Published online Jun 21, 2008. doi: 10.3748/wjg.14.3739
Revised: May 9, 2008
Accepted: May 16, 2008
Published online: June 21, 2008
AIM: To investigate the relationship between the expression of P120 and the clinicopathologic parameters in intrahepatic cholangiocarcinoma (ICC).
METHODS: An immunohistochemical study of E-cadherin and P120 catenin was performed on 42 specimens of ICC with a Dako Envision kit.
RESULTS: The expression of E-cadherin and P120 was reduced in 27 cases (64.3%) and 31 cases (73.8%), respectively. Both E-cadherin and P120 expressions were significantly correlated with the tumor histological grade (χ2 = 9.333, P = 009 and χ2 = 11.71, P = 0.003), TNM stage (χ2= 8.627, P = 0.035 and χ2 = 13.123, P = 0.004), intrahepatic metastasis (χ2= 7.292, P = 0.007 and χ2 = 4.657, P = 0.041, respectively) and patients’ survival (χ2= 6.351, P = 0.002 and χ2 = 4.023, P = 0.000, respectively). In addition, the expression of P120 was in concordance with that of E-cadherin (χ2 = 13.797, P = 0.000), indicating that the expression of P120 may be dependent on that of E-cadherin. Finally, only P120 expression was found to be an independent prognostic factor in Cox regression model (r = 0.088, P = 0.049).
CONCLUSION: Down-regulated expression of E-cadherin and P120 occurs frequently in ICC and contributes to the progression and development of tumor. Both of them may be valuable biologic markers for predicting tumor invasion, metastasis and patients’ survival, but only P120 is an independent prognostic factor for ICC.
- Citation: Zhai B, Yan HX, Liu SQ, Chen L, Wu MC, Wang HY. Reduced expression of P120 catenin in cholangiocarcinoma correlated with tumor clinicopathologic parameters. World J Gastroenterol 2008; 14(23): 3739-3744
- URL: https://www.wjgnet.com/1007-9327/full/v14/i23/3739.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3739
P120-catenin is a member of the Armadillo (ARM)/β-catenin gene family and is essential for mesenchymal cadherin-mediated regulation of cell motility and invasiveness[12]. Cadherin, one of the transmembrane cell-cell adhesion receptors involved in development, and morphogenesis of ICC[3], is necessary and sufficient for P120 targeting cell-cell junctions.
A main function of P120 is to stabilize cadherins at the cell membrane by regulating cadherin turnover and degradation. In this way, P120 level acts as a set point mechanism underlying cell-cell adhesive interactions. P120 may function as a “cap” to bind to the cadherin cytoplasmic tail and prevent cadherin interactions with endocytic membrane trafficking machinery. Alternatively, P120 may stabilize cell junctions or regulate membrane trafficking machinery through interactions with small GTPases, such as Rho A, Rac and Cdc42. Through these mechanisms, P120 exerts its influence over a wide range of biological processes that are dependent upon tight regulation of cell surface cadherin levels[4].
Intrahepatic cholangiocarcinoma (ICC) is the second most common tumor of primary liver cancers in adults worldwide, accounting for about 15% of liver cancers, and its incidence has increased in recent years[5]. Despite improved diagnostic and operative techniques, the prognosis of ICC remains poor. In addition, molecular events involving the development of ICC are not well understood. Some studies examined the expression of E-cadherin/catenin complex in ICC, but the conclusion is still controversial. Moreover, to our knowledge, no study has demonstrated the expression characteristics of P120 and the relationship between the expression of P120, and the clinicopathologic parameters in ICC. Therefore, in the present study, we used immunohistochemical staining for the E-cadherin/P120 complex in primary ICC to correlate its expression with its clinicopathologic features.
In this study, we selected 42 specimens of intrahepatic cholangiocarcinoma collected and diagnosed at the Eastern Hepatobilliary Surgery Hospital, the Second Military Medical University from October 1997 to March 2004. The patients were consisted of 32 men and 10 women. Their age ranged from 27 to 73 years, with an average age of 51 years. Cancer tissue and non-tumorous liver tissue were obtained from each patient for pathological examination. The detailed pathologic data were obtained from the Department of Pathology of Eastern Hepatobilliary Surgery Hospital. Background liver showed cirrhosis in 19 (45.2%) patients, and chronic hepatitis in 15 (35.7%) patients.
