Published online Sep 7, 2008. doi: 10.3748/wjg.14.5176
Revised: July 21, 2008
Accepted: July 28, 2008
Published online: September 7, 2008
AIM: To investigate the combined effects of K-ras antisense oligodeoxynucleotide (K-ras ASODN) specific to GTT point mutation at codon 12 and type Iinsulin-like growth factor receptor (IGF-IR) antisense oligodeoxynucleotide (IGF-IR ASODN) on proliferation and apoptosis of human pancreatic cancer Patu8988 cells in vitro and in vivo.
METHODS: K-ras gene point mutation and its style at codon 12 of human pancreatic cancer cell line Patu8988 were detected by using polymerase chain reaction with special sequence primers (PCR-SSP) and sequence analysis. According to the mutation style, K-ras mutation ASODN specific to K-ras point mutation at codon 12 was designed and composed. After K-ras ASODN and IGF-IR ASODN treated on Patu8988 cells respectively or cooperatively, the proliferation and morphological change of Patu8988 cells were analyzed by 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, colony forming assay and transmission electron microscopy; the expression of K-ras and IGF-IR mRNA and protein in the treated cells was measured by reverse-transcript polymerase chain reaction (RT-PCR) and flow cytometry respectively; apoptosis was determined by flow cytometry. The combined antitumor activity of K-ras ASODN and IGF-IR ASODN was evaluated in BALB/c nude mice bearing human pancreatic cancer inoculated with Patu8988 cells.
RESULTS: The results of PCR-SSP and sequence analysis showed that the human pancreatic cancer cell line Patu8988 had point mutation at codon 12, and the mutation style was GGT→GTT. 2-32 μg/mL K-ras ASODN and 2-32 μg/mL IGF-IR ASODN could inhibit Patu8988 cells’ growth, induce apoptosis and decrease the expression of K-ras and IGF-IR mRNA and protein alone. However, there was much more effective inhibition of growth and induction of apoptosis by their combination than by each one alone. In tumor bearing mice, the combination of K-ras ASODN and IGF-IR ASODN showed a significant inhibitory effect on the growth of transplanted pancreatic cancer, resulting in a statistically significant difference compared with each alone.
CONCLUSION: It has been found that K-ras ASODN combined with IGF-IR ASODN could cooperatively inhibit the growth of Patu8988 cells, and induce their apoptosis via reinforcing specific down regulation of K-ras and IGF-IR mRNA and protein expression.
- Citation: Shen YM, Yang XC, Yang C, Shen JK. Enhanced therapeutic effects for human pancreatic cancer by application K-ras and IGF-IR antisense oligodeoxynucleotides. World J Gastroenterol 2008; 14(33): 5176-5185
- URL: https://www.wjgnet.com/1007-9327/full/v14/i33/5176.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5176
Pancreatic cancer is the fatal cancer of the digestive system with the worst prognosis. The 5-year survival rate is approximately 1%-2%, and the median survival time after diagnosis ranges only 4-6 mo[1-3]. The reasons for poor prognosis include: (1) the difficulty of early diagnosis due to its anatomical location, and lack of specific early syndromes; (2) the high potential to infiltrate to the surrounding tissues and metastasize even in the early stage; and (3) the poor responsiveness to conventional treatments such as chemotherapy, radiotherapy and immunotherapy[4-6]. Surgery represents the only opportunity for possible cure, but it is restricted to early stage pancreatic cancer and most patients who undergo tumor resection show recurrence or distant metastases and die within a few years. At least, at the present time, an emphasis on early diagnosis alone may not be sufficient for significant improvement in the current poor prognosis of pancreatic cancer, which necessitates the search for novel treatment strategies to improve the prognosis.
Previous studies have demonstrated that a high percentage of pancreatic cancers harbors K-ras gene point mutation and overexpresses insulin-like growth factor receptor type 1 (IGF-IR)[7-11]. These alterations may together contribute to the progression and aggressiveness of pancreatic cancer from different pathways. Consequently, targeting expression of K-ras or IGF-IR has a potential value in pancreatic cancer therapy, and has led to the development of new therapeutic strategies based on the use of agents able to selectively inhibit targeted gene expression. In particular, antisense oligodeoxynucleotides (ASODNs) have proved their efficacy as targeted therapy, and are able to modulate target protein expression in pancreatic cancer studies[12-15]. In the practical application of the ASODN approach, many key problems need to be solved: selection of a single agent does not seem particularly promising because of the multigenic alterations of pancreatic cancer; finding a targeting site of K-ras mRNA or IGF-IR mRNA that is likely to be accessible to ASODNs; selection of an adaptable vector for mediating ASODNs; optimization of transfection concentration in a cell line, etc. Based on these considerations, we used polymerase chain reaction with special sequence primers (PCR-SSP) and sequence analysis to detect a K-ras point mutation at codon 12, and its mutation style on pancreatic cancer Patu8988 cells, designed and prepared ASODN (K-ras ASODN) specific for the K-ras point mutation at codon 12, and then combined it with strongest efficient IGF-IR ASODN designed by Resnicoff et al[16] to transfect pancreatic cancer Patu8988 cells with highly efficient vector Lipofectamine 2000. 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, reverse-transcript polymerase chain reaction (RT-PCR), flow cytometry and transmission electron microscope were used to evaluate the effects of cell proliferation, apoptosis and target gene expression. Therapeutic efficacy of the combination treatment was also evaluated in xenografts.
