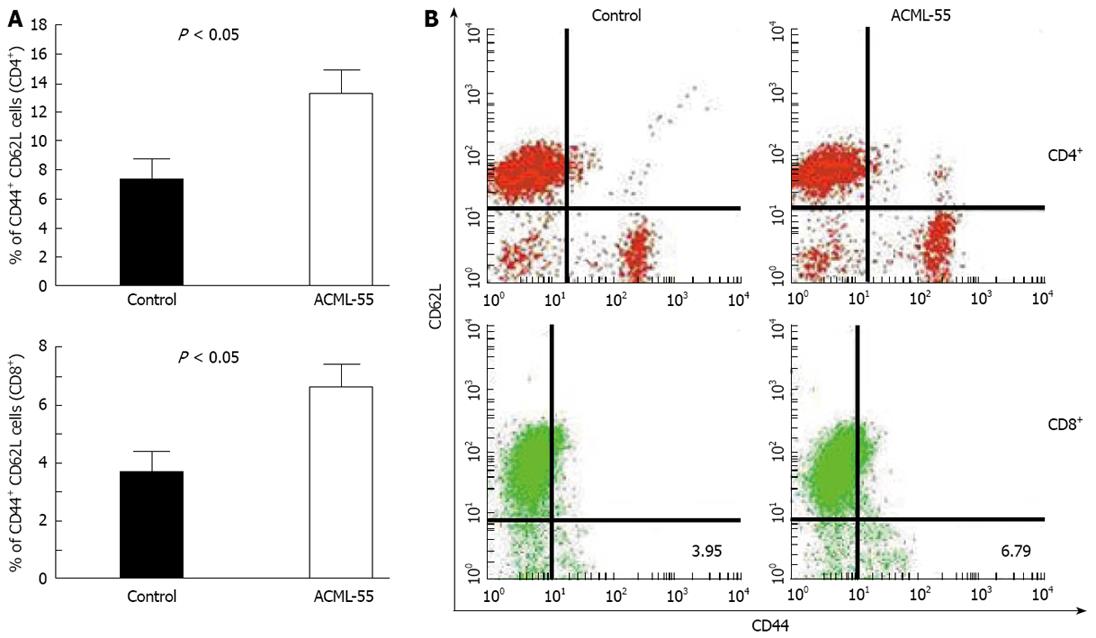
To define the underlying molecular mechanisms of ACML-55 mediated anti-tumor immune response, we first tested the effect of ACML-55 on the adaptive immune response. Sex- and age- matched BALB/c mice were given ACML-55 or PBS daily for 2 wk, followed by intraperitoneal inoculation with EG7 tumor cells (EG7 tumor cells expressing OVA, 1.0 × 106 cell/mouse)[20]. On day 10 post-inoculation, harvested splenocytes were used for analysis of CD4+ and CD8+ T cell activation using specific activation markers. ACML-55 treatment significantly increased the number of activated CD4+ and CD8+ T cells. According to our findings, the percentage of CD62Llow CD44high population in the spleen for each T cell subset was significantly higher in ACML-55 treated mice compared to those treated with PBS [14.29 ± 4.3 vs 7.63 ± 2.95 for CD4+ T cells, and 6.79 ± 1.41 vs 3.95 ± 1.97 for CD8+ T cells (Figure 3A), P = 0.0008 for CD4 and P = 0.0002 for CD8]. Representative data of the FACS profile for CD4+ and CD8+ T cells from ACML-55 or PBS treated mice are represented in Figure 3B.
