Published online Sep 14, 2008. doi: 10.3748/wjg.14.5237
Revised: August 10, 2008
Accepted: August 17, 2008
Published online: September 14, 2008
Despite significant advances over the last decade, mucosal lesions of the small bowel are poorly detected by imaging studies such as CT scan, MRI-enteroclysis and contrast-enhanced abdominal ultrasound. Capsule endoscopy (CE) has dramatically changed the diagnostic approach to intestinal diseases. Moreover, the use of CE can be extended to include other conditions. However, it is difficult to assess the positive influence of CE on patient outcomes in conditions involving a small number of patients, or in critically ill and difficult to examine patients. CE has the advantage of diagnosing intestinal lesions and of directing the use of double balloon enteroscopy (DBE) in order to obtain biopsy specimens. Moreover, CE allows repeated assessment in chronic conditions, especially to detect relapse of an infectious disease.
- Citation: Gay G, Delvaux M, Frederic M. Capsule endoscopy in non-steroidal anti-inflammatory drugs-enteropathy and miscellaneous, rare intestinal diseases. World J Gastroenterol 2008; 14(34): 5237-5244
- URL: https://www.wjgnet.com/1007-9327/full/v14/i34/5237.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5237
| Frequency | |
| Crohn's disease | ++++ |
| Drug-related injuries (NSAIDs) | +++ |
| Mesenteric ischaemia | ++ |
| Coeliac disease (jejunitis) | ++ |
| Cryptogenic multifocal ulcerous stenosing enteritis | + |
| Radiation enteritis | + |
| Lymphoma, ulcerated cancer | + |
| Vasculitides (lupus, polyarthritis, PAN) | + |
| Behçet's disease | +/- |
| Eosinophilic enteritis | +/- |
| Infections (CMV, Whipple, yersinia etc) | +/- |
| Gastrointestinal signs | Extra-intestinal signs |
| Giardia lamblia infection | Recurrent respiratory tract infections |
| Small bowel bacterial overgrowth | Increased risk of lymphomas |
| Viral and infectious diarrhoea | Increased risk of gastric cancer |
| Coeliac disease | |
| Frequent nodular lymphoid hyperplasia, not premalignant |
| Disease | Frequency of digestive manifestations |
| Primary vasculitides | |
| Periarteritis nodosa. | 30%-50% |
| Churg-Strauss Syndrome | 25%-50% |
| Behçet’s disease | Up to 30% |
| Takayasu’s arteritis | Up to 15% |
| Wegenre’s disease (granulomatosis) | 5%-10% |
| Lymphomatous granulomatosis | 1.5% |
| Horton’s disease | 1% |
| Henoch-Schönlein purpura | 50%-90% |
| Secondary vasculitides | |
| Systemic lupus erythematosus | Up to 50% |
| Rheumatoid arthritis | Up to 10% |
| Connective tissue disorders | |
| Systemic sclerosis | 75%-90% |
| Systemic lupus erythematosus (except vascular lesions) | 25% |
Since it was introduced by Iddan and Meron in 2000[1], capsule endoscopy (CE) has dramatically changed the diagnostic approach to intestinal diseases. Despite significant advances over the last decade, mucosal lesions of the small bowel are poorly detected by techniques such as CT scan, MRI-enteroclysis and contrast-enhanced abdominal ultrasound[2]. The diagnostic superiority of CE over these methods is related to its ability to provide a complete examination of the small bowel mucosa. On the other hand, the drawback of CE is the inability to obtain biopsies. However, this deficiency has been overcome with the use of double balloon enteroscopy, which permits obtaining biopsies from lesions detected by CE[3].
Therefore, the diagnostic approach to conditions such as obscure GI bleeding, Crohn’s disease and coeliac sprue has been dramatically altered by CE[4-6]. Moreover, the use of CE has resulted in significant advances in the understanding and diagnosis of several gastrointestinal diseases including assessment of the effect of medications on the small bowel, intestinal lesions secondary to systemic diseases, and some rare conditions[7].
In this review, the role of CE will be discussed in the following conditions: (I) Intestinal consequences of medications such as non-steroidal anti-inflammatory drugs (NSAIDs). (II) Rare conditions mainly involving the small bowel. (1) Primary lymphangiectasia such as Waldmann’s disease. (2) Common variable immunodeficiency disorder. (3) Familial polyposis syndromes with small bowel involvement. (III) Immunological disorders with small bowel involvement. (1) Acute and chronic graft versus host disease. (2) Hypobetalipoproteinaemia. (IV) General diseases with intestinal lesions, such as vasculitides. (V) Infectious intestinal diseases such as Whipple’s disease and CMV infection in immunosuppressed patients.
For each of these conditions, the clinical and biological characteristics will be discussed when needed, to understand the role of CE in diagnosis. The typical endoscopic patterns observed with CE will be described, and the use of CE will be integrated in a global diagnostic approach.