Human pancreatic cancer cell lines Patu8988 and BXPC-3 used in this study were preserved in our laboratory. The cells were grown in RPMI 1640 medium (Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum (Sijiqing, Hangzhou, China), 5 mmol/L HEPES, 100 U/mL penicillin and 100 U/mL streptomycin in 5% atmospheric CO2 at 37°C. Cells were passaged every 3 d, checked routinely, and found to be free of contamination. When cells grew to 75% confluence, they were digested and used for in vitro and in vivo studies.
Genomic DNAs for Patu8988 cells and BXPC-3 cells were extracted according to the protocol. With regard to the sequences of K-ras cDNA in Genbank and the three high frequency mutation styles (CGT, GTT and GAT) at codon 12, three kinds of special sequence primers (SSP) for polymerase chain reaction were designed to detect K-ras gene point mutation at codon 12 for Patu8988 cells and BXPC-3 cells. Primers were as following: R1: 5'-GGTAGTTGG-AGCTC-3'; R2: 5'-GTAGTTGGAGCTGT-3'; R3: 5'-GTAGTTGGAGCTGA-3'; R4: 5'-CTATTGTTGGATCATATTCG-3'. The pairing of R1-R4 amplified CGT mutation with a 89 base pair fragment; the pairing R2-R4 amplified GTT mutation with a 88 base pair fragment; the pairing R3-R4 amplified GAT mutation with a 88 base pair fragment. The amplification products were loaded on 8% acrylamide gels, and stained with ethidium bromide to detect mutation styles. In addition, K-ras gene was amplified from Patu8988 cells and BXPC-3 cells using RT-PCR, and the PCR products were directly sequenced.
Based on the results of PCR-SSP and sequence analysis, the antisense phosphorothioate oligodeoxynu-cleotides 5'-TACGCCAACAGCTCCAAC-3' (K-ras ASODN) specific to the K-ras gene point mutation at codon 12 were designed and synthesized. The antisense phosphorothioate oligodeoxynucleotides 5'-TCCTCCGGAGCCAGACTT-3' (IGF-IR ASODN) specific to IGF-IR gene were synthesized according to the report from Resnicoff et al[16]. Exponentially growing Patu8988 cells at 1 × 105/well were seeded in 96-well microtiter plate, and treated with K-ras ASODN or IGF-IR ASODN mediated by LipofectamineTM 2000 at concentration of 2-32 mg/L for 24 , 48, 72 and 96 h. The culture medium was changed every 24 h with fresh RPMI 1640 medium, which contained the same concentration of K-ras ASODN or IGF-IR ASODN. The control cultures were left untreated at 37°C for the same period of time, with triplicate wells for each concentration. After incubating for 24, 48, 72 and 96 h, 20 μL of 5 g/L MTT (Sigma, USA) in PBS was added to each well, followed by incubation for 4 h at 37°C. Formazan crystals were dissolved in DMSO for 15 min at 37°C. Absorbance was determined with an enzyme-linked immunosorbent assay reader at 570 nm. The cell proliferation curves were drawn according to the absorbance. The optimal concentration able to inhibit cell growth was selected for further experiments.
Patu8988 cells were seeded in a 96-well plate at a concentration of 1 × 105/well, and divided into three groups: (1) 16 mg/L K-ras ASODN group; (2) 16 mg/L IGF-IR ASODN group; (3)16 mg/L K-ras ASODN + 16 mg/L IGF-IR ASODN group. The cell cultures were measured for cell proliferation at different time points (0, 24, 48, 72 and 96 h after transfection) using MTT assay as described above. The cell proliferation curves were drawn according to the absorbance.
Cells at the concentration of 1 × 105/mL were plated in 6-well plates, divided into three groups as described above. After being incubated for 48 h at 37°C, cells were harvested by trypsinization and rinsed with cold PBS twice. After centrifugation, cells were suspended by 250 μL conjugated buffer solution and then treated with 5 μL Annexin V-FITC and 10 μL propidium iodide (PI) for 15 min in the dark at room temperature. Finally, each sample was added into 300 μL of conjugated buffer solution and analyzed with flow cytometry. The experiments were performed in triplicate and the results were given as mean ± SE.
Patu8988 cells, treated as described above, were removed from the plate by brief trypsinization with 0.25% trypsin, and then washed with PBS twice, stained with primary K-ras Ab or IGF-IR Ab, followed by FITC-conjugated goat anti-mouse IgG. After two rinses with PBS containing 2% FBS, these cells were analyzed with flow cytometry. Controls consisted of incubation with no antibodies or incubation with only the secondary antibody. The experiment was repeated three times.
Cells were plated in 6-well plates and performed as described above. Total cellular RNA was extracted by using Trizol Reagent (Invitrogen, USA) according to the manufacturer’s instructions. The purity and concentration were determined by measuring the absorbance (A) at 260 nm and 280 nm (A260/A280). To generate first-strand cDNA, an oligo (dT) 18 was used as primer, and 2 μg RNA was reverse-transcribed in the light of MMLV First Strand cDNA Synthesis Kit (Fermentas, USA) protocols. Amplification of human β-actin served as an internal control. The primers used were 5'-GGACCTGACTGACTACCTC-3' (forward) and 5'-TCATACTCCTGCTTGCTG-3' (reverse). The amplification products were 540 bp. The primers for K-ras were 5'-CGCGGATCCATGACTGAATATAAACTTGTG-3' (forward) and 5'-CGCAAGCTTTTACATAATTACACACTTTGT-3' (reverse). The amplification products were 585 bp. The primers for IGF-IR were 5'-CCAAAACTGAAGCCGAGAAG-3' (forward) and 5'-TGCAGCTGTGGATATCGATG-3' (reverse). The amplification products were 300 bp. K-ras was amplified 35 cycles under the following conditions: denaturing at 94°C for 5 min followed by 94°C for 1 min, annealing at 51°C for 30 s and extension at 72°C for 1 min; the final extension was at 72°C for 10 min. IGF-IR gene and β-actin were amplified 30 cycles under the following conditions: denaturing at 94°C for 5 min followed by 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 90 s; the final extension was at 72°C for 10 min. PCR products were separated in 1.5% agarose gels, stained with ethidium bromide, and visualized by UV absorption. Densitometric scanning of the bands was performed, and the relative amount of each gene mRNA expression was estimated by normalization to the β-actin mRNA detected in the same sample.