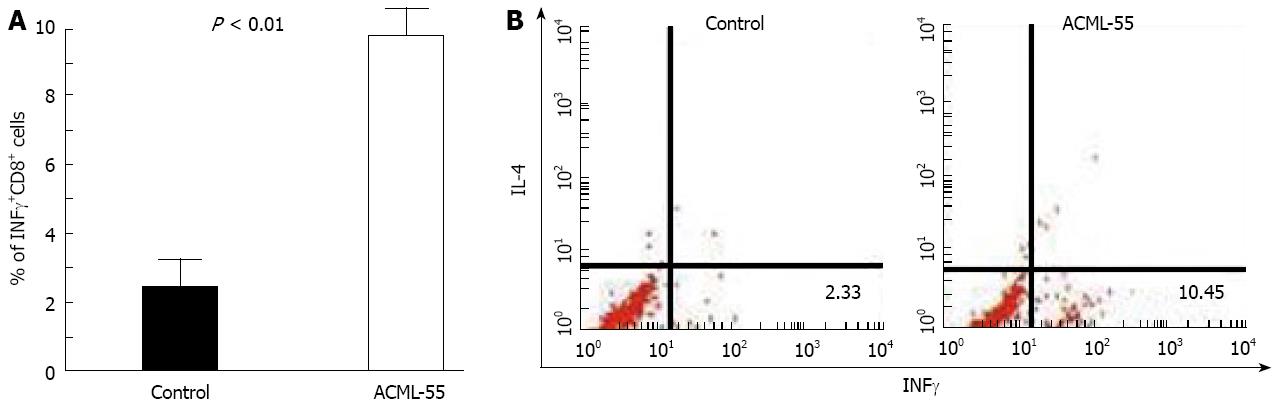
IFN-γ has been shown previously to be a critical cytokine in tumor immunosurveillance[21]. To define the effect of ACML-55 on tumor antigen specific IFN-γ production, ACML-55 or PBS was administered to sex- and age- matched BALB/c mice (n = 6 for each group) as mentioned above for 2 wk, and then immunized in the hind footpad with CT26 tumor lysate in CFA. Eight days post-immunization, lymphocytes from the draining lymph nodes were isolated, cultured with 200 μg/mL tumor lysate for 24 h, with brefeldin A added during the last 3 h of culture. These cells were then fixed and permeabilized with 0.5% saponin for intracellular cytokine staining. The percentage of IFN-γ producing CD8+ T cells from ACML-55 treated mice (mean ± SD) was significantly higher than that of PBS treated mice (10.05 ± 2.3 and 2.30 ± 1.013, respectively, P = 0.0001; Figure 4A). An example of FACS analysis is represented in Figure 4B. In the same cultures, the percentage of IFN-γ producing CD4+ T cells from ACML-55 treated mice was also higher than those from PBS treated mice, although it did not reach significance (data not shown). ACML-55 treatment did not enhance the percentage of IFN-γ producing CD8+ T cells responding to control tumor lysate (different tumor cell line, data not shown). Our results demonstrate that ACML-55 not only enhances activation and proliferation of T cells, but also increases their capacity to produce IFN-γ cytokine.
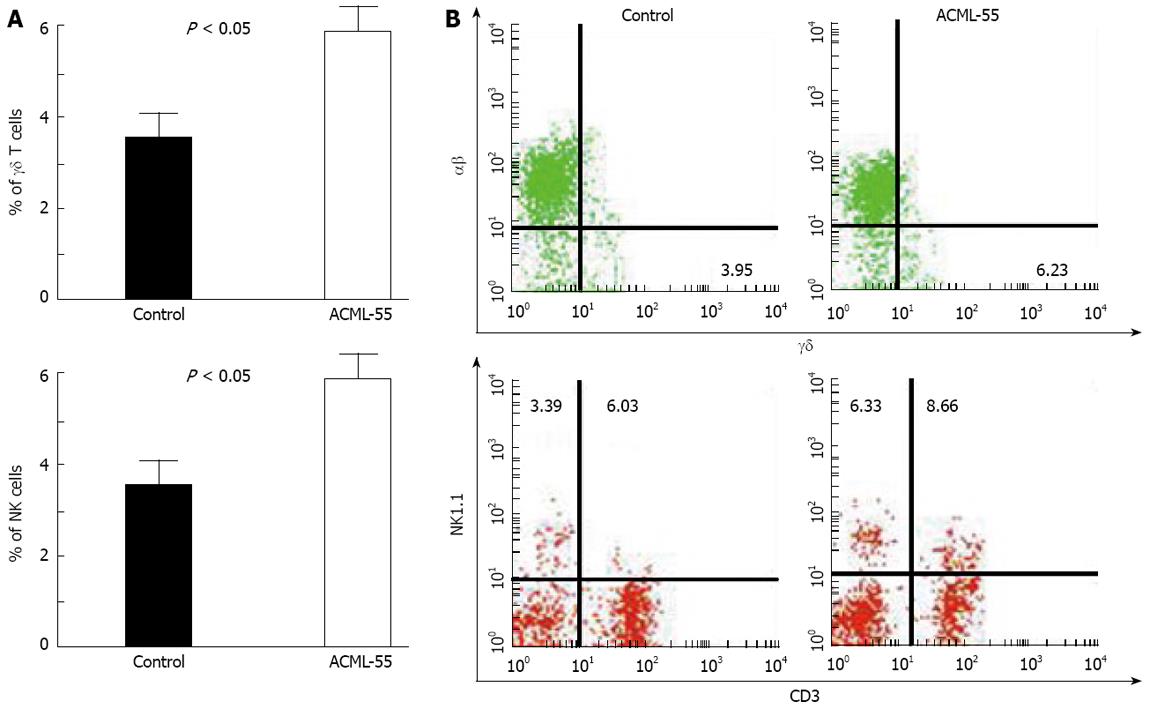
Both NK cells and γδ T cells play a critical role in tumor immune surveillance. To test the effect of ACML-55 on these cell types, sex- and age- matched BALB/c mice were treated with ACML-55 or PBS (n = 6 for each group) as above for 2 wk, and the percentages of NK and γδ T cells in the spleen were analyzed by flow cytometry. Treatment with ACML-55 significantly increased the numbers of splenic NK cells and γδ T cells, with the percentage (mean ± SD) of NK1.1+ cells in ACML-55 treated mice vs control being 6.28 ± 0.90 vs 3.48 ± 0.77, and the percentage of CD3+ γδ+ cells for ACML-55 vs control being 6.51 ± 0.59 vs 3.85 ± 0.59, (Figure 5A, P = 0.0001). A representative result of FACS analysis for CD3 and NK1.1 as well as αβ/γδ T cell staining is presented in Figure 5B. Splenocytes from ACML-55 or PBS treated mice were cultured with anti-CD3 and anti-CD28 antibodies in the presence of brefeldin A for 6 h, and the percentage of IFN-γ + γδ+ T cells was analyzed by intracellular cytokine staining after gating TCR γδ positive cells. The results showed there is no significant difference in the percentage of IFN-γ producing γδ T cells between two groups (data not shown). NKT cells (CD3+ NK1.1+) were also higher in ACML-55 treated mice compared to controls, although it did not reach significance. These results indicate that ACML-55 may enhance the antitumor immune response not only through modulating the adaptive immune response, but also working on innate immunity.