Clinicopathologic parameters included histological grade, pTNM stage, tumor size, capsular and vascular invasion, satellite nodules, intrahepatic metastasis, lymph node status and patients’ survival. Histological grade of ICC was sub-classified into well, moderately and poorly differentiated ICC based on the criteria for a liver cancer study in Japan[6]. Tumor staging was performed according to the pTNM staging system of the International Union against Cancer UICC[7].
Formalin-fixed and paraffin-embedded tissues were cut to 5-&mgr;m thick sections. Immunohistochemical staining for E-cadherin and P120 was performed with a Dako EnVisionTM kit (Dakocytomation Company, Denmark). The sections were dewaxed, incubated with methanol containing 30% H2O2 for 20 min to block endogenous peroxidase activity, immersed in 0.01 mol/L citrate buffer(pH 6.0), heated at 100°C in a microwave oven for 20 min, washed three times with distilled water and blocked with 1% BSA for 30 min. The sections were then incubated overnight at 4°C with rabbit polyclonal IgG of E-cadherin (H-297, SC-7870, Santa Clauze Corporation, USA ) and rabbit polyclonal IgG of P120 (H-90, SC-13957, Santa Clauze Corporation, USA) at a 1:200 dilution. A subsequent reaction was carried out using second antibodies (Dakocytomation Company, Denmark) at 37°C for 30 min. Then, the sections were washed three times with phosphate-buffered saline (PBS) and subsequently the color was displayed with DAB (Dakocytomation Company, Denmark) for about 5 min. Nuclei were lightly counterstained with hematoxylin. No staining was obtained when immune serum or PBS was used instead of primary antibodies, thus confirming the specificity of each primary antibody.
A scoring system was used to semiquantitatively evaluate the immunoexpression of E-cadherin and P120 in ICC as described previously[8]. The expression of E-cadherin and P120 in nontumorous tissue was used as an internal control. Briefly, immune activities of E-cadherin and P120 were assessed by the extent (broadness) and intensity (color strength). Depending on the percentage of positive cells, the extent was scored as follows: 0 = no positive cells or less than 5%, +1 = 5%-25% positive cells, +2 = 26%-50% positive cells, +3 = 51%-75% positive cells, and +4 = 76%-100% positive cells. The intensity was also scored as follows: 0 = no immunoreaction, +1 = mild immunoreaction, +2 = moderate immunoreaction, +3 =marked immunoreaction. E-cadherin or P120 expression was defined as positive when the composite score was 6 or 7, and as “absent or loss” when the total score was 0.
Results from immunohistochemistry were analyzed by χ2 or Fisher’s exact test. P < 0.05 was considered statistically significant. Survival analysis was performed using the log-rank test (P < 0.05). Survival curves were plotted according to the method of Kaplant and Meier. The prognosis value of E-cadherin and P120 for ICC was evaluated with univariate (log-rank test) and multivariate analysis (Cox regression model). SPSS 10.1 software package for Windows (SPSS, Inc., Chicago, IL) was used.
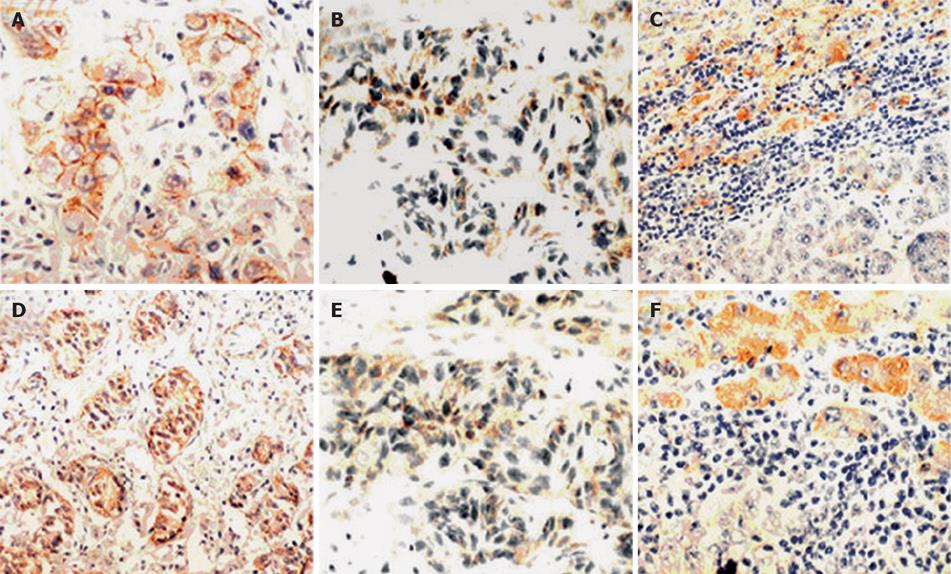
In nontumorous liver tissue, both E-cadherin and P120 were expressed strongly on cell membranes, but the staining intensity was gradually decreased. In addition, these molecules were normally expressed on cell membranes of bile ducts, proliferating ductules and intra-hepatic vessels. No expression was found in other types of cells in the liver.