Patu8988 cells treated with the combination of K-ras ASODN and IGF-IR ASODN for 48 h were harvested, and washed in PBS. The cell pellets were prefixed in 2.5% glutaraldehyde, postfixed in 1% osmic acid, dehydrated in gradient acetone and embedded in the resin. Ultrathin sections were cut, stained with lead citrate and assessed for the morphological changes under transmission electron microscope.
Twenty Patu8988 cells treated with 16 mg/L K-ras ASODN, 16 mg/L IGF-IR ASODN or 16 mg/L K-ras ASODN + 16 mg/L IGF-IR ASODN were seeded in 6-well plate and cultured in 5% atmospheric CO2 at 37°C for 2 wk. The control was with the same volume of culture medium.
To investigate whether the combination of K-ras ASODN with IGF-IR ASODN would alter the tumorigenicity of Patu8988, male 4-wk -old BALB/c nude mice were purchased from the Animal Center of Shanghai. 1 × 107 cells in 0.1 mL PBS were injected subcutaneously into the right flank of nude mice. Fourteen days later, 16 mice with about the same tumor size were divided into four groups randomly. Intratumoral injections were given with K-ras ASODN, IGF-IR ASODN or K-ras ASODN + IGF-IR ASODN, and the control with 100 μL physiological saline. The injection was repeated every 48 h and 5 times in all. Tumor sizes were measured every 7 d and calculated by the formula: volume (mm3) = 1/2(width)2× length. After a 49-d follow-up period, mice were sacrificed. The tumors were removed, fixed by 4% polyformaldehyde, paraffin embedded and sectioned for immunohistochemical analysis.
All experiments were performed in triplicate and data were expressed as mean ± SD. Statistical analyses were conducted by one-factor analysis of variance and performed with SPSS 10.0 software. P < 0.05 was considered statistically significant.
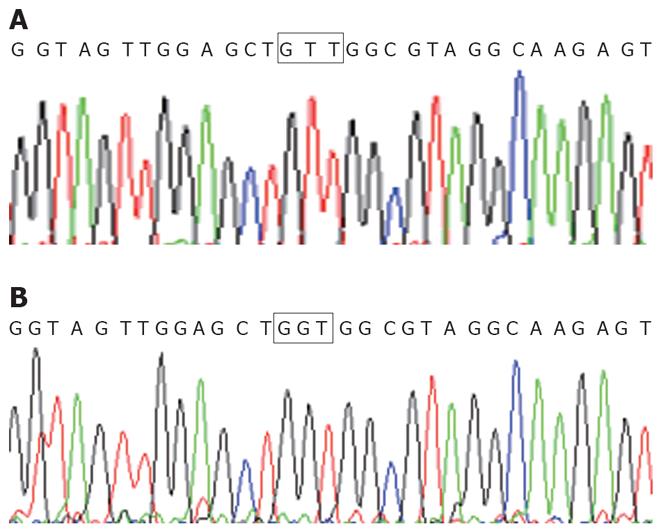
Detection of K-ras point mutation at codon 12 in the pancreatic cancer cell line Patu8988 is shown as Figure 1. The pairing R2-R4 had the amplification product of GTT mutation with an 88 bp fragment. But, the pairings of R1-R4 and R3-R4 had no amplification product of any mutation. Therefore, K-ras point mutation at codon 12 was found in pancreatic cancer cell line Patu8988, and the mutation style was GTT; no other mutation styles were found. For wild type pancreatic cancer cell line BXPC-3, no amplification products were found in pairings of R1-R4, R2-R4 and R3-R4. The direct sequencing results were consistent with the results from the PCR-SSP (Figure 2).
As shown in Figure 3A and B, when Patu8988 cells were exposed to K-ras ASODN and IGF-IR ASODN respectively, the growth of the cells was suppressed as compared to untreated cells (P < 0.01) except at the concentration of 2 mg/L. Moreover, when cells were exposed to different doses of K-ras ASODN and IGF-IR ASODN individually, growth inhibition was dose dependent: obvious inhibition was seen at the concentration of 16 mg/L, and the greatest effect was seen at a concentration of 32 mg/L. However, no statistical significance was found between 16 mg/L and 32 mg/L (P > 0.05). So, combination treatment with 16 mg/L K-ras ASODN and 16 mg/L IGF-IR ASODN was employed, and the ASODNs were transfected into Patu8988 cells for 24, 48, 72 and 96 h. Patu8988 cell growth was inhibited at a significantly higher rate in the combination treatment than that in K-ras ASODN or IGF-IR ASODN alone at different transfection times (P < 0.01) (Figure 3C). The inhibition peak was reached at 48 h. Subsequently, the inhibition ability wore off, and the tumor cells recovered proliferation. Further experiments were conducted to assess the combined effects on the expression of K-ras or IGF-IR mRNA and protein, apoptosis, clone formation and tumor growth inhibition in vivo with the combination treatment of 16 mg/L K-ras ASODN and 16 mg/L IGF-IR ASODN at 37°C for 48 h.
