Published online Feb 28, 2007. doi: 10.3748/wjg.v13.i8.1162
Revised: December 28, 2006
Accepted: January 29, 2007
Published online: February 28, 2007
AIM: To investigate the effect of (-)-epigallocatechin-3-gallate (EGCG) on growth of gastric cancer and its possible mechanism.
METHODS: Heterotopic tumors were induced by subcutaneously injection of SGC-7901 cells in nude mice. Tumor growth was measured by calipers in two dimensions. Tumor angiogenesis was determined with tumor microvessel density (MVD) by immunohistology. Vascular endothelial growth factor (VEGF) protein level and activation of signal transducer and activator of transcription 3 (Stat3) were examined by Western blotting. VEGF mRNA expression was determined by RT-PCR and VEGF release in tumor culture medium by ELISA. VEGF-induced cell proliferation was studied by MTT assay, cell migration by gelatin modified Boyden chamber (Transwell) and in vitro angiogenesis by endothelial tube formation in Matrigel.
RESULTS: Intraperitoneal injection of EGCG inhibited the growth of gastric cancer by 60.4%. MVD in tumor tissues treated with EGCG was markedly reduced. EGCG treatment reduced VEGF protein level in vitro and in vivo. Secretion and mRNA expression of VEGF in tumor cells were also suppressed by EGCG in a dose-dependent manner. This inhibitory effect was associated with reduced activation of Stat3, but EGCG treatment did not change the total Stat3 expression. EGCG also inhibited VEGF-induced endothelial cell proliferation, migration and tube formation.
CONCLUSION: EGCG inhibits the growth of gastric cancer by reducing VEGF production and angiogenesis, and is a promising candidate for anti-angiogenic treatment of gastric cancer.
- Citation: Zhu BH, Zhan WH, Li ZR, Wang Z, He YL, Peng JS, Cai SR, Ma JP, Zhang CH. (-)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J Gastroenterol 2007; 13(8): 1162-1169
- URL: https://www.wjgnet.com/1007-9327/full/v13/i8/1162.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i8.1162
Gastric cancer is the leading cause of cancer-related death in most countries, but the incidence has steadily decreased throughout the world in recent decades[1]. Although the exact reason for the decline is uncertain, it was reported that dietary factors play a role[2]. Epidemiological studies showed that regular drinking of green tea significantly reduces the occurrence and mortality of many types of cancer[3]. Case-control studies have provided evidence for protective effect of green tea against gastric cancer[4]. Experiment and animal model studies also demonstrated that green tea and its components have anti-cancer effect against gastric cancer[5,6], but conclusive results are not available at present[7].
(-)-Epigallocatechin-3-gallate (EGCG) is the most abundant and active component of green tea, and the beneficial properties of green tea appear to be ascribed to EGCG. The effect of EGCG on cell proliferation and apoptosis has been well established. EGCG suppresses tumor growth by inhibiting proliferation and inducing apoptosis through a variety of mechanisms[8]. Recent studies showed that EGCG has anti-angiogenic property and reduces tumor growth in mouse model by inhibiting angiogenesis[9,10]. Angiogenesis, the growth of new blood vessels from preexisting capillaries, is necessary for solid tumor growth and metastasis. Angiogenesis is initiated by the release of certain angiogenic factors from tumor cells. Vascular endothelial growth factor (VEGF), which has been shown to be the most potent angiogenic factor, is associated with tumor-induced angiogenesis. Angiogenesis is closely associated with progression and prognosis of gastric cancer, and VEGF expression is a predictive and prognostic factor of gastric cancer[11]. Anti-angiogenic therapy targeting VEGF can inhibit growth and metastasis of gastric cancer[12]. However, whether EGCG suppresses growth of gastric cancer by anti-angiogenesis remains to be elucidated.
In this study, we investigated the effect of EGCG on angiogenesis and growth of gastric cancer with an in vitro and in vivo model. Data reported here show that EGCG suppresses growth of gastric cancer by reducing VEGF production and VEGF-induced angiogenesis.