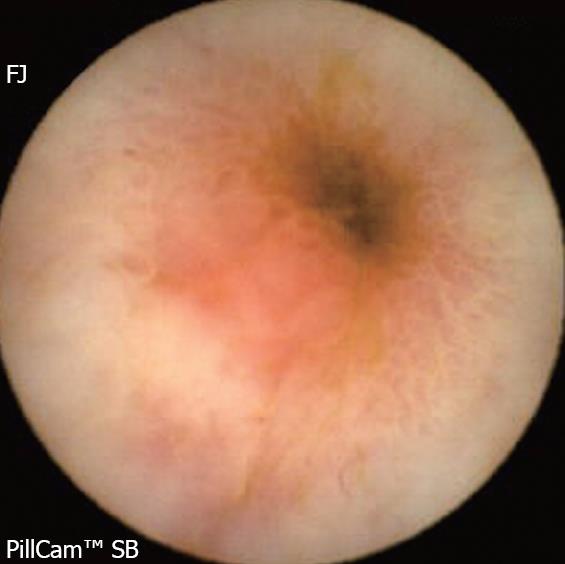
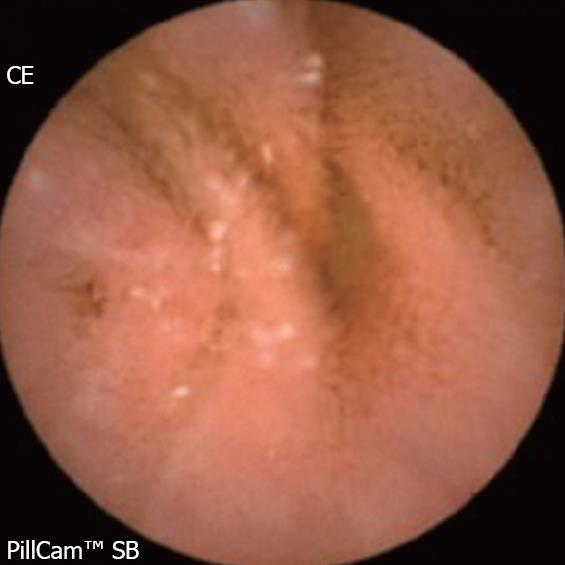
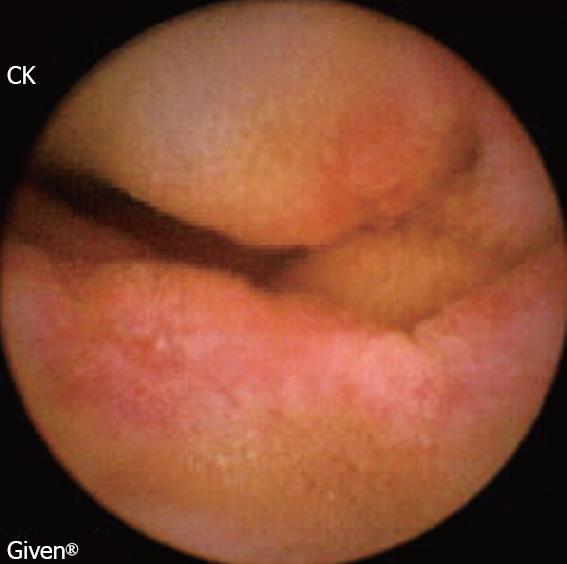
Background: NSAIDs account for 5%-10% of all drug prescriptions in developed countries and about 25% of the reported side effects caused by all classes of medications, including gastroduodenal ulcers seen in 10%-30% patients with intestinal lesions[8]. The prevalence of severe side effects involving the small bowel and colon is 0.89 per 100 patient-years with naproxen, and 0.41 with rofecoxib, corresponding to 39.4% and 42.0%, respectively, of all the severe gastrointestinal side effects[9]. NSAIDs alter the intestinal permeability, about 12 hours after intake and result in mucosal inflammation within 10 days. A variety of endoscopic lesions have been described, ranging from asymptomatic enteropathy to severe lesions such as ulcers, perforation, stenosis (Figure 1), diaphragms and villous atrophy. CE may be able to help detect these lesions and clarify their pathophysiology.
Description of the lesions: One of the most interesting studies was reported by Maiden et al[10], who evaluated the number and the type of intestinal lesions induced by NSAIDs in 40 healthy volunteers between the ages of 21 and 61 years, using CE and fecal calprotectin. The fecal calprotectin test and CE were performed before and after a 2-week course of treatment with diclofenac (75 mg) and omeprazole (20 mg twice a day). The CE recordings were read by three independent investigators, and the lesions were regarded as significant if indicated by at least two of them. The lesions were classified into six categories: (1) reddened folds; (2) denuded area with loss of villous architecture; (3) petechiae; (4) mucosal breaks; (5) presence of blood without a lesion being visualized; and (6) other findings, mainly lymphangiectasia and angiodysplasia. Before treatment, lymphangiectasia and arteriovenous malformations were observed in three patients each. After treatment with nonsteroidal anti-inflammatory drugs (NSAIDs), lesions were seen in 27 patients, 15 of whom had more than one lesion: 16 mucosal breaks (40%), including two cases of bleeding; 14 reddened folds (35%); 13 petechiae and red spots (33%); eight denuded areas (20%), including two patients with mucosal breaks; and three patients with blood in the intestinal lumen (8%). After 2 weeks, the fecal calprotectin level increased significantly in 30 subjects (+ 75-82 μg; P < 0.0001) but this increase did not correlate with the number of lesions detected by CE. The authors concluded that short-term NSAID treatment was associated with a high level of intestinal inflammation, and an increased frequency of intestinal lesions.
NSAID-related lesions were located equally in the proximal and distal parts of the small bowel. The study by Maiden et al[10] was interesting, as it showed that significant lesions can occur even after a short course of NSAID treatment in healthy individuals. However, the study had several methodological limitations: (1) The interpretation of CE images was often difficult[11] and the clinical relevance of lesions detected by CE was not always established, since such lesions could be found in the absence of any treatment in healthy individuals[12]. (2) Other causes of intestinal lesions such as chronic ischemia were not excluded. (3) A standardized terminology allowing a consistent description of the lesions and comparisons between different studies was lacking[11]. Moreover, DBE provides a direct access to intestinal lesions detected by CE and biopsy specimens can be obtained. Therefore, our understanding of the pathophysiology of early lesions induced by NSAIDs is likely to improve.