Patu8988 cell proliferation treated in different groups was analyzed by soft agar colony formation assays. The average numbers of colonies in the control, K-ras ASODN, IGF-IR ASODN and combination group were 18.8, 11, 12 and 3, respectively. The Patu8988 cells in the combination groups formed significantly fewer colonies (6 fold decrease) in soft agar than those in the control groups did (P < 0.05 vs control). However, there were no statistical differences between K-ras ASODN groups and IGF-IR ASODN groups, although the number of colonies of IGF-IR ASODN groups was a little larger than those of K-ras ASODN groups (P > 0.05). At the same time, we noticed that the size of most of the colonies in the combination groups were much smaller than those in the control groups.
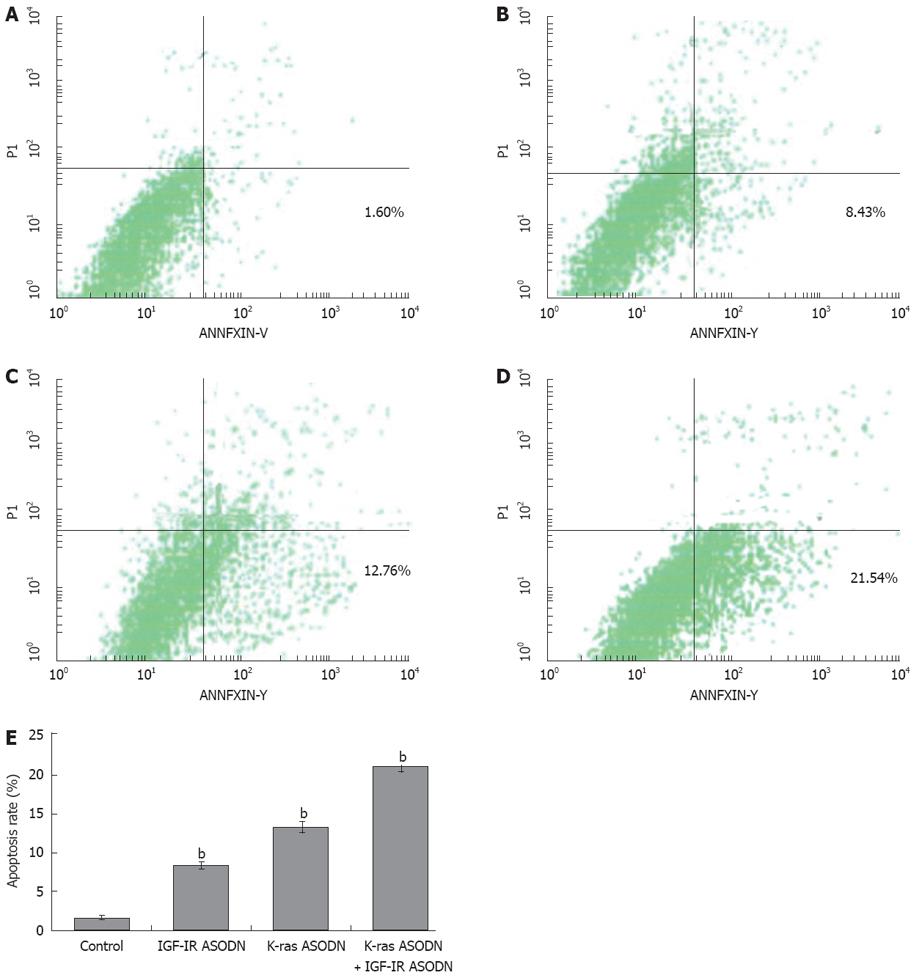
To further confirm the occurrence of apoptosis, we subjected the ASODNs-treated cells (48 h of ASODNs exposure) to annexin V-FITC/PI dual staining followed by flow cytometry analyses. The ratios of apoptosis cells were 21.54% ± 0.93%, 12.76% ± 0.74%, 8.43% ± 0.51% and 1.60% ± 0.19% in combination group, K-ras ASODN group, IGF-IR group and control group, respectively. Compared with the control group, statistically significant differences were observed (P < 0.01). The apoptotic rate of combination group was significantly higher than that of K-ras ASODN group alone or IGF-IR ASODN alone (P < 0.05). No difference existed between K-ras ASODN group and IGF-IR group (P > 0.05) (Figure 4).
Flow cytometry, results showed that the positive rate of K-ras protein was 76.15% ± 1.62% and 69.18% ± 0.87% in control group and IGF-ASODN group, respectively. No statistical difference was found between the two groups (P > 0.05). But, in K-ras ASODN group and the combination group, K-ras protein was significantly decreased by 25.95% ± 0.18% and 19.69% ± 1.15%, respectively, compared with that of control group (P < 0.01). Flow cytometric analysis by using IGF-IR antibody showed that there was high expression in K-ras ASODN group, and control group with a positive rate of 91.53% ± 1.62% and 85.25% ± 0.99%, respectively. But, in IGF-IR ASODN, and combination group, IGF-IR protein expression was reduced to 40.78% ± 1.42% and 38.25% ± 1.22%, respectively. Significant differences were found when compared with control group (P < 0.01). All above results revealed that antisense oligodeoxynucleotides can inhibit corresponding protein expression. But, K-ras ASODN can not obviously inhibit the expression of IGF-IR protein, and IGF-IR ASODN can not obviously inhibit the expression of K-ras protein (Table 1).