Extracts from European mistletoe are used widely in the treatment of cancer, but the mechanism of antitumor properties has not yet been clearly elucidated. Consumers often use EM as a complementary therapy for cancer treatment, and in some cases as an alternative to conventional cancer treatment[22,23]. Although Korean mistletoe, a subspecies of European mistletoe, has been used as a medicinal herb and shown to be cytotoxic against tumor cells as well[24], there are fewer systemic controlled studies to define the effect of EM in tumor immunity. In this study, we demonstrate that ACML-55 enhances tumor immune surveillance against both melanoma and lymphoma by regulating both innate and adaptive immune responses. We first illustrated that oral administration of ACML-55 prior to tumor inoculation could significantly delay the tumor growth and reduce tumor size (Figure 2A and B).The anti-tumor effect of ACML-55 is not limited to melanoma.
It has been shown that lymphocytes and IFN-γ both are essential components of tumor immune surveillance[25,26]. Different subsets of lymphocytes contribute to anti-tumor immune responses at different stages. CD4+ and CD8+ T cells are critical elements for the adaptive anti-tumor immune response. CD4+ T cells, especially Th1 subsets, produce IFN-γ to facilitate innate and adaptive immune responses[27]. These cells are also in favor of CD8+ T cells to develop memory response, whereas CD8+ T cells provide cytokines (IFN-γ and TNF-α) and cytotoxicity to exert their function to kill tumor cells directly. To explain the molecular mechanisms through which ACML-55 could mediate anti-tumor immune response, we sought to determine whether ACML-55 modulates the adaptive immune response. Our results indicate that ACML-55 treatment enhances activation and proliferation of both CD4+ and CD8+ T cells (Figure 3A and B). Moreover, administration of ACML-55 significantly increases the frequency of tumor antigen specific CD8+ T cells and their ability to produce higher level of IFN-γ (Figure 4A and 3B). Finally, ACML-55 treatment can make antigen specific CD8+ T cells expand more actively. The increasing number of CD8+ T cells partially contributes to the tumor resistance of ACML-55 treated mice. However, it is unclear how ACML-55 enhances the function of these T cells. It is possible that the mixture of polysaccharides in ACML-55 may activate the innate immune response through undefined signaling pathways, such as Toll-like receptors and the downstream NF-κB pathway, which in turn helps to regulate the adaptive immune response. Consistently, it has been reported that ACML-55 enhances IL-12 production from macrophages[10] and increases nitric oxide concentration[12]. Further studies are needed to clarify the underlying mechanisms that mediate the effect of ACML-55 on the adaptive immune response.