In ICC, the expression of E-cadherin and P120 catenin was reduced in 27 (64.3%) and 31 cases (73.8%), being absent in 8 and 10 cases, respectively. In addition, P120 was expressed in 17 cases (40.5%) (Figure 1).
As shown in Table 1, the membranous expression of E-cadherin and P120 was significantly correlated with the tumor grade (P = 0.009 and P = 0.003, respectively). The expression of E-cadherin and P120 tended to be reduced in poorly-differentiated tumors compared with well- and moderately-differentiated tumors. In addition, the expression of E-cadherin and P120 was inversely associated with the pTNM stage of tumors (P = 0.035 and P = 0.004, respectively).
| E-cadherin | P120 catenin | ||||||
| n | + | - | P value | + | - | P value | |
| Differentiation grade | |||||||
| Well | 3 | 3 (100) | 0 (0) | 0.009 | 3 (100) | 0 (0) | 0.003 |
| Mediate | 14 | 7 (50.0) | 7 (50.0) | 5 (35.7) | 9 (64.3) | ||
| Poor | 25 | 5 (20.0) | 20 (80.0) | 3 (12.0) | 22 (88.0) | ||
| pTNM | |||||||
| I | 2 | 2 (100) | 0 (0) | 0.035 | 2 (100) | 0 (0) | 0.004 |
| II | 9 | 5 (55.6) | 4 (44.4) | 5 (55.6) | 4 (44.4) | ||
| III | 25 | 8 (32.0) | 17 (68.0) | 4 (16.0) | 21 (84.0) | ||
| IV | 6 | 0 (0) | 6 (100) | 0 (0) | 6 (100) | ||
As shown in Table 2, the expression of E-cadherin or P120 was significantly associated with intra-hepatic matestasis of ICC (P = 0.007 and P = 0.041, respectively). No statistically significant difference was observed between the expression level of E-cadherin or P120 and tumor size, capsular and vascular invasion, lymph node permission and satellite nodules.
| E-cadherin | P120 catenin | ||||||
| n | + | - | P value | + | - | P value | |
| Size | |||||||
| < 5 cm | 17 | 7 (41.2) | 10 (58.8) | 0.826 | 6 (35.3) | 11 (64.7) | 0.584 |
| 5-10 cm | 16 | 5 (31.3) | 11 (68.7) | 3 (18.8) | 12 (81.2) | ||
| > 10 cm | 9 | 3 (33.3) | 6 (66.7) | 2 (22.2) | 7 (77.8) | ||
| Capsular invasion | |||||||
| + | 6 | 4 (66.7) | 2 (33.3) | 0.164 | 3 (50) | 3 (50) | 0.391 |
| - | 36 | 11 (30.6) | 25 (69.4) | 8 (22.2) | 28 (77.8) | ||
| Satellite nodules | |||||||
| + | 11 | 4 (36.4) | 7 (65.6) | 1 | 3 (27.3) | 8 (72.7) | 0.314 |
| - | 31 | 11 (35.5) | 20 (64.5) | 8 (25.8) | 23 (74.2) | ||
| Vascular invasion | |||||||
| + | 13 | 2 (15.4) | 11 (84.6) | 0.089 | 1 (7.7) | 12 (92.3) | 0.127 |
| - | 29 | 13 (44.8) | 16 (55.2) | 10 (34.5) | 19 (65.5) | ||
| L.N.P | |||||||
| + | 7 | 1 (14.3) | 6 (85.7) | 0.39 | 0 (0) | 7 (100) | 0.161 |
| - | 35 | 14 (40) | 21 (60) | 11 (31.4) | 24 (68.6) | ||
| I.M. | |||||||
| + | 10 | 0 (0) | 10 (100) | 0.007 | 0 (0) | 10 (100) | 0.041 |
| - | 32 | 15 (46.9) | 17 (53.1) | 11 (34.4) | 21 (65.6) | ||
As shown in Table 3, positive and negative expression of E-cadherin and P120 was found in 9 and 25 cases, respectively. However, negative expression of P120 was observed in 7 cases. There was a significant concordance between the expressions of E-cadherin and P120 (P = 0.000).