Shunji and Nakamura showed in a randomized trial on 16 patients that rebaminide significantly decreased the prevalence of diclofenac-induced small intestinal mucosal injury[13]. These results need to be confirmed in larger studies. On the other hand, the etiological diagnosis of intestinal lesions observed by CE is often difficult. The distinction of lesions related to NSAIDs or those seen in Crohn’s disease is particularly difficult, as recently shown by Voderholzer who reported a rate of misdiagnosed lesions in as many as 25% of the 40 patients with 146 intestinal lesions detected by CE[14].
Table 1 shows how difficult the differential diagnosis is of intestinal erosions and ulcers, since they may be related to a number of pathological conditions and diseases. Therefore, DBE with biopsy of the lesions will often be necessary to obtain a correct diagnosis.
Intestinal lymphangiectasia appears on CE recordings as whitish areas, often diffusely spread over the intestinal mucosa. The endoscopic appearance of lymphangiectasia is the result of an accumulation of chylomicrons in the dilated lymphatic vessels, varying in size from a few millimeter white spots to large white nodular areas. Lymphangiectasia may be diffuse or may involve a localized segment of the small bowel. Clinically, lymphangiectasia is classified as primary, secondary or functional. Functional lymphangiectasia are not clinically relevant and it is often encountered in patients with functional digestive disorders. Secondary lymphangiectasia is a consequence of intestinal or extra-intestinal diseases causing compression of the gut. By contrast, Waldmann’s disease is primary idiopathic lymphangiectasia, which results in an exudative enteropathy. This condition may present as a diffuse disease or may involve only a localized segment of the small bowel. Localized lymphangiectasia can be treated surgically, by resection of the pathological segment. However, diffuse disease requires medical treatment (diet containing medium chain fatty acids). DBE shows thickened mucosal folds without villous atrophy in the duodenum, jejunum and ileum. Small size nodules (spots) may be observed as well as large confluent areas[15]. CT scan may show thickening of the mucosal folds.
This condition is characterized by hypogamma-globulinaemia, recurrent bacterial infections, mainly pulmonary, and is frequently associated with autoimmune and neoplastic disorders. Table 2 summarizes the clinical features. CE shows small (millimetre size) nodular lesions spread diffusely over the intestinal mucosa. In some cases, the lesions are polypoidal, of variable sizes[16]. DBE shows similar findings and may also demonstrate areas of atrophic mucosa, in the event of an associated infection. The nodules correspond to hypertrophic lymphoid follicles, without plasma cells.
In most instances, the lesions (either nodules or polyps) are disseminated throughout the gut. There is an increased risk of intestinal lymphoma in these patients. CE allows regular surveillance of the small bowel. When CE reveals a change in the endoscopic findings, DBE with biopsy should be performed or the patient should refered to a surgeon for intestinal resection.
Background: CE is very effective in detecting intestinal tumours. Intestinal tumours account for 3% to 6% of all digestive neoplasms, less than 2% of which are malignant. The data collected since the introduction of CE, shows a higher frequency of intestinal tumours, between 6.3% and 12.3% in patients investigated for obscure digestive bleeding by CE[17]. Since the use of CE permits earlier diagnosis, the clinical course of intestinal tumours has changed. These tumours are now seen as ulcerated, haemorrhagic lesions, before the occurrence of obstructive signs. Therefore, one may assume that CE can be used for the detection of intestinal tumours in patients with familial polyposis syndromes. There are two conditions that are of particular concern: Peutz-Jeghers syndrome and familial adenomatous polyposis syndrome.
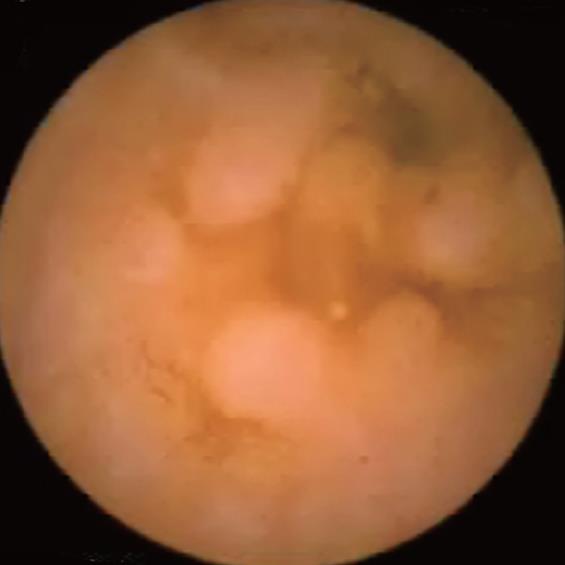
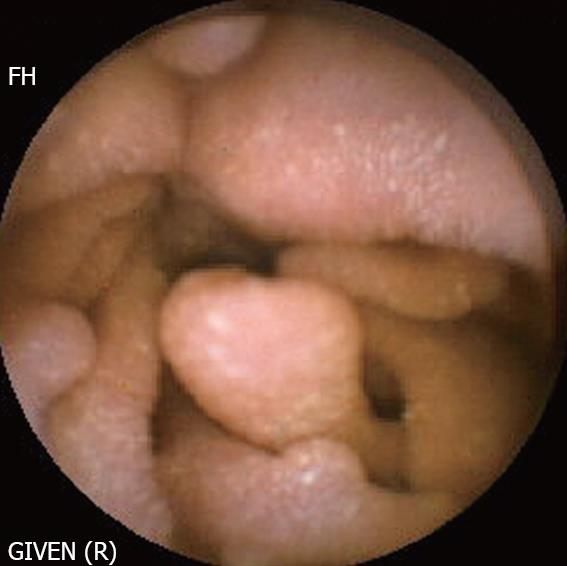
Peutz-Jeghers syndrome (Figure 4): In patients with Peutz-Jeghers syndrome, the prevalence of small bowel cancer is particularly high. Intussusception of the polyps may cause intestinal obstruction, while ulcerated lesions may be responsible for acute or chronic gastrointestinal bleeding. CE can detect polyps in the entire length of the small intestines, with a higher diagnostic yield compared to CT scan and MRI with enteroclysis, particularly for lesions < 1 cm in diameter[18]. The role of CE has recently been established in the initial work-up, as well as for the follow-up of patients with Peutz-Jeghers syndrome[18]. However, it is difficult to determine the size of the polyps on capsule recordings, which is an important factor in selecting patients for removal by DBE. It should be noted that polyps selected for removal are of a large size, and those at high risk of ulceration, malignancy and intussusception.