A potential target of ACML-55 modulation within the innate immune system may be γδ T cells. These cells belong to a unique subset of T cells. They recognize protein or peptide independent of antigen presentation and function as innate like cells[28]. Our earlier studies have demonstrated that γδT cells predominantly produce IFN-γ upon activation[12,29] and play a critical role in tumor immune surveillance by providing an early source of IFN-γ[14]. Interestingly, ACML-55 treatment significantly increases the number of γδ T cells compared to those of PBS-treated mice (Figure 5). Since ACML-55 was given orally, it is possible that the effective components in ACML-55 might directly encounter γδ T cells lining the epithelial layer of the intestine resulting in their activation. In addition to γδ T cells, it has been well established that NK cells play an essential role in tumor immune surveillance. Interestingly, we found that treatment with ACML-55 also upregulates the number of NK cells upon tumor inoculation. Although the changes of NK T cells did not reach significance, the trend is clear. These results indicate that ACML-55 has multiple effects on the immune system.
Given the findings that ACML-55 could efficiently enhance several immune parameters (CD4+, CD8+ and γδ T cells), which were shown previously to be positive for tumor immune surveillance, it is likely that ACML-55 mediates its potential effects on tumor surveillance, at least in part, by upregulation these particular parameters. Future studies using different T cell subset from deficient mice will help to illuminate these questions.
In summary, we have presented clear picture that, as an active lectin from Chinese mistletoe, ACML-55 enhances tumor immune surveillance by regulating both the innate and the adaptive immune responses. Further studies are needed to define the molecular mechanisms mediating the effect of ACML-55 in tumor immunity.
Mistletoe is a semiparasitic plant with many unusual properties. In our prior study, a mistletoe lectin was purified from Chinese mistletoe and the effect of the active Chinese mistletoe lectin-55 (ACML-55 ) on human γδ T cell cytotoxicity, apoptosis and modulation of the cytokine network was reported. Although these investigations suggest that ACML-55 may modulate the immune response against tumor development, the precise mechanism by which ACML-55 regulates the immune function has not been studied systematically.
In this study, an experimental model was established by hypodermically inoculating the colon cancer cell line CT26 into Balb/c mice. Intracellular cytokine staining used to detect tumor antigen specific CD8+ T cell IFN-γ production. The FACS profile for CD4+ and CD8+ T cells and NK or γδ T Cells composition and activation.
This study is investigates the potential effect of active Chinese mistletoe lectin-55 to enhance colon cancer immune surveillance through regulating both innate and adaptive immune responses.
The results demonstrate that ACML-55 can enhance colon cancer immune surveillance through regulating both innate and adaptive immune responses and also suggest that ACML-55 may be a useful complementary therapy for treating colon cancer.
The study investigates the potential effect of ACML-55 to enhance colon cancer immune surveillance through regulating both innate and adaptive immune responses. Its scientific contents can reflect the advanced levels of basic research and the first report on Chinese mistletoe lectin-55.
Peer reviewer: Yoshiharu Motoo, MD, PhD, FACP, FACG,Professor and Chairman, Department of Medical Oncology, Kanazawa Medical University,1-1 Daigaku, Uchinada, Ishikawa 920-0293, Japan
S- Editor Zhong XY L- Editor Negro F E- Editor Zhang WB
| 1. | Bussing A, Suzart K, Bergmann J, Pfuller U, Schietzel M, Schweizer K. Induction of apoptosis in human lymphocytes treated with Viscum album L. is mediated by the mistletoe lectins. Cancer Lett. 1996;99:59-72. [Cited in This Article: ] |
| 2. | Hajto T. Immunomodulatory effects of iscador: a Viscum album preparation. Oncology. 1986;43 Suppl 1:51-65. [Cited in This Article: ] |
| 3. | Ribereau-Gayon G, Dumont S, Muller C, Jung ML, Poindron P, Anton R. Mistletoe lectins I, II and III induce the production of cytokines by cultured human monocytes. Cancer Lett. 1996;109:33-38. [Cited in This Article: ] |
| 4. | Beuth J. Clinical relevance of immunoactive mistletoe lectin-I. Anticancer Drugs. 1997;8 Suppl 1:S53-S55. [Cited in This Article: ] |