Human gastric cancer cells (SGC-7901 cells, Cell Bank of Sun Yat-Sen University, Guangzhou, China) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco BRL, Gaithersburg, MD) and incubated at 37°C in a humidified incubator containing 50 mL/L CO2. Human umbilical vein endothelial cells (HUVECs) were prepared from fresh human umbilical cord and grown in human endothelial-serum free medium (endothelial-SFM, Gibco BRL, Gaithersburg, MD) supplemented with 10% FBS, 100 U penicillin, streptomycin and fungizone, and incubated at 37°C in a humidified incubator containing 50 mL/L CO2. To maintain uniform conditions, all experiments were carried out between cell passages 4-6.
SGC-7901 cells (5 × 106 cells/0.2 mL) in SFM were inoculated subcutaneously into the dorsal area of 6-8-wk female BALB/c nude mice, weighing 18-22 g (Experimental Animal Center of Sun Yet-Sen University, Guangzhou, China). When tumors reached a volume of 50 mm3, intraperitoneal injection of EGCG was carried out at the dosage of 1.5 mg/d per mouse (n = 6). The control group (n = 6) was treated with the same volume of PBS. Tumor growth was monitored by external measurement in two dimensions every other day with calipers. Tumor volume was determined according to the equation: V = (L × W2)/2, where V is volume, L is length and W is width. Twenty-eight days after EGCG injection, all animals were sacrificed, tumors were excised and weighed. The tumor inhibition ratio was calculated as follows: inhibition ratio (%) = [(C-T)/C] × 100%, where C is the average tumor weight of the control group and T is the average tumor weight of the EGCG-treated group. Some tumor tissues were frozen at -80°C for Western blot analysis and others fixed in 4% formaldehyde for immunohistochemistry analysis.
All animal studies were performed according to the guides for the Care and Use of Laboratory Animals approved by Sun Yat-Sen University.
Histologic 4-μm thick sections prepared from form-aldehyde fixed and paraffin embedded tumor tissues were used for immunohistochemical analysis. After deparaffinization, rehydration, antigen retrieval and blocking of endogenous peroxidase, the specimens were incubated overnight at 4°C with goat polyclonal anti-CD34 antibodies diluted at 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The sections were rinsed and incubated with peroxidase-conjugated second antibody followed by enzyme conjugate (HRP-streptavidin). Microvessels were revealed with diaminobenzidine (DAB) and the sections were counterstained with Mayer's hematoxylin. In negative-control staining, the primary antibodies were omitted. Tumor vasculature was quantified by the Weidner’s method[13]. The area of tumor containing the maximum number of microvessels to be counted was identified by scanning the entire tumor at low power (100 ×). The number of highlighted vessels from 3 fields was then counted in this area at high power (200 ×).
SGC-7901 cells were seeded in 90 mm plates and cultured in growth medium until 70%-80% confluence. The culture medium was replaced with SFM supplemented with different concentrations of EGCG (0, 5, 10, 20, and 40 μmol/L). The cells were incubated for another 24 h. Total protein from cell lysates and tumor tissue homogenizations was extracted with mammalian cell lysis kit (Bio Basic Inc., Ontario, Canada). Protein levels were quantified with Bio-Rad protein assay kit (Bio-Rad Laboratories, Richmond, CA). Total protein (100 μg) was separated in 12% SDS–PAGE, and transferred onto PVDF membrane (Invitrogen, Carlsbad, CA, USA). The membrane was blocked with 5% skim milk and incubated at 4°C overnight with rabbit polyclonal anti-VEGF antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-Stat3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), or goat polyclonal anti-p-Stat3 antibody (Phospho-Stat3 [tyr-705], Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing three times with 0.1% Tween 20 in Tris-saline, the membranes were incubated with biotin-labeled anti-rabbit or anti-goat IgG for 1 h at room temperature with agitation. The probe proteins were detected using enhanced chemiluminescence system (Amersham International, Piscataway, N.J., USA).