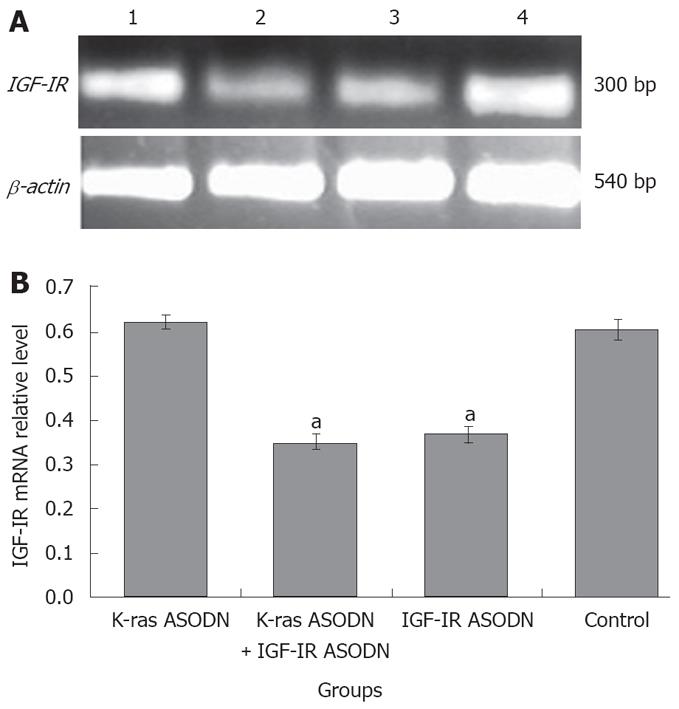
The mRNA expression intensities of K-ras gene and IGF-IR gene were analyzed by semiquantitive RT-PCR. The mRNA levels were normalized by internal control β-actin. At 48 h post-transfection, K-ras mRNA intensity levels were 0.389 ± 0.018 for IGF-IR ASODN group, 0.213 ± 0.027 for K-ras ASODN + IGF-IR ASODN group, 0.275 ± 0.023 for K-ras ASODN group and 0.391 ± 0.021 for control group. The statistical analysis showed that K-ras mRNAs of Patu8988 cells in K-ras ASODN group, and combination group were reduced significantly, compared with that of control group (P < 0.05). The inhibition rate reached 45.5% in the combination group. IFG-IR ASODN had no significant inhibitory effect on the expression of K-ras mRNA (P > 0.05, vs control) (Figure 5). As for IGF-IR gene, the relative mRNA levels were 0.642 ± 0.017 for K-ras ASODN, 0.355 ± 0.020 for the combination group, 0.387 ± 0.025 for IGF-IR ASODN group, and 0.630 ± 0.029 for control group. The statistical analysis showed that both IGF-IR ASODN group, and combination group could have a significant down-regulation effect on the mRNA expression of IGF-IR in Patu8988 cells (P < 0.05, vs control). The inhibition rate was 43.7% in the combination group. However, K-ras ASODN showed no obvious inhibition for IGF-IR mRNA expression (P > 0.05 vs control) (Figure 6).
All nude mice were bearing pancreatic tumors from 7 to 10 d, and survived during the therapy with no red swelling, and disruption at the inoculation point. Before therapy, there were no significant difference for nude mice in weight and volume. As shown in Figure 3D, the tumor volume increased gradually in control group. The long diameter reached above 1.0 cm, and the volume reached 2230.0 ± 65.6 mm3 on the 49th d after inoculation. The tumor growth in K-ras ASODN group, IGF-IR ASODN group and combined group was inhibited with significant difference when compared with control group (P < 0.01). The therapeutic effect in the combined group was greater than that of K-ras ASODN or IGF-IR ASODN alone (P < 0.01). These results indicated that combination group exerted a strong growth-suppressive effect on pancreatic cancer. However, between K-ras ASODN and IGF-IR ASODN group, there was no obvious difference (P > 0.05). The results of immunohistochemical showed that K-ras and IGF-IR protein expression decreased in tumor tissues (data not shown).