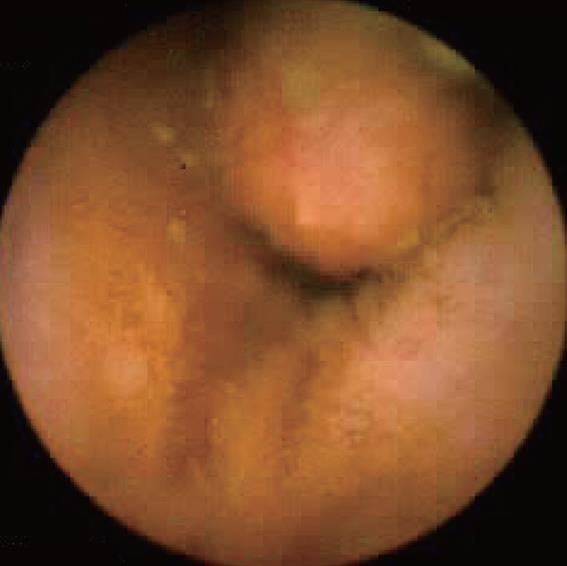
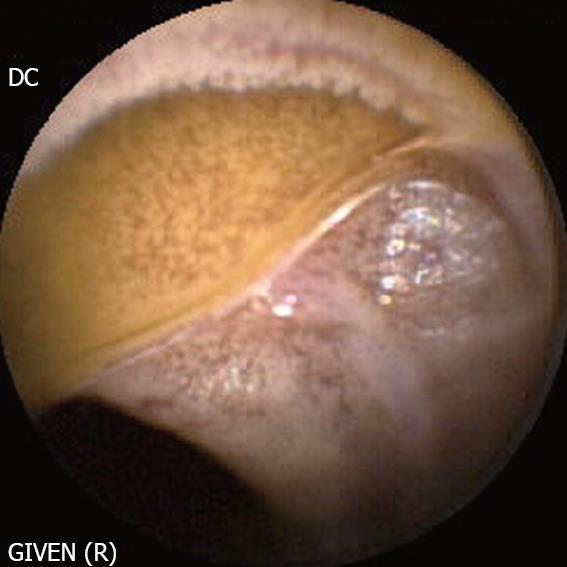
Familial adenomatous polyposis syndrome (FAP, Figure 5): There is currently no established indication for small bowel CE in the initial work-up of patients with FAP. By contrast, CE may be indicated in FAP patients with multiple duodenal polyps (Spiegelmann III or IV) as these patients have more frequent intestinal polyps[19]. Moreover, polyps larger than 10 mm in diameter should be removed because of the risk of malignancy, either by DBE or intra-operative enteroscopy. On the other hand, examination of the duodenum, in particular the area of the papilla is often difficult with CE because of the rapid transit of the capsule through this segment. Therefore, assessment of the duodenum with a lateral viewing endoscope is the preferred approach.
Capsule endoscopy should be used with caution in familial polyposis syndromes, since many of these patients have undergone multiple abdominal surgeries which may delay capsule progression or even cause its retention.
B-cell lymphoma: Finally, in this section on rare intestinal tumours, it is important to mention the role of CE in the investigation of patients with suspected B-cell lymphoma. CE not only allows the diagnosis of such tumours but also helps to evaluate the extent of the intestinal disease[20]. CE is also useful in evaluating the response to chemotherapy.
Bone marrow transplantation is widely used for the treatment of a number of haematological diseases. Acute GVHD is a severe complication that requires quick initiation of treatment with immunosuppressive drugs. The small bowel is often involved in acute GVHD, with the development of hematochezia, diarrhoea, abdominal pain, nausea and vomiting. The diagnosis is difficult and GVHD must be distinguished from other conditions sharing a similar clinical picture such as cytomegalovirus enteritis and Clostridium difficile infection.
The diagnosis is easier in the presence of multi-system disease, especially when the skin lesions can be biopsied. In patients without skin involvement, an endoscopic work-up is needed, with esogastroduodenoscopy and ileo-colonoscopy. Until recently, esogastroduodenoscopy was regarded as the gold standard for the examination of the duodenum, with findings of mucosal oedema, denuded mucosa, erosions, erythema and bleeding ulcers. These lesions are classified into 4 grades, grade I refers to focal erythema, grade II diffuse erythema and oedema, grade III severe oedema and friable mucosa with erosions, and grade IV presence of exudate, ulcers and active bleeding[21].