The effect of EGCG on VEGF release in tumor cells was measured by ELISA. SGC-7901 cells seeding in 90 mm plates were cultured until 70%-80% confluence. The cells were washed three times with PBS and the culture medium was replaced with SFM. EGCG was added to the medium to various concentrations (0, 5, 10, 20 and 40 μmol/L) and incubated with the cells for 24 h. The conditioned medium was harvested and centrifuged. VEGF concentration in the supernatant was measured using a VEGF ELISA kit (R & D systems, Minneapolis, Minn., USA).
SGC-7901 cells were seeded in 90 mm plates and cultured in growth medium until 90% confluence. The culture medium was replaced with SFM supplemented with EGCG at different concentrations (0, 5, 10, 20 and 40 μmol/L). The cells were incubated for another 24 h. Total RNA was extracted from the cell lysates. The levels of human VEGF cDNAs were evaluated by RT-PCR and normalized by the levels of human cytoplasmic β-actin. Reverse transcription was performed with 1 μg total RNA and 100 pmol random hexamers in a total volume of 20 μL to produce first-strand cDNA. PCR experiments were performed with 1 μL of the first-strand cDNA in a 50 μL reaction mixture. Human VEGF cDNA was amplified with specific primers (sense primer: 5’TGC ATT CAC ATT TGT TGT GC 3’; antisense primer: 5’AGA CCC TGG TGG ACA TCT TC-3’, a 200 bp product) and β-actin specific primers (sense primer: 5’-TCA TCA CCA TTG GCA ATG AG-3’; antisense primer: 5’-CAC TGT GTT GGC GTA CAG GT-3’; a 150 bp product). Amplification conditions were as follows: denaturation at 94°C for 1 min, annealing at 60°C (for β-actin at 55°C) for 1 min, and extension at 72°C for 1 min. All PCRs were linear up to 30 cycles.
The effect of EGCG (Sigma, St. Louis, MO., USA) on endothelial cell proliferation induced by VEGF was determined by MTT assay (Sigma, St. Louis, MO., USA). Briefly, 2.5 × 104 HUVECs were plated in 24-well plates pre-coated with 2.0% gelatin in triplicate and cultured overnight in growth medium. Then the culture medium was changed with endothelial-SFM supplemented with 2% FBS and 20 ng/mL recombinant human vascular endothelial growth factor (hrVEGF; R & D Systems, Minneapolis, Minn., USA) in the presence of EGCG at various concentrations (0, 5, 10, 20 and 40 μmol/L). The viable cells were quantified by MTT assay at indicated time point following the manufacturer’s instructions.
Endothelial cell migration induced by VEGF was assessed using a modified Boyden chamber (8 μmol/L pores, Transwell®; Corning Costar Corp., Cambridge, MA). The transwell inserts were coated with 2.0% gelatin. HUVECs were treated with EGCG at various concentrations (0, 5, 10, 20 and 40 μmol/L) for 24 h. Cells were harvested by trypsinization, washed twice with PBS, and resuspended in endothelial-SFM containing 2% FBS and EGCG at the indicated concentrations. Then the treated cells were added to the upper chamber at 2 × 105 cells per well and 0.6 mL endothelial-SFM containing 2% FBS and 20 ng/mL VEGF was added to the bottom chamber. After 4-h incubation at 37°C, cells on the upper surface of the membrane were mechanically removed, and the migrated cells on the lower surface of the membrane were fixed and stained with hematoxylin. The average number of migrated cells from 5 randomly chosen fields (× 200) on the lower surface of the membrane was counted. Each experiment was performed in triplicate.
Matrigel was thawed at 4°C in an ice-water bath, and 300 μL/well was carefully added to a pre-chilled 24-well plate using a cold pipette. Matrigel was allowed to polymerize for 1 h at 37°C. After polymerization, 5 × 104 cells/well in endothelial-SFM with 2% serum, 20 ng/mL VEGF and EGCG at different concentrations (0, 5, 10, 20 and 40 μmol/L) was layered on top of polymerized gel. Cells were incubated for 48 h and photographed. For quantification of tube formation, the total length of tubes formed in a unit area was measured using an NIH Image program.
Student’s t test was used in all statistical analyses. P < 0.05 was considered statistically significant.