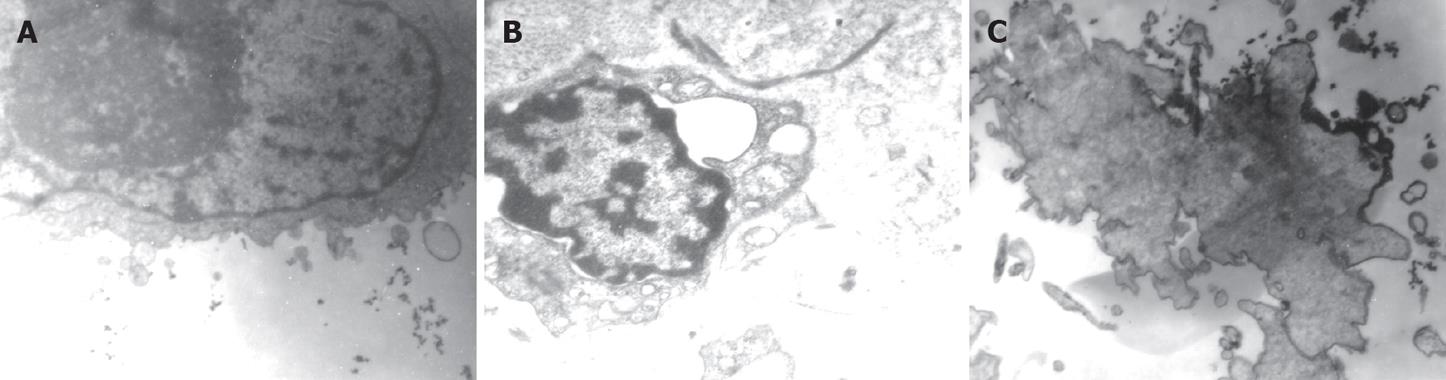
Using transmission electron microscope, we saw that the normal Patu8988 cells had intact cell membranes and nuclear membranes, distributed nuclear chromosomes, distinct organelles, big nuclei and excessive nuclear division, which indicated that Patu8988 cells were highly malignant (Figure 7A). When Patu8988 cells were treated with K-ras ASODN combined with IGF-IR ASODN for 48 h, changes such as apoptosis, cell shrinkage, separation from neighboring cells, plasma condensation, plasma vacuolation, karyopyknosis, margination of condensed chromatin and membrane-bounded apoptotic bodies were observed (Figure 7B); some cells exhibited distinct deformation and disruption (Figure 7C).
The rapid development of molecular techniques has made it clear that tumorigenesis is actually a process of gene abnormalities. The strong invasiveness and rapid diffusive ability of pancreatic cancer are also closely associated with gene abnormalities. The study results of many years show that many genes’ cooperation and many factors’ participation contribute to the development of pancreatic cancer. Gene therapy brings hope for patients of pancreatic cancer. But, single gene therapy does not achieve ideal results. If two or more genes are combined to treat pancreatic cancer, in theory therapeutic effects will be better.
Since Almoguera et al[17] first reported that K-ras mutation occurred in patients with pancreatic cancer, 85%-95% patients with pancreatic cancer have been found to have K-ras mutation, and most of these were point mutations at codon 12. Among those point mutations, GAT, GTT and CGT mutation styles comprised more than 95% of the point mutations at codon 12[18]. Therefore, K-ras point mutation at codon 12 is an early event for pancreatic cancer, which can be used as a target for early diagnosis and gene therapy[19,20]. K-ras gene mutation destroys the GTP enzyme activity of ras protein and makes K-ras active constantly, which makes K-ras protein unable to block signals for growth. Recently, K-ras antisense oligodeoxynucleotids (ASODNs) have been transfected into pancreatic cells in China and abroad. Wang et al[21] first detected and confirmed K-ras gene mutation type CGT in human pancreatic carcinoma cell line PC-2. Then, K-ras mutation ASODN specific to CGT at codon 12 was used to explore its inhibitory effects on target gene in cell line PC-2. The results show that ASODN specific to K-ras point mutation CGT had significant inhibitory effects on target gene expression in human pancreatic carcinoma cells in vitro. Nakada et al[22] used ASODN specific to K-ras point mutation GAT at codon 12 to transfect into human pancreatic cancer cell line PANC-1 (with GAT mutation at codon 12), and the invasive activity was investigated using in vitro chemoinvasion assay. The results show that K-ras mutation ASODN specific to GAT at codon 12 strongly inhibited the invasive activity of the cell line PANC-1, but not in that with a wild type K-ras (BxPC-3). So, ASODNs specific to mutated K-ras genes can inhibit the proliferation and invasiveness of human pancreatic cancer cell lines. Specific antisense therapy to the point mutation of K-ras might be a new anticancer strategy for pancreatic cancer. However, these studies also indicate that adopting K-ras ASODN alone could not eradicate all the tumor cells. For exploring more effective therapy methods, some scholars abroad recently started to explore therapeutic alliance with diverse antisense oligodeoxynucleotides to treat pancreatic cancer, such as simultaneous transfection with mda-7 ASODN and K-ras ASODN into pancreatic cancer in vitro and in vivo. The results of their studies showed that the therapeutic effects of combination methods were better than that of one alone[23,24].
IGF-IR is a receptor protein tyrosine kinase (RPTK) expressed in a wide variety of cell types including mesenchymal, epithelial, and hematopoietic cells. The receptor is a transmembrane heterotetramer consisting of two α-subunits and two β-subunits linked by disulfide bonds. The binding of IGF-I to its receptor results in receptor oligomerization, activation of PTK, inter-molecular receptor autophosphorylation and phosphorylation of cellular substrates that consequently lead to gene activation, DNA synthesis and cell proliferation. Overexpression of IGF-IR stimulated cells not only to transform toward malignance and sustain malignant phenotype, but also to promote tumor cells’ anti-apoptosis, mitosis, proliferation and invasiveness. Min et al[10] reported that IGF-IR overexpressed in pancreatic cancer and down-regulation of IGF-IR expression using monoclonal antibodies or antisense oligodeoxynucleotides could inhibit tumor cell growth both in vitro and in vivo[25-27]. However, the inhibition ratio was not high[24].