| P120 | |||
| E-cadherin | + | - | P value |
| + | 9 | 6 | 0.000 |
| - | 2 | 25 | |
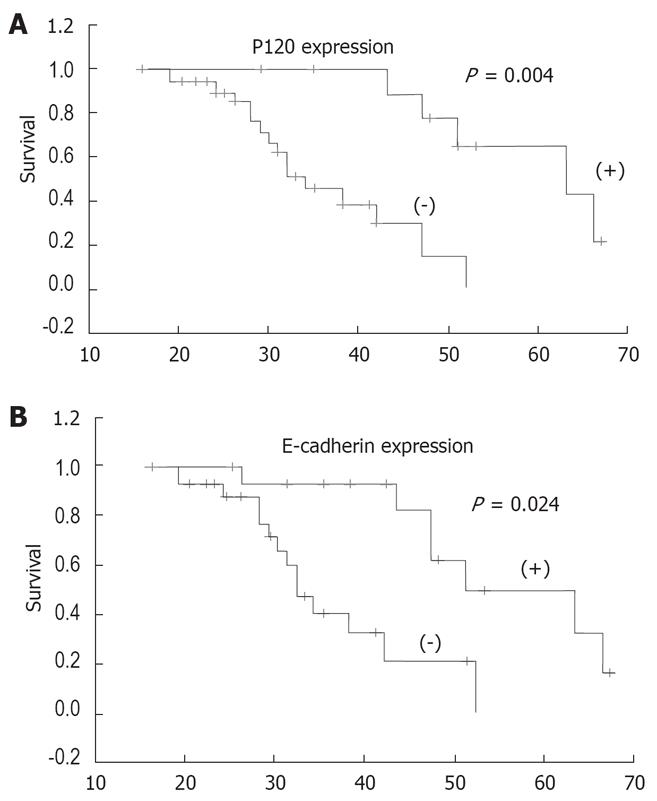
The patients were followed up for 4-67 months. The overall survival rate of patients according to the expression of E-cadherin and P120 in tumor is shown in Figure 2. Analysis of the survival of all patients showed that abnormal expression of E-cadherin and P120 was significantly correlated with the poor survival of patients (P = 0.024 and P = 0.004, respectively). However, when the expression of E-cadherin or P120 and the clinicopathological parameters were analyzed by the Cox regression model, abnormal expression of P120 was found to be an independent prognostic factor for ICC patients (P = 0.049) (Table 4).
| Sig | RR | 95% CI | ||
| Lower | Upper | |||
| E-cadherin expression | 0.724 | 1.525 | 0.147 | 15.827 |
| P120 expression | 0.049 | 0.088 | 0.008 | 0.991 |
| Differentiation | 0.194 | 0.407 | 0.105 | 1.583 |
| pTNM stage | 0.073 | 2.898 | 0.904 | 9.288 |
| Tumor size | 0.037 | 0.387 | 0.159 | 0.944 |
| Capsular invasion | 0.052 | 17.046 | 0.981 | 6.166 |
| Satellite nodules | 0.709 | 1.597 | 0.137 | 18.578 |
| Vascular invasion | 0.948 | 0.961 | 0.284 | 3.247 |
| Limph node invasion | 0.087 | 4.72 | 0.8 | 27.855 |
| Intrahepatic metastasis | 0.786 | 1.352 | 0.154 | 11.899 |
Usually, ICC is an adenocarcinoma and may arise from the large intra-hepatic bile ducts near the hepatic hilus or from the bile ducts at the border of hepatic parenchyma. It was reported that altered expression of E-cadherin/catenins complex in ICC occurs frequently and is significantly correlated with tumor histological features and/or vascular invasion and metastasis[9–14].