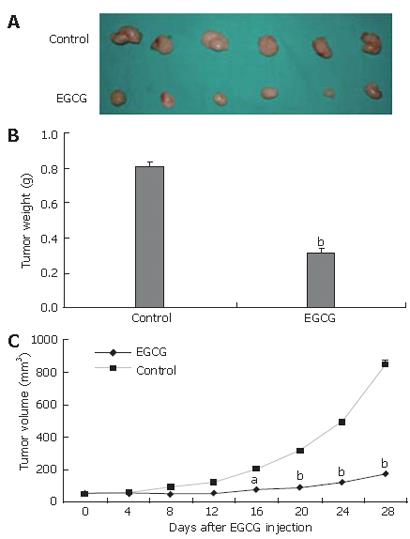
To evaluate the effect of EGCG on tumor growth, tumor xenografts were created by implanting SGC-7901 cells into the dorsal area of athymic mice. Nine days after implantation, tumors reached a size of 50 mm3. The mice were randomized into two groups and received intraperitoneal injection of EGCG or PBS, respectively. EGCG treatment markedly inhibited tumor growth when compared to control group (Figure 1A). The mean weight of tumors of EGCG treatment was significantly lower than that of control group, and an average of 60.4% suppression of primary tumor growth was observed (P < 0.01, Figure 1B). From d 16 after EGCG injection, the average volume of tumors was significantly lower in EGCG treatment group than in control group (Figure 1C).
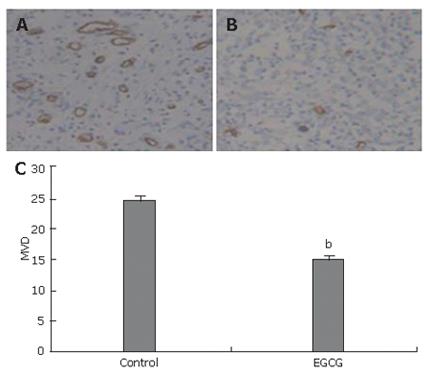
To elucidate the anti-angiogenic activity of EGCG in vivo, the effect of EGCG on tumor angiogenesis was evaluated by CD34 immunostaining for capillaries in tumor tissues. The density of CD34-stained capillaries was significantly lower in EGCG treatment group (Figure 2A) than in control group (Figure 2B). Microvessel density in tumors receiving EGCG treatment was markedly reduced (P < 0.01, Figure 2C), and an average of 38.2% suppression was observed.
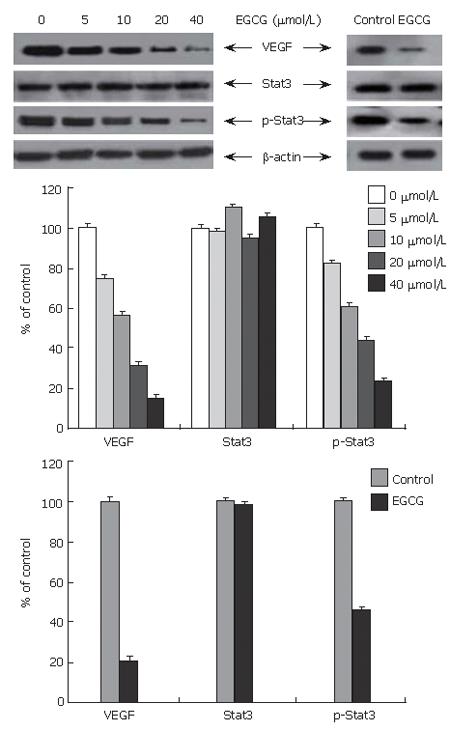
Gastric cancer had a high expression level of VEGF and Stat3, and activation of Stat3. To determine the effect of EGCG on Stat3 activation and expression of VEGF and total Stat3, the protein level of VEGF, total Stat3 and phosphorylated Stat3 in tumor cells and tissues was examined by Western blot. As shown in Figure 3, EGCG treatment down-regulated VEGF expression in cultured tumor cells in a dose-dependent manner. VEGF expression in tumor tissues treated with EGCG was also markedly reduced. Consistent with the results of VEGF expression, activation of Stat3 in cultured tumor cells was decreased in a concentration-dependent manner, and also apparently reduced in EGCG treated tumor tissues. However, EGCG treatment did not change the expression of total Stat3 either in cultured cells or in tumor tissues.