Considering the important effects of K-ras gene point mutation at codon 12 in pancreatic cancer and the broad tumorigenesis of IGF-IR gene, our study explored the effects of Patu8988 cell proliferation, apoptosis and target gene expression using combined antisenses with K-ras ASODN against K-ras point mutation at codon 12 and IGF-IR ASODN against insulin-like growth factor-1. We noticed that different doses for K-ras ASODN or IGF-IR ASODN could inhibit Patu8988 cell growth. But, combinations could produce greater effects (P < 0.01). The results were also confirmed in animal experiments. Compared with the single method, combination could obviously induce Patu8988 cell apoptosis, and reduce protein and mRNA expression of K-ras and IGF-IR. In our study, the inhibition effects of K-ras ASODN were better than that of IGF-IR ASODN. Ras protein is not only one of the signal pathways of IGF-IR, but also the pathway for many other growth factors, such as VEGF. Therefore, mutated ras protein not only amplifies the IGF-IR signal, but also amplifies signals for many other growth factors to inhibit cell apoptosis, and also induces vascular growth and cell proliferation. IGF-IR ASODN can inhibit only IGF-IR signal. But, K-ras ASODN can inhibit signals for many factors. Therefore, the inhibition effects of K-ras ASODN was better than that of IGF-IR ASODN. So, combined therapy can inhibit signals on two sides. After being treated with K-ras ASODN and IGF-IR ASODN together, some cells appeared in the form of apoptosis, some others in the shape of edema or deformation, which indicated that antisense not only induced apoptosis, but also promoted cell death directly.
Our study shows that K-ras ASODN combined with IGF-ASODN obviously inhibited Patu8988 cell growth, and induced cell apoptosis and death. The mechanism may be associated with the inhibition of mRNA, and protein expression of K-ras and IGF-IR in Patu8988 cells. Cooperation with two synergistic antisense oligodeoxynucleotides could provide a new gene therapeutic strategy against pancreatic cancer. Meanwhile, the results of our study show that K-ras ASODN and IGF-IR ASODN inhibited tumor growth alone or in combination. However, a rapid cell proliferation tendency was seen in later stage of combined therapy. We speculated that this phenomenon might be associated with the degradation of ASODN in the late stage of treatment. We need to do further study to learn the relationship between dose-effect and time-effect. On the other hand, the development of pancreatic cancer involves many genes; we can not inhibit tumor growth completely by suppressing two genes of K-ras and IGF-IR, only partial.
Pancreatic carcinoma is the cancer that has the highest K-ras gene mutation rate. 95% of mutations happen at codon 12. Three major mutation types have been reported, including CGT, GAT and GTT. Antisense oligodeoxynucleotides (ASODNs) specific to CGT and GAT point mutations in human pancreatic cancer cell lines were reported; the ASODN against GTT point mutation in pancreatic cancer remains unclear. Some studies reported that type I insulin-like growth factor receptor (IGF-IR) is overexpressed in pancreatic cancer and down-regulation of IGF-IR expression using ASODNs could inhibit tumor cell growth. In this article, whether K-ras ASODN specific to GTT mutation in alliance with IGF-IR ASODN regulate Patu8988 proliferation, apoptosis, target gene expression in vitro and in vivo was investigated.
In previous studies, antisense oligodeoxynucleotides (ASODNs) specific to CGT and GAT point mutation of K-ras gene were demonstrated to inhibit proliferation in pancreatic cancer.
It was found in the present study that K-ras ASODN combined with IGF-IR ASODN could cooperatively inhibit the growth of Patu8988 cells and induce their apoptosis via reinforcing specific down regulation of K-ras and IGF-IR mRNA and protein expression.
Cooperation with two synergistic antisense oligodeoxynucleotides could provide a new gene therapeutic strategy against pancreatic cancer.
PCR-SSP is polymerase chain reaction with special sequence primers; K-ras ASODN is an antisense oligodeoxynucleotide against K-ras gene; IGF-IR ASODN is an antisense oligodeoxynucleotide against IGF-IR gene; GAT, CGT and GGT are three major point mutation types at codon 12 of K-ras gene.
This is an interesting study that identifies molecular pathways that may be therapeutically targeted to inhibit pancreatic cancer growth.
Peer reviewer: Minoti Vivek Apte, Associate Professor, Pancreatic Research Group, South Western Sydney Clinical School, the University of New South Wales, Level 2, Thomas and Rachel Moore Education Centre, Liverpool Hospital, New South Wales 2170, Liverpool, Australia
S- Editor Li DL L- Editor Li M E- Editor Zhang WB
| 1. | Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59-66. [Cited in This Article: ] |
| 2. | Coppola D. Molecular prognostic markers in pancreatic cancer. Cancer Control. 2000;7:421-427. [Cited in This Article: ] |
| 3. | Welsch T, Kleeff J, Friess H. Molecular pathogenesis of pancreatic cancer: advances and challenges. Curr Mol Med. 2007;7:504-521. [Cited in This Article: ] |