The clinical usefulness of CE in acute GVHD was demonstrated in two studies. Neumann et al[22] evaluated 14 patients with clinical signs of acute GVHD after bone marrow transplantation, and observed typical intestinal lesions with histological confirmation on biopsy obtained by endoscopy, performed subsequently. This study also showed that the lesions involved the entire length of the small bowel and were more intense in the ileum compared to in the jejunum. The most important finding was the very high negative predictive value of CE since patients with a negative CE did not develop acute GVHD during the 2-month follow-up period after CE. These findings were confirmed by Aghai et al[23] in a study on a larger cohort of patients.
Although it is worth mentioning that CE is well tolerated by such critically ill patients, certain limitations should be noted. In some patients, the capsule may be retained in the stomach. The Rapidview system can be used to monitor the progression of the capsule[24]. In the event of delayed gastric clearance, erythromycin may be administered. Moreover, it should be emphasized that in some patients, CE was normal but these patients had acute GVHD[25].
Despite the limited data available in the literature, CE should now be regarded as an alternative to esogastroduodenoscopy in the workup of patients with suspected GVHD, particularly in critically ill patients requiring quick therapeutic decisions. Indeed, CE appears to be as effective as esogastroduodenoscopy with biopsy for the diagnosis of acute GVHD[25-27].
Background: Hypobetalipoproteinemia is an autosomal dominant disorder caused by a mutation or deletion in the apoB gene which produces a truncated apolipoprotein B. The plasma concentrations of the two forms of apolipoprotein B, that is, Apo B-100 synthesized by the liver and Apo-B48 synthesized by the intestine, are undetectable. The biological and clinical picture is similar to that of abetalipoproteinemia. Heterozygous hypobetalipoproteinemia is present in 0.1%-0.8% of the general population, while homozygous hypobetalipoproteinemia is rare. The prognosis of Apo B-related disorders depends mainly on the consequences of the malabsorption syndrome, and on the progression of neurological alterations caused by the deficiency of soluble vitamin E[28].
Diagnostic procedure: Laboratory tests show hypocholesterolemia, acanthocytosis and undetectable ApoB lipoprotein. CT scan shows massive and diffuse infiltration of the liver. CE demonstrates diffuse whitish pattern of the intestinal mucosa with occasional yellow areas seen in the entire length of the small bowel, but without any villous atrophy. Enteroscopy, formerly performed with push enteroscopes and currently with DBE shows a similar pattern as CE and moreover, biopsy specimens can be obtained. These lesions are produced by the accumulation of fat vesicles in the enterocytes in the jejunum and ileum, without causing villous atrophy[29]. Immunohistchemistry shows migration of truncated Apo-B and the absence of normal Apo-B hypobetalipoproteinemia.
General considerations: Systemic diseases include several very heterogeneous pathological conditions which share some common features: (1) symptoms suggesting the involvement of multiple organs, (2) specific immunological and biological alterations, (3) a tendency to chronicity, and (4) clinical response to immunosuppressive therapy. Systemic diseases are divided into two main categories[30,31]: connective tissue or collagenous diseases with predominant abnormal production or deregulated synthesis of collagen, and vasculitides with predominant inflammation of the blood vessels resulting in abnormal vascular permeability, thrombosis and tissue ischaemia. These conditions share some pathophysiological mechanisms such as abnormal production of proteins or cytokines, and abnormal humoral or cellular immunity that results in muscular inflammation, atrophy and fragmentation. In the end, the vascular endothelial changes become predominant and are responsible for the muscular, neurological and digestive abnormalities.
Digestive manifestations of vasculitides and systemic diseases include a number of clinical conditions (Table 3)[32]. The development of GI symptoms raises three main questions: Do the digestive symptoms correspond to the initial manifestation of the condition or to a new acute phase of the disease? Are the digestive symptoms secondary to a complication of the treatment? Is the observed complication a component of the primary disease? To answer these questions, the clinician may use diagnostic techniques such as CT scan, biological and immunological tests, and CE. The use of CE will be illustrated by a few examples.
Behçet’s disease: Behçet’s disease is a systemic inflammatory condition, with 10% to 25% patients developing GI manifestations. The symptoms consist mainly of abdominal pain, diarrhoea and acute or chronic bleeding. CE may show erosions and aphthous ulcers in the small bowel, as reported by Fylejk in a series of 20 patients[33]. Intestinal lesions may be present in symptomatic and asymptomatic patients, and do not show a specific endoscopic pattern.