| 5. | Stein GM, Berg PA. Mistletoe extract-induced effects on immunocompetent cells: in vitro studies. Anticancer Drugs. 1997;8 Suppl 1:S39-S42. [Cited in This Article: ] |
| 6. | Fischer S, Scheffler A, Kabelitz D. Activation of human gamma delta T-cells by heat-treated mistletoe plant extracts. Immunol Lett. 1996;52:69-72. [Cited in This Article: ] |
| 7. | Fischer S, Scheffler A, Kabelitz D. Stimulation of the specific immune system by mistletoe extracts. Anticancer Drugs. 1997;8 Suppl 1:S33-S37. [Cited in This Article: ] |
| 8. | Hauer J, Anderer FA. Mechanism of stimulation of human natural killer cytotoxicity by arabinogalactan from Larix occidentalis. Cancer Immunol Immunother. 1993;36:237-244. [Cited in This Article: ] |
| 9. | Mueller EA, Anderer FA. A Viscum album oligosaccharide activating human natural cytotoxicity is an interferon gamma inducer. Cancer Immunol Immunother. 1990;32:221-227. [Cited in This Article: ] |
| 10. | Mueller EA, Anderer FA. Synergistic action of a plant rhamnogalacturonan enhancing antitumor cytotoxicity of human natural killer and lymphokine-activated killer cells: chemical specificity of target cell recognition. Cancer Res. 1990;50:3646-3651. [Cited in This Article: ] |
| 11. | Mockel B, Schwarz T, Zinke H, Eck J, Langer M, Lentzen H. Effects of mistletoe lectin I on human blood cell lines and peripheral blood cells. Cytotoxicity, apoptosis and induction of cytokines. Arzneimittelforschung. 1997;47:1145-1151. [Cited in This Article: ] |
| 12. | Thies A, Nugel D, Pfuller U, Moll I, Schumacher U. Influence of mistletoe lectins and cytokines induced by them on cell proliferation of human melanoma cells in vitro. Toxicology. 2005;207:105-116. [Cited in This Article: ] |
| 13. | Harmsma M, Gromme M, Ummelen M, Dignef W, Tusenius KJ, Ramaekers FC. Differential effects of Viscum album extract IscadorQu on cell cycle progression and apoptosis in cancer cells. Int J Oncol. 2004;25:1521-1529. [Cited in This Article: ] |
| 14. | Lee HS, Kim YS, Kim SB, Choi BE, Woo BH, Lee KC. Isolation and characterization of biologically active lectin from Korean mistletoe, Viscum album var. Coloratum. Cell Mol Life Sci. 1999;55:679-682. [Cited in This Article: ] |
| 15. | Gong F, Ma Y, Ma A, Yu Q, Zhang J, Nie H, Chen X, Shen B, Li N, Zhang D. A lectin from Chinese mistletoe increases gammadelta T cell-mediated cytotoxicity through induction of caspase-dependent apoptosis. Acta Biochim Biophys Sin (Shanghai). 2007;39:445-452. [Cited in This Article: ] |
| 16. | Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433-442. [Cited in This Article: ] |
| 17. | Yin Z, Zhang DH, Welte T, Bahtiyar G, Jung S, Liu L, Fu XY, Ray A, Craft J. Dominance of IL-12 over IL-4 in gamma delta T cell differentiation leads to default production of IFN-gamma: failure to down-regulate IL-12 receptor beta 2-chain expression. J Immunol. 2000;164:3056-3064. [Cited in This Article: ] |
| 18. | Jung ML, Baudino S, Ribereau-Gayon G, Beck JP. Characterization of cytotoxic proteins from mistletoe (Viscum album L.). Cancer Lett. 1990;51:103-108. [Cited in This Article: ] |
| 19. | Gao Y, Tao J, Li MO, Zhang D, Chi H, Henegariu O, Kaech SM, Davis RJ, Flavell RA, Yin Z. JNK1 is essential for CD8+ T cell-mediated tumor immune surveillance. J Immunol. 2005;175:5783-5789. [Cited in This Article: ] |
| 20. | Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118-1122. [Cited in This Article: ] |
| 21. | Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95-109. [Cited in This Article: ] |
| 22. | deVere White RW, Hackman RM, Soares SE, Beckett LA, Li Y, Sun B. Effects of a genistein-rich extract on PSA levels in men with a history of prostate cancer. Urology. 2004;63:259-263. [Cited in This Article: ] |
| 23. | deVere White RW, Hackman RM, Soares SE, Beckett LA, Sun B. Effects of a mushroom mycelium extract on the treatment of prostate cancer. Urology. 2002;60:640-644. [Cited in This Article: ] |
| 24. | Khil LY, Kim W, Lyu S, Park WB, Yoon JW, Jun HS. Mechanisms involved in Korean mistletoe lectin-induced apoptosis of cancer cells. World J Gastroenterol. 2007;13:2811-2818. [Cited in This Article: ] |
| 25. | Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137-148. [Cited in This Article: ] |
| 26. | Li NL, Nie H, Yu QW, Zhang JY, Ma AL, Shen BH, Wang L, Bai J, Chen XH, Zhou T. Role of soluble Fas ligand in autoimmune diseases. World J Gastroenterol. 2004;10:3151-3156. [Cited in This Article: ] |
| 27. | Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107-1111. [Cited in This Article: ] |
| 28. | Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336-345. [Cited in This Article: ] |