| 4. | Boeck S, Heinemann V. The role of second-line chemotherapy after gemcitabine failure in patients with advanced pancreatic cancer. Future Oncol. 2008;41-50. [Cited in This Article: ] |
| 5. | Brasiuniene B, Juozaityte E. The effect of combined treatment methods on survival and toxicity in patients with pancreatic cancer. Medicina (Kaunas). 2007;43:716-725. [Cited in This Article: ] |
| 6. | Plate JM. Current immunotherapeutic strategies in pancreatic cancer. Surg Oncol Clin N Am. 2007;16:919-943, xi. [Cited in This Article: ] |
| 7. | Deramaudt T, Rustgi AK. Mutant KRAS in the initiation of pancreatic cancer. Biochim Biophys Acta. 2005;1756:97-101. [Cited in This Article: ] |
| 8. | Fryzek JP, Garabrant DH, Schenk M, Kinnard M, Greenson JK, Sarkar FH. The Association Between Selected Risk Factors for Pancreatic Cancer and the Expression of p53 and K-ras Codon 12 Mutations. Int J Gastrointest Cancer. 2006;37:139-145. [Cited in This Article: ] |
| 9. | Jiao L, Zhu J, Hassan MM, Evans DB, Abbruzzese JL, Li D. K-ras mutation and p16 and preproenkephalin promoter hypermethylation in plasma DNA of pancreatic cancer patients: in relation to cigarette smoking. Pancreas. 2007;34:55-62. [Cited in This Article: ] |
| 10. | Min Y, Adachi Y, Yamamoto H, Ito H, Itoh F, Lee CT, Nadaf S, Carbone DP, Imai K. Genetic blockade of the insulin-like growth factor-I receptor: a promising strategy for human pancreatic cancer. Cancer Res. 2003;63:6432-6441. [Cited in This Article: ] |
| 11. | Liu W, Bloom DA, Cance WG, Kurenova EV, Golubovskaya VM, Hochwald SN. FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis. 2008;29:1096-1107. [Cited in This Article: ] |
| 12. | Wang YX, Gao L, Ji ZZ. Inhibitary effects of antisense oligonucleotide specific to K-ras point mutation on the target gene expression in human pancreatic carcinoma cells. Chin Med J (Engl). 2007;120:1448-1450. [Cited in This Article: ] |
| 13. | Zhou NX, Fen YQ, Huang ZQ, Wang YZ, Xu QS. Growth inhibition of pancreatic cancer by antisense oligonucleotide against the insulin-like factor-I receptor. Zhonghua Putong Waike Zazhi. 1999;29:247-251. [Cited in This Article: ] |
| 14. | Masui T, Hosotani R, Ito D, Kami K, Koizumi M, Mori T, Toyoda E, Nakajima S, Miyamoto Y, Fujimoto K. Bcl-XL antisense oligonucleotides coupled with antennapedia enhances radiation-induced apoptosis in pancreatic cancer. Surgery. 2006;140:149-160. [Cited in This Article: ] |
| 15. | Hotz HG, Hines OJ, Masood R, Hotz B, Foitzik T, Buhr HJ, Gill PS, Reber HA. VEGF antisense therapy inhibits tumor growth and improves survival in experimental pancreatic cancer. Surgery. 2005;137:192-199. [Cited in This Article: ] |
| 16. | Resnicoff M, Coppola D, Sell C, Rubin R, Ferrone S, Baserga R. Growth inhibition of human melanoma cells in nude mice by antisense strategies to the type 1 insulin-like growth factor receptor. Cancer Res. 1994;54:4848-4850. [Cited in This Article: ] |
| 17. | Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549-554. [Cited in This Article: ] |
| 18. | Berndt C, Haubold K, Wenger F, Brux B, Muller J, Bendzko P, Hillebrand T, Kottgen E, Zanow J. K-ras mutations in stools and tissue samples from patients with malignant and nonmalignant pancreatic diseases. Clin Chem. 1998;44:2103-2107. [Cited in This Article: ] |
| 19. | Fleming JB, Shen GL, Holloway SE, Davis M, Brekken RA. Molecular consequences of silencing mutant K-ras in pancreatic cancer cells: justification for K-ras-directed therapy. Mol Cancer Res. 2005;3:413-423. [Cited in This Article: ] |
| 20. | Zhu H, Liang ZY, Ren XY, Liu TH. Small interfering RNAs targeting mutant K-ras inhibit human pancreatic carcinoma cells growth in vitro and in vivo. Cancer Biol Ther. 2006;5:1693-1698. [Cited in This Article: ] |
| 21. | Wang YX, Gao L, Ji ZZ. [The study of the effect of antisense oligonucleotide specific to K-ras point mutation on human pancreatic carcinoma cell PC-2]. Zhonghua Waike Zazhi. 2005;43:1387-1390. [Cited in This Article: ] |
| 22. | Nakada Y, Saito S, Ohzawa K, Morioka CY, Kita K, Minemura M, Takahara T, Watanabe A. Antisense oligonucleotides specific to mutated K-ras genes inhibit invasiveness of human pancreatic cancer cell lines. Pancreatology. 2001;1:314-319. [Cited in This Article: ] |
| 23. | Su Z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci USA. 2001;98:10332-10337. [Cited in This Article: ] |
| 24. | Lebedeva IV, Su ZZ, Emdad L, Kolomeyer A, Sarkar D, Kitada S, Waxman S, Reed JC, Fisher PB. Targeting inhibition of K-ras enhances Ad.mda-7-induced growth suppression and apoptosis in mutant K-ras colorectal cancer cells. Oncogene. 2007;26:733-744. [Cited in This Article: ] |
| 25. | Maloney EK, McLaughlin JL, Dagdigian NE, Garrett LM, Connors KM, Zhou XM, Blattler WA, Chittenden T, Singh R. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63:5073-5083. [Cited in This Article: ] |
| 26. | Pan YZ, Sun CY, Wang YZ. The growth inhibition of human pancreatic cancer cells by lipofectin mediated IGF-1R antisense oligonucleotides. Zhonghua Heyixue Zazhi;. 2005;25:212-215. [Cited in This Article: ] |
| 27. | Adachi Y, Lee CT, Carbone DP. Genetic blockade of the insulin-like growth factor 1 receptor for human malignancy. Novartis Found Symp. 2004;262:177-189; discussion 190-192, 265-268. [Cited in This Article: ] |