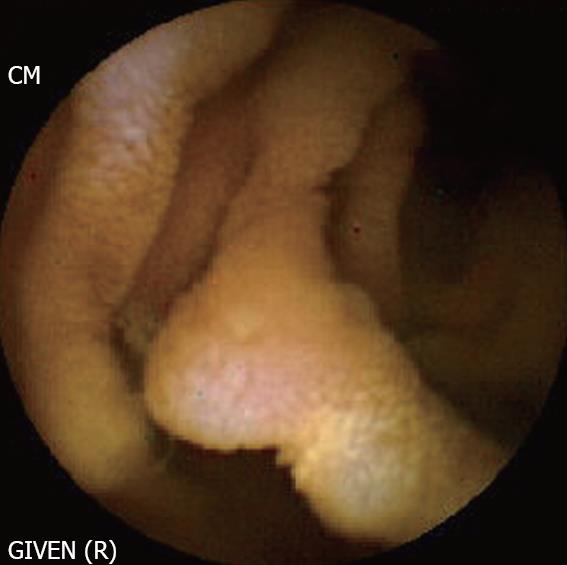
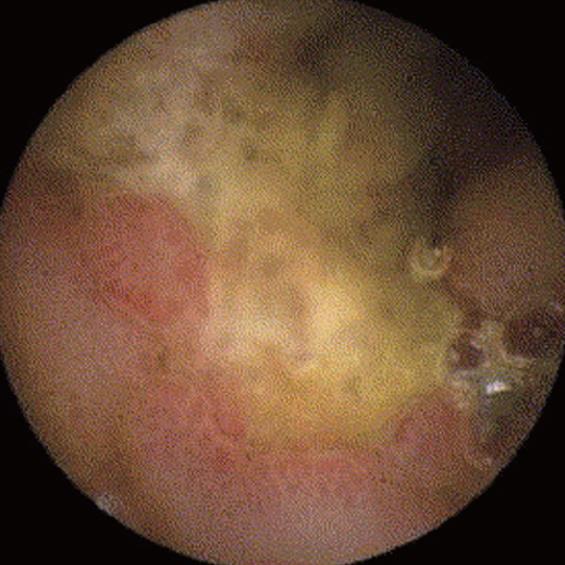
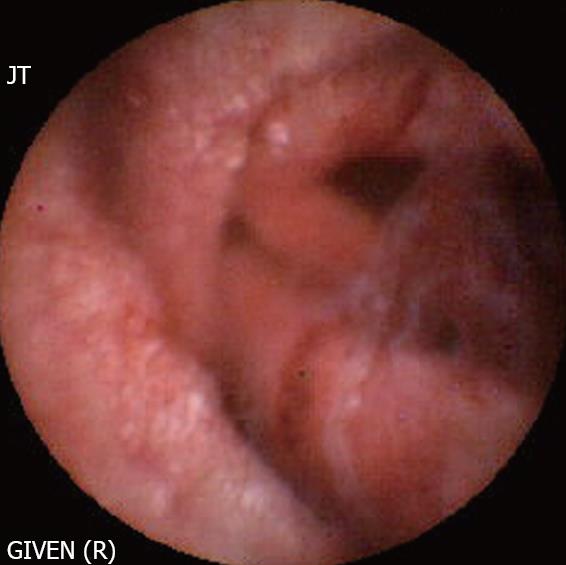
Churg et Strauss disease (Figure 8): Churg et Strauss disease is a systemic disease characterized by eosinophilia and NTE manifestations suggesting an allergenic reaction. The small bowel is involved in nearly 50% patients, presenting with gastrointestinal bleeding, diarrhoea and abdominal pain. Intestinal ulcers are not uncommon and often manifest as bleeding or perforation. Sanchez [34] have shown that the ulcers detected by CE are deep, involve the jejunum and ileum, and respond well to immunosuppressive treatment. Therefore, CE is also useful in evaluating the response to treatment.
Henoch-Schönlein purpura: The clinical picture of Henoch-Schönlein purpura is characterized by the occurrence of purpuric lesions, abdominal pain, polyarthralgia and renal failure. The disease is a consequence of the deposition of IgA in the blood vessels. CE may reveal intestinal involvement, with findings of oedematous mucosa, multiple ulcers, fibrin deposits and bleeding. Lesions observed by CE are often diffuse, involving the jejunum and ileum. If the clinical picture suggests Henoch-Schönlein purpura, the presence of intestinal lesions at CE should be regarded as diagnostic, and further endoscopic assessment is not required[35].
Antral vascular ectasia associated with vasculitides: Antral vascular ectasia are a common cause of bleeding and anaemia, particularly in patients with liver cirrhosis and portal hypertension. However, portal hypertension is not observed in nearly 70% patients. In these patients, the condition is related to immunological disorders such as systemic sclerosis and rheumatoid arthritis, renal failure and diabetes. Antral vascular ectasia can be diagnosed by CE with a typical endoscopic pattern of “watermelon stomach”. Thus, CE is useful not only in diagnosing the cause of anaemia but also for the follow-up of patients treated with endoscopic haemostasis[36].
The clinical manifestation of intestinal infection is usually an acute episode of diarrhoea, which resolves spontaneously, and endoscopic assessment is not indicated in most patients. By contrast, patients with persistent diarrhoea or malabsorption require further workup. Recently, there has been an increase in the incidence of certain viral infections in patients with immunodeficiency due to HIV infection or those on immunosuppressive therapy. Endoscopy is the main diagnostic procedure as it allows examination of the mucosa, and biopsy specimens can be obtained for identification of the causal virus. Biopsies are frequently performed during esogastroduodenoscopy and ileo-colonoscopy. These procedures have a limited range of examination, since a large part of the small bowel is excluded. CE is a very useful complement to these investigations, as it allows assessment of the intestinal mucosa with a high diagnostic yield, and can have a direct impact on the management of such patients[37]. Moreover, lesions detected by CE can be subsequently biopsied during DBE.
CE can detect ulcers involving the intestinal mucosa in patients with viral, fungal or bacterial infections. Cytomegalovirus infection can be diagnosed by the presence of one or two large jejunal ulcers in immunodeficient patients, as for example in transplant patients. These ulcers may bleed and are the source of haematochezia when located in the distal ileum or the colon[38]. Fungal infection in HIV patients, such as histoplasmosis is characterized by the presence of multiple deep ulcerations accompanied with submucosal nodules protruding into the lumen. The diagnosis is confirmed by the detection of the pathogenic agent on biopsies obtained during enteroscopy[39]. In patients with tuberculosis, CE may reveal deep ulcers, often appearing as serpiginous. The lesions are difficult to differentiate from those of Crohn’s disease. The pathological examination of the biopsies may show a typical granuloma. Studies using CE have shown that the lesions may not be restricted to the ileum but are also seen in the jejunum[40].