It was recently reported that P120 plays a role in the occurrence of various cancers, and that P120 may behave either as a tumor suppressor or as a metastasis promoter, depending on the loss of E-cadherin and P120. If E-cadherin is lost first, P120 may directly and actively promote metastasis. If P120 is lost first, E-cadherin levels would fall significantly, which is likely to be parallel to the reduced levels of α-and β-catenins[15]. P120 down-regulation results in a striking dose-dependant loss of endogenous cadherins, indicating that P120 is essential for cadherin stability. Moreover, P120 down-regulation occurs frequently in almost all carcinomas[16]. P120 loss is often associated with the stage and poor prognosis of tumors, suggesting that its loss may be associated with biological aggressiveness and progression of tumors. Nevertheless, to our knowledge, no report is available on the expression of P120 in human intrahepatic chola-giocarcinoma.
The present study showed that reduced or absent expression of E-cadherin and P120 was associated with the histological grade of tumors, which is consistent with reported data[17–21]. In well-differentiated tumors, there were obvious and strong staining along the cell-cell boundaries, whereas in poorly- differentiated tumors, the immunohistostaining was focally and heterogeneously distributed, with patchy or spotty features along the cell-cell boundaries, indicating that the staining of E-cadheri and P120 is related with the differentiation of ICC, namely both E-cadherin and P120 may be regarded as differentiation markers of tumor. In addition, the staining intensity of the E-cadheri and P120 complex was gradually decreased, suggesting that P120 may play a critical role in ICC progression.
Microscopy revealed that E-cadherin was located on the membrane either in non-tumor tissues or in tumor cells, whereas P120 was expressed on the membrane or in cytoplasm of tumor cells. However, it was reported that P120 is also in nuclei[22], suggesting that P120 plays an important role in cell signal transduction. P120 has an intrinsic nucleocytoplasmic shuttling activity that is modulated, in part, by extrinsic factors such as cadherin binding and interactions with the microtubule network[22]. Julia and his colleagues reported[23] that P120 displays up-regulation and nuclear expression in pancreatic cancer. No expression of P120 in nuclei of cancer cells, however, was observed in our study, suggesting that it is necessary to further investigate the mechanism underlying P120 expression in nuclei of cancer cells.
In this study, we observed the relationship between reduced expression of E-cadherin and P120 and several clinicopathologic parameters of ICC. The expression of P120 and E-cadherin was significantly associated with tumor pTNM stage and intrahepatic metastasis (IM), but not with tumor stage and size, capsular and vascular invasion, and lymph node invasion. Osada and his colleagues[24] revealed that E-cadherin is involved in intra-hepatic metastasis of hepatocellular carcinoma. Asayama et al[13] detected the expression of E-cadherin in hepatocellular carcinoma and cholangiocarcinoma, and found that reduced expression of E-cadherin is significantly correlated with the grade and IM of ICC. Therefore, E-cadherin and P120 may be important mediators in tumor progression, and can be considered as invasion and metastasis markers of ICC.
Several studies on other cancers have evaluated the relationship between the expression of E-cadherin/P120 and the survival of patients, but the results remain debatable[25–29]. In the present study, reduced expression of both E-cadherin and P120 was significantly related with the survival of patients. However, when the expression of E-cadherin/P120 and the clinicopathological parameters of ICC were analyzed by the Cox regression model, only the abnormal expression of P120 was found to be an independent prognostic factor for ICC, suggesting that P120 can be considered a valuable biological marker for predicting the prognosis of ICC patients.
In summary, abnormal expression of E-cadherin and P120 catenin occurs frequently in intrahepatic cholan-giocarcinoma. Reduced expression of P120 catenin and E-cadherin is correlated with tumor differentiation, pTNM stage, intrahepatic metastasis and survival of patients. Both P120 catenin and E-cadherin may play an important role in the development and progression of human intrahepatic cholangiocarcinoma.
P120-catenin is a member of the E-cadherin/catenin complex family and may be associated with biological aggressiveness and progression of tumors. However, no report is available on the expression of P120 catenin in human intra-hepatic cholangiocarcinoma.