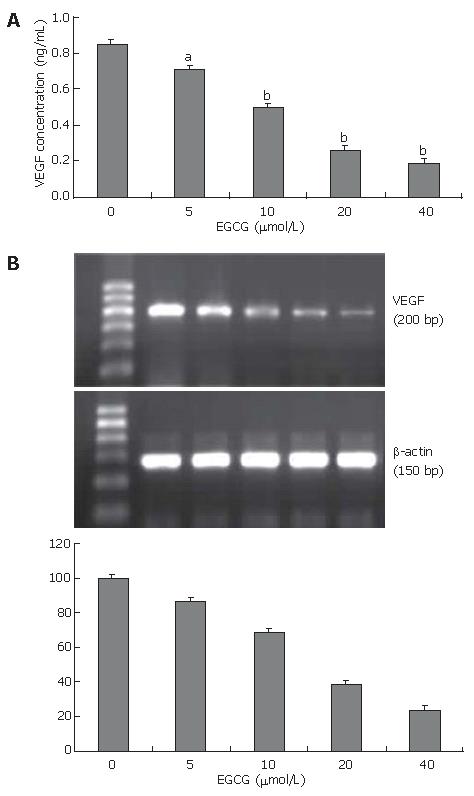
Angiogenesis is initiated by the release of angiogenic factors from tumor cells. VEGF is the most potent angiogenic factor and associated with tumor-induced angiogenesis. To examine the effect of EGCG on VEGF secretion, VEGF protein level in the conditioned medium was measured by ELISA. EGCG treatment reduced VEGF secretion in the culture medium in a dose-dependent manner (Figure 4A). EGCG at the concentration of 5 μmol/L significantly reduced VEGF secretion by 16.0% (P < 0.05).
To determine whether the inhibitory effect of EGCG on VEGF expression is at the transcriptional level, we examined VEGF mRNA expression in tumor cells by RT-PCR, showing that VEGF mRNA expression in EGCG treated cells was reduced in a dose-dependent manner (Figure 4B). These results suggested that EGCG reduced VEGF expression by inhibiting VEGF gene transcription and decreasing VEGF mRNA expression.
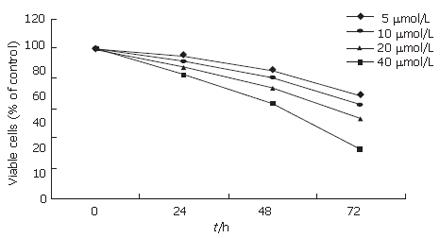
Tumor angiogenesis is induced by VEGF mainly produced by tumor cells. VEGF-induced angiogenesis is very important for tumor growth and metastasis. To further investigate whether EGCG inhibits VEGF-induced angiogenesis, we examined the effect of EGCG on VEGF-induced endothelial proliferation, migration and tuber formation. The effect of EGCG on VEGF-induced proliferation of HUVECs was determined by MTT assay. HUVECs were treated with 20 ng/mL VEGF and EGCG at the indicated concentrations for 24, 48 and 72 h. As shown in Figure 5, EGCG treatment abrogated VEGF-induced proliferation of HUVECs in a time- and dose-dependent manner.
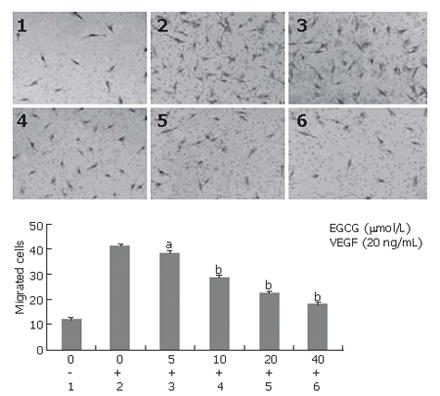
Endothelial cell migration is one of the key steps in angiogenesis. We examined the inhibitory effect of EGCG on endothelial cell migration induced by VEGF. As shown in Figure 6, EGCG treatment significantly and dose-dependently inhibited migration of HUVECs induced by VEGF. EGCG at the concentration of 5 μmol/L could effectively reduce VEGF-induced endothelial cell migration (P < 0.05).