Whipple’s disease is a multisystem infectious condition caused by Tropheryma whippelii. GI symptoms are often present and the diagnosis is made by demonstrating the presence of the infectious agent on duodenal biopsy. The most typical endoscopic finding is the presence of an oedematous, friable and haemorrhagic mucosa with multiple erosions and serpiginous ulcers. CE has shown that the intestinal lesions are diffuse, involving the jejunum and ileum. This is explained by the fact that previous investigative techniques allowed only limited access to the intestinal mucosa, i.e. before CE and DBE became available[41]. Moreover, CE is also a useful non-invasive method to determine the response to treatment, which has to be maintained for at least two years, and occasionally five years in order to avoid relapse, especially of the neurological manifestations[42].
Since it was introduced in 2000, the use of CE in several clinical situations has been validated. However, the indications for CE can be extended to other diseases, although an assessment of a positive influence of CE on patient outcomes is difficult obtain in conditions which occur either in a small number of patients, or in critically ill and difficult to examine patients. Clearly, CE has the advantage of detecting lesions in the small intestine and directing DBE to the correct location in order to obtain biopsies. Moreover, repeated assessment can be made in chronic conditions, especially to detect the response to treatment.
Peer reviewer: Giovanni D De Palma, Professor, Department of Surgery and Advanced Technologies, University of Naples Federico II, School of Medicine, Naples 80131, Italy
S- Editor Zhong XY L- Editor Anand BS E- Editor Lin YP
| 1. | Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. [Cited in This Article: ] |
| 2. | Orjollet-Lecoanet C, Menard Y, Martins A, Crombe-Ternamian A, Cotton F, Valette PJ. [CT enteroclysis for detection of small bowel tumors]. J Radiol. 2000;81:618-627. [Cited in This Article: ] |
| 3. | Gay G, Delvaux M, Fassler I. Outcome of capsule endoscopy in determining indication and route for push-and-pull enteroscopy. Endoscopy. 2006;38:49-58. [Cited in This Article: ] |
| 4. | Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418. [Cited in This Article: ] |
| 5. | Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease. Am J Gastroenterol. 2006;101:954-964. [Cited in This Article: ] |
| 6. | Cellier C, Green PH, Collin P, Murray J. ICCE consensus for celiac disease. Endoscopy. 2005;37:1055-1059. [Cited in This Article: ] |
| 7. | Delvaux M, Gerard Gay. Capsule endoscopy in 2005: facts and perspectives. Best Pract Res Clin Gastroenterol. 2006;20:23-39. [Cited in This Article: ] |
| 8. | Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247-1255. [Cited in This Article: ] |
| 9. | Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520-1528, 2 p following 1528. [Cited in This Article: ] |
| 10. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [Cited in This Article: ] |
| 11. | Korman LY, Delvaux M, Gay G, Hagenmuller F, Keuchel M, Friedman S, Weinstein M, Shetzline M, Cave D, de Franchis R. Capsule endoscopy structured terminology (CEST): proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy. 2005;37:951-959. [Cited in This Article: ] |
| 12. | Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Aisenberg J, Bhadra P, Berger MF. Small bowel mucosal injury is reduced in healthy subjects treated with celecoxib compared with ibuprofen plus omeprazole, as assessed by video capsule endoscopy. Aliment Pharmacol Ther. 2007;25:1211-1222. [Cited in This Article: ] |
| 13. | Nakamura M, Niwa Y, Ohmiya N, Goto H. Protective effect of rebaminide for dictofenac-induced small intestinal mucosal injury: A cross over randomized, double blind, placebo-controlled study. 6th ICCE Report. 2007;70. [Cited in This Article: ] |
| 14. | Voderholzer W, Maiden L, Adler S. N, Thjodleifsson B, Lochs H, Bjarnason I. Inter observer variability of wireless capsule endoscopy (WCE) in patients with Crohn’s disease and NSAID enteropathy. 6th ICCE Report. 2007;13. [Cited in This Article: ] |
| 15. | Toth E, Keuchel M, Riemann JF. Intestinal lymphangiectasia. Atlas of video capsule endoscopy. Heildelberg: Springer 2006; 101-106. [Cited in This Article: ] |
| 16. | Mihaly E, Nemeth A, Zagoni T, Nemet A, Werling K, Racz I, Tulassay Z. Gastrointestinal manifestations of common variable immunodeficiency diagnosed by video- and capsule endoscopy. Endoscopy. 2005;37:603-604. [Cited in This Article: ] |
| 17. | Bailey AA, Debinski HS, Appleyard MN, Remedios ML, Hooper JE, Walsh AJ, Selby WS. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a three-center Australian experience. Am J Gastroenterol. 2006;101:2237-2243. [Cited in This Article: ] |