P120 down-regulation occurs frequently in almost all carcinomas. P120 loss is often associated with the stage and poor prognosis of tumors.
Our results suggest that down-regulated expression of E-cadherin and P120 catenin occurred frequently in intrahepatic cholangiocarcinoma (ICC) and contributed to the progression and development of tumors. Both E-cadherin and P120 catenin may be valuable biologic markers for predicting tumor invasion, metastasis and survival of patients, but only P120 catenin is an independent prognostic factor for ICC.
Because down-regulated expression of P120 contributes to the progression and development of ICC, P120 can be used as a valuable biologic marker for predicting the invasion and metastasis of ICC, and the survival of patients.
This is an interesting report on E-cadherin and P120 catenin in human intra-hepatic cholangiocarcinoma. The study was performed on 42 specimens of ICC with a Dako Envision kit, indicating that. Both E-cadherin and P120 catenin may be valuable biological markers for predicting tumor invasion, metastasis and survival of patients. However, its clinical application should be further studied.
| 1. | Reynolds AB, Herbert L, Cleveland JL, Berg ST, Gaut JR. p120, a novel substrate of protein tyrosine kinase receptors and of p60v-src, is related to cadherin-binding factors beta-catenin, plakoglobin and armadillo. Oncogene. 1992;7:2439-2445. [Cited in This Article: ] |
| 2. | Yanagisawa M, Anastasiadis PZ. p120 catenin is essential for mesenchymal cadherin-mediated regulation of cell motility and invasiveness. J Cell Biol. 2006;174:1087-1096. [Cited in This Article: ] |
| 3. | Nollet F, Berx G, van Roy F. The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol Cell Biol Res Commun. 1999;2:77-85. [Cited in This Article: ] |
| 4. | Xiao K, Oas RG, Chiasson CM, Kowalczyk AP. Role of p120-catenin in cadherin trafficking. Biochim Biophys Acta. 2007;1773:8-16. [Cited in This Article: ] |
| 5. | Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357. [Cited in This Article: ] |
| 6. | The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg. 1989;19:98-129. [Cited in This Article: ] |
| 7. | Hermanek P, Hutter RVP, Sobin LH. TNM Atlas, UICC. 4 th ed. Berlin:. Springer. 1997;115-123. [Cited in This Article: ] |
| 8. | Gamallo C, Palacios J, Suarez A, Pizarro A, Navarro P, Quintanilla M, Cano A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. Am J Pathol. 1993;142:987-993. [Cited in This Article: ] |
| 9. | Ashida K, Terada T, Kitamura Y, Kaibara N. Expression of E-cadherin, alpha-catenin, beta-catenin, and CD44 (standard and variant isoforms) in human cholangiocarcinoma: an immunohistochemical study. Hepatology. 1998;27:974-982. [Cited in This Article: ] |
| 10. | Sato K, Murai H, Ueda Y, Katsuda S. Intrahepatic sarcomatoid cholangiocarcinoma of round cell variant: a case report and immunohistochemical studies. Virchows Arch. 2006;449:585-590. [Cited in This Article: ] |
| 11. | Settakorn J, Kaewpila N, Burns GF, Leong AS. FAT, E-cadherin, beta catenin, HER 2/neu, Ki67 immuno-expression, and histological grade in intrahepatic cholangiocarcinoma. J Clin Pathol. 2005;58:1249-1254. [Cited in This Article: ] |
| 12. | Tokumoto N, Ikeda S, Ishizaki Y, Kurihara T, Ozaki S, Iseki M, Shimizu Y, Itamoto T, Arihiro K, Okajima M. Immunohistochemical and mutational analyses of Wnt signaling components and target genes in intrahepatic cholangiocarcinomas. Int J Oncol. 2005;27:973-980. [Cited in This Article: ] |
| 13. | Asayama Y, Taguchi Ki K, Aishima Si S, Nishi H, Masuda K, Tsuneyoshi M. The mode of tumour progression in combined hepatocellular carcinoma and cholangiocarcinoma: an immunohistochemical analysis of E-cadherin, alpha-catenin and beta-catenin. Liver. 2002;22:43-50. [Cited in This Article: ] |