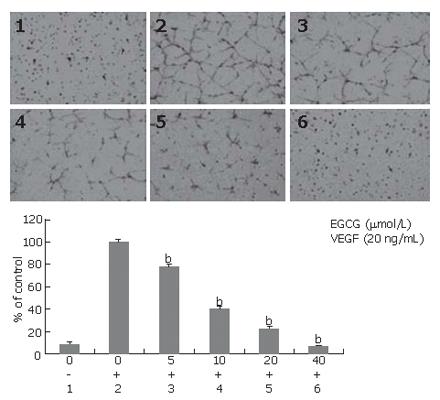
We determined the effect of EGCG on tube formation induced by VEGF. When treated with EGCG, tube formation induced by VEGF was inhibited in a concentration-dependent manner. EGCG reduced the length of endothelial tubes even at the concentration of 5 μmol/L, and an average of 24.2% inhibition was observed (P < 0.01). At the concentration of 40 μmol/L, EGCG abrogated almost all tube formations induced by VEGF (Figure 7).
Despite significant advance in treatment, gastric cancer remains one of the leading causes of cancer-related death in many countries such as China[14]. Tremendous efforts have been made to identify new chemopreventive and chemotherapeutic agents and strategies. Anti-angiogenic therapy is one of the most promising novel strategies and many attempts have been made to prevent or delay tumor growth by anti-angiogenesis. As the most potent angiogenic factor, VEGF has become one of the most common targets in anti-angiogenic therapy. In this study, we demonstrated that EGCG suppressed growth of gastric cancer by reducing VEGF production and VEGF-induced angiogenesis.
Green tea has been shown to have anti-angiogenic activity and drinking tea can significantly prevent VEGF-induced corneal neovascularization[15]. As the most abundant and active component of green tea, EGCG has also been shown to have anti-angiogenic property[9]. Treatment with EGCG can inhibit tumor growth[10]. In this study, intraperitoneal injection of EGCG markedly suppressed growth of gastric cancer, and an average of 60.4% inhibition was observed. MVD in tumor tissues treated with EGCG were significantly decreased. These results suggest that EGCG inhibits growth of gastric cancer.
Previous studies indicated that EGCG inhibits VEGF expression in tumor cells[10,16]. Treatment with EGCG also dose-dependently suppresses the secretion of VEGF both in endothelial cells and in tumor cells[17]. To evaluate the effect of EGCG on expression of VEGF in gastric cancer, we determined the expression of VEGF in cultured cells and tumor tissues. As shown in Figure 3, EGCG treatment dose-dependently decreased the expression of VEGF in cultured tumor cells. In tumor tissues treated with EGCG, the expression of VEGF was markedly reduced. Treatment with EGCG also reduced VEGF secretion in tumor cells in a dose-dependent manner. EGCG dose-dependently suppressed VEGF mRNA expression (Figure 4B). These findings suggest that EGCG inhibits angiogenesis in gastric cancer by reducing production of VEGF at transcriptional level.
VEGF expression is associated with a variety of transcription factors, genes and modulators[18]. Several mechanisms have been proposed for the inhibitory effect of EGCG on VEGF expression. EGCG strongly inhibits the transcriptional activity of transcription factors, such as NF-kappa B[16,19] and activator protein-1[20]. Treatment with EGCG also decreases the constitutive activation of EGFR[16] and the expression of protein kinase C[19], transcription modulators of VEGF, suggesting that these changes are associated with inhibition of VEGF promoter activity and cellular production of VEGF. Recent studies showed that EGCG strongly inhibits the constitutive activation of Stat3 in tumor cells[16]. Stat3 is one of the key transcription factors in regulation of VEGF expression[18]. Stat3 activation directly promotes VEGF expression and stimulates tumor angiogenesis[21]. In this study, high activation level of Stat3 was observed in gastric cancer, showing that abnormally activated Stat3 expression significantly correlates with VEGF expression and microvessel density (MVD), and is an independently prognostic factor of poor survival[22]. EGCG can reduce VEGF expression by inhibiting activation of Stat3 in human head, neck and breast cancer[16]. In this study, EGCG dose-dependently inhibited activation of Stat3. In tumor cells and tissues treated with EGCG, the protein level of phosphorylated Stat3 was markedly reduced, but the total Stat3 level remained unchanged, suggesting that EGCG down-regulates VEGF expression at least in part by inhibiting Stat3 activation in gastric cancer.
It was reported that EGCG is most effective in inhibiting angiogenesis among the four main catechins of green tea[23]. Angiogenesis is initiated by the release of angiogenic factors from tumor cells, and VEGF is the most potent angiogenic factor. In this study, EGCG directly inhibited VEGF-induced angiogenesis. As shown in Figure 5, treatment with EGCG inhibited VEGF-induced HUVEC proliferation in a time- and dose-dependent manner and endothelial cell migration and tuber formation were also abrogated by EGCG, suggesting that EGCG inhibits angiogenesis in gastric cancer by inhibiting VEGF-induced angiogenesis and reducing production of VEGF.
VEGF induces angiogenesis by binding to its receptors on endothelial cell surface. It was reported that EGCG dose-dependently inhibits VEGF binding to its receptors on endothelial cell surface, and EGCG is the only catechin of green tea that could disrupt VEGF receptor binding[23]. Importantly, EGCG at low doses (0.5-10 μmol/L) markedly inhibits formation of VEGFR-2 complex[19]. The exact mechanism underlying the inhibition of VEGF receptor binding is unclear. A recent report suggested that green tea extract dose-dependently inhibits expression of VEGFR-1 and -2 on HUVECs[24]. But in our study, EGCG could not modulate VEGFR-1 and VEGFR-2 expression (data not shown). Disruption of VEGF receptor binding would block receptor tyrosine phosphorylation and VEGF-induced growth and survival signaling. Treatment with EGCG inhibits VEGF-induced VEGFR-1 and VEGFR-2 phosphorylation on endothelial cells in a time- and dose-dependent manner[25]. This disruption decreases PI3-kinase activity in a dose-dependent manner[19], suppresses VE-cadherin tyrosine phosphorylation and Akt activation[26], and also decreases the PI3 kinase-dependent activation and DNA-binding ability of NF-kappaB[19], suggesting that EGCG inhibits VEGF-induced angiogenesis by disrupting VEGF signaling pathway.
In the present study, EGCG suppressed growth of gastric cancer by targeting multiple steps of angiogenesis. EGCG at the concentration 5 μmol/L effectively inhibited VEGF production and VEGF-induced angiogenesis. EGCG at 40 μmol/L almost totally abolished tube formation induced by VEGF. Previous studies also showed that concentrations of EGCG used in anti-angiogenesis experiments are usually lower than those in anti-cancer experiments, thus strongly supporting the concept that EGCG represents a potent angiogenic inhibitor and EGCG has dual anti-angiogenesis and anti-cancer activities. EGCG might mainly act as an angiogenic inhibitor in vivo, and this might in part explain the discrepancy between cell line and animal model studies. The concentration of EGCG tested in cell line system usually is 10-100 μmol/L, or higher. It is much higher than that observed in plasma or tissues of animals or human beings after ingestion of tea or related tea preparations for the poor bioavailability of EGCG[27].
As an angiogenic inhibitor targeting tumor-induced angiogenesis, EGCG might have some advantages over conventional cytotoxic chemotherapy for genetic stability of endothelial cells and abnormality of tumor vasculature. EGCG is less likely to induce drug resistance and has little or no toxicity. During the treatment, EGCG treated mice did not show weight loss or unusual behavior, histopathological examination also did not reveal any detectable toxicity to liver or kidney (data not shown), suggesting that EGCG at the concentrations used does not cause any detectable toxicity. Previous studies also showed that EGCG induces apoptosis and cell cycle arrest in many cancer cells without affecting normal cells[28]. It was reported that EGCG has anti-cancer effect without any severe side effects[29]. Taken together, as a natural and non-toxic product, EGCG might be a promising candidate for anti-angiogenic treatment of gastric cancer.
S- Editor Liu Y L- Editor Wang XL E- Editor Lu W
| 1. | Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1065] [Cited by in F6Publishing: 1127] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 2. | Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 546] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 3. | Chen D, Daniel KG, Kuhn DJ, Kazi A, Bhuiyan M, Li L, Wang Z, Wan SB, Lam WH, Chan TH. Green tea and tea polyphenols in cancer prevention. Front Biosci. 2004;9:2618-2631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Kono S, Ikeda M, Tokudome S, Kuratsune M. A case-control study of gastric cancer and diet in northern Kyushu, Japan. Jpn J Cancer Res. 1988;79:1067-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 209] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Yamane T, Takahashi T, Kuwata K, Oya K, Inagake M, Kitao Y, Suganuma M, Fujiki H. Inhibition of N-methyl-N'-nitro-N-nitrosoguanidine-induced carcinogenesis by (-)-epigallocatechin gallate in the rat glandular stomach. Cancer Res. 1995;55:2081-2084. [PubMed] [Cited in This Article: ] |
| 6. | Horie N, Hirabayashi N, Takahashi Y, Miyauchi Y, Taguchi H, Takeishi K. Synergistic effect of green tea catechins on cell growth and apoptosis induction in gastric carcinoma cells. Biol Pharm Bull. 2005;28:574-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Hoshiyama Y, Kawaguchi T, Miura Y, Mizoue T, Tokui N, Yatsuya H, Sakata K, Kondo T, Kikuchi S, Toyoshima H. Green tea and stomach cancer--a short review of prospective studies. J Epidemiol. 2005;15 Suppl 2:S109-S112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Yang CS, Lambert JD, Hou Z, Ju J, Lu G, Hao X. Molecular targets for the cancer preventive activity of tea polyphenols. Mol Carcinog. 2006;45:431-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Jung YD, Ellis LM. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int J Exp Pathol. 2001;82:309-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844-850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Kakeji Y, Koga T, Sumiyoshi Y, Shibahara K, Oda S, Maehara Y, Sugimachi K. Clinical significance of vascular endothelial growth factor expression in gastric cancer. J Exp Clin Cancer Res. 2002;21:125-129. [PubMed] [Cited in This Article: ] |
| 12. | Shishido T, Yasoshima T, Denno R, Sato N, Hirata K. Inhibition of liver metastasis of human gastric carcinoma by angiogenesis inhibitor TNP-470. Jpn J Cancer Res. 1996;87:958-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4014] [Cited by in F6Publishing: 4027] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 14. | Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17-20. [PubMed] [Cited in This Article: ] |
| 15. | Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398:381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 528] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 16. | Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132:2307-2311. [PubMed] [Cited in This Article: ] |
| 18. | Xie K, Wei D, Shi Q, Huang S. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev. 2004;15:297-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Rodriguez SK, Guo W, Liu L, Band MA, Paulson EK, Meydani M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int J Cancer. 2006;118:1635-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Dong Z, Ma W, Huang C, Yang CS. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (-)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997;57:4414-4419. [PubMed] [Cited in This Article: ] |
| 21. | Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000-2008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 877] [Cited by in F6Publishing: 912] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 22. | Gong W, Wang L, Yao JC, Ajani JA, Wei D, Aldape KD, Xie K, Sawaya R, Huang S. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005;11:1386-1393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Kondo T, Ohta T, Igura K, Hara Y, Kaji K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002;180:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Kojima-Yuasa A, Hua JJ, Kennedy DO, Matsui-Yuasa I. Green tea extract inhibits angiogenesis of human umbilical vein endothelial cells through reduction of expression of VEGF receptors. Life Sci. 2003;73:1299-1313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Lamy S, Gingras D, Béliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62:381-385. [PubMed] [Cited in This Article: ] |
| 26. | Tang FY, Nguyen N, Meydani