| 18. | Caspari R, von Falkenhausen M, Krautmacher C, Schild H, Heller J, Sauerbruch T. Comparison of capsule endoscopy and magnetic resonance imaging for the detection of polyps of the small intestine in patients with familial adenomatous polyposis or with Peutz-Jeghers' syndrome. Endoscopy. 2004;36:1054-1059. [Cited in This Article: ] |
| 19. | Schulmann K, Hollerbach S, Kraus K, Willert J, Vogel T, Moslein G, Pox C, Reiser M, Reinacher-Schick A, Schmiegel W. Feasibility and diagnostic utility of video capsule endoscopy for the detection of small bowel polyps in patients with hereditary polyposis syndromes. Am J Gastroenterol. 2005;100:27-37. [Cited in This Article: ] |
| 20. | Flieger D, Keller R, May A, Ell C, Fischbach W. Capsule endoscopy in gastrointestinal lymphomas. Endoscopy. 2005;37:1174-1180. [Cited in This Article: ] |
| 21. | Thompson B, Salzman D, Steinhauer J, Lazenby AJ, Wilcox CM. Prospective endoscopic evaluation for gastrointestinal graft-versus-host disease: determination of the best diagnostic approach. Bone Marrow Transplant. 2006;38:371-376. [Cited in This Article: ] |
| 22. | Neumann S, Schoppmeyer K, Lange T, Wiedmann M, Golsong J, Tannapfel A, Mossner J, Niederwieser D, Caca K. Wireless capsule endoscopy for diagnosis of acute intestinal graft-versus-host disease. Gastrointest Endosc. 2007;65:403-409. [Cited in This Article: ] |
| 23. | Yakoub-Agha I, Maunoury V, Wacrenier A, Couignoux S, Depil S, Desreumaux P, Bauters F, Colombel JF, Jouet JP. Impact of Small Bowel Exploration Using Video-Capsule Endoscopy in the Management of Acute Gastrointestinal Graft-versus-Host Disease. Transplantation. 2004;78:1697-1701. [Cited in This Article: ] |
| 24. | Delvaux M, Gay G. Real-Time Viewing of Capsule Endoscopy Recordings: Principle and Clinical Potential. Techn. Gastrointest Endosc. 2006;8:160-163. [Cited in This Article: ] |
| 25. | Eisen GM. Using capsule endoscopy to diagnose graft-versus-host disease: seeing is believing? Gastrointest Endosc. 2007;65:410-411. [Cited in This Article: ] |
| 26. | Terdiman JP, Linker CA, Ries CA, Damon LE, Rugo HS, Ostroff JW. The role of endoscopic evaluation in patients with suspected intestinal graft-versus-host disease after allogeneic bone-marrow transplantation. Endoscopy. 1996;28:680-685. [Cited in This Article: ] |
| 27. | Shapira M, Adler SN, Jacob H, Resnick IB, Slavin S, Or R. New insights into the pathophysiology of gastrointestinal graft-versus-host disease using capsule endoscopy. Haematologica. 2005;90:1003-1004. [Cited in This Article: ] |
| 28. | Gay G, Delmotte JS. Abeta and hypobetalipoproteinemias in Atlas of Enteroscopy. Rossini FP, Gay G (Eds). Milan: Springer 1998; 119-120. [Cited in This Article: ] |
| 29. | Gay G, Barth E, Keuchel M. Involvement of the small intestine in systemic disease. In Keuchel L, Hagenmüller F, Fleischer D. Atlas of video capsule endoscopy. Heildelberg: Springer 2006; 136-144. [Cited in This Article: ] |
| 30. | Lie JT. Nomenclature and classification of vasculitis: plus ca change, plus c'est la meme chose. Arthritis Rheum. 1994;37:181-186. [Cited in This Article: ] |
| 31. | Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CG. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187-192. [Cited in This Article: ] |
| 32. | Muller-Ladner U. Vasculitides of the gastrointestinal tract. Best Practice Res Gastroenterol. 2001;15:59-82. [Cited in This Article: ] |
| 33. | Fylyk S, Fylyk SN, Safatle-Riberio AV, Neves F, Goncalves CR, Sipahi AM, Ishiok AS, Sakai P, Galvao-Neto M. Small intestine involvement in Behcet’s disease: A capsule endoscopy approach. 6 th ICCE Repor. 2007;19. [Cited in This Article: ] |
| 34. | Sanchez R, Aparicio JR, Baeza T, Calero Y. Capsule endoscopy diagnosis of intestinal involvement in a patient with Churg-Strauss syndrome. Gastrointest Endosc. 2006;63:1082-1084. [Cited in This Article: ] |
| 35. | Skogestad E. Capsule endoscopy in Henoch-Schonlein purpura. Endoscopy. 2005;37:189. [Cited in This Article: ] |
| 36. | Tang SJ, Zanati S, Kandel G, Marcon NE, Kortan P. Gastric intestinal vascular ectasia syndrome: findings on capsule endoscopy. Endoscopy. 2005;37:1244-1247. [Cited in This Article: ] |
| 37. | Keuchel M, Soares J, Reddy DN. Infectious diseases of the small intestine. Atlas of video capsule endoscopy. Heildelberg: Springer 2006; 126-135. [Cited in This Article: ] |
| 38. | Bramwell NH, Davies RA, Koshal A, Tse GN, Keon WJ, Walley VM. Fatal gastrointestinal hemorrhage caused by cytomegalovirus duodenitis and ulceration after heart transplantation. J Heart Transplant. 1987;6:303-706. [Cited in This Article: ] |
| 39. | Gupta R, Reddy D. Infections diseases of the small intestine. Techn. Gastrointest Endosc. 2006;8:182-187. [Cited in This Article: ] |
| 40. | Reddy DN, Sriram PV, Rao GV, Reddy DB. Capsule endoscopy appearances of small-bowel tuberculosis. Endoscopy. 2003;35:99. [Cited in This Article: ] |