| 14. | Sugimachi K, Taguchi K, Aishima S, Tanaka S, Shimada M, Kajiyama K, Sugimachi K, Tsuneyoshi M. Altered expression of beta-catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod Pathol. 2001;14:900-905. [Cited in This Article: ] |
| 15. | Thoreson MA, Reynolds AB. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation. 2002;70:583-589. [Cited in This Article: ] |
| 16. | Reynolds AB, Carnahan RH. Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer. Semin Cell Dev Biol. 2004;15:657-663. [Cited in This Article: ] |
| 17. | Bremnes RM, Veve R, Gabrielson E, Hirsch FR, Baron A, Bemis L, Gemmill RM, Drabkin HA, Franklin WA. High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J Clin Oncol. 2002;20:2417-2428. [Cited in This Article: ] |
| 18. | Sarrio D, Perez-Mies B, Hardisson D, Moreno-Bueno G, Suarez A, Cano A, Martin-Perez J, Gamallo C, Palacios J. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene. 2004;23:3272-3283. [Cited in This Article: ] |
| 19. | Ishizaki Y, Omori Y, Momiyama M, Nishikawa Y, Tokairin T, Manabe M, Enomoto K. Reduced expression and aberrant localization of p120catenin in human squamous cell carcinoma of the skin. J Dermatol Sci. 2004;34:99-108. [Cited in This Article: ] |
| 20. | Qian ZR, Sano T, Yoshimoto K, Asa SL, Yamada S, Mizusawa N, Kudo E. Tumor-specific downregulation and methylation of the CDH13 (H-cadherin) and CDH1 (E-cadherin) genes correlate with aggressiveness of human pituitary adenomas. Mod Pathol. 2007;20:1269-1277. [Cited in This Article: ] |
| 21. | Bremnes RM, Veve R, Gabrielson E, Hirsch FR, Baron A, Bemis L, Gemmill RM, Drabkin HA, Franklin WA. High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J Clin Oncol. 2002;20:2417-2428. [Cited in This Article: ] |
| 22. | Roczniak-Ferguson A, Reynolds AB. Regulation of p120-catenin nucleocytoplasmic shuttling activity. J Cell Sci. 2003;116:4201-4212. [Cited in This Article: ] |
| 23. | Mayerle J, Friess H, Buchler MW, Schnekenburger J, Weiss FU, Zimmer KP, Domschke W, Lerch MM. Up-regulation, nuclear import, and tumor growth stimulation of the adhesion protein p120 in pancreatic cancer. Gastroenterology. 2003;124:949-960. [Cited in This Article: ] |
| 24. | Osada T, Sakamoto M, Ino Y, Iwamatsu A, Matsuno Y, Muto T, Hirohashi S. E-cadherin is involved in the intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 1996;24:1460-1467. [Cited in This Article: ] |
| 25. | Bellovin DI, Bates RC, Muzikansky A, Rimm DL, Mercurio AM. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 2005;65:10938-10945. [Cited in This Article: ] |
| 26. | Wang EH, Liu Y, Xu HT, Dai SD, Liu N, Xie CY, Yuan XM. Abnormal expression and clinicopathologic significance of p120-catenin in lung cancer. Histol Histopathol. 2006;21:841-847. [Cited in This Article: ] |
| 27. | Wijnhoven BP, Pignatelli M, Dinjens WN, Tilanus HW. Reduced p120ctn expression correlates with poor survival in patients with adenocarcinoma of the gastroesophageal junction. J Surg Oncol. 2005;92:116-123. [Cited in This Article: ] |
| 28. | Bantis A, Giannopoulos A, Gonidi M, Liossi A, Aggelonidou E, Petrakakou E, Athanassiades P, Athanassiadou P. Expression of p120, Ki-67 and PCNA as proliferation biomarkers in imprint smears of prostate carcinoma and their prognostic value. Cytopathology. 2004;15:25-31. [Cited in This Article: ] |
| 29. | Nakopoulou L, Gakiopoulou-Givalou H, Karayiannakis AJ, Giannopoulou I, Keramopoulos A, Davaris P, Pignatelli M. Abnormal alpha-catenin expression in invasive breast cancer correlates with poor patient survival. Histopathology. 2002;40:536-546. [Cited in This Article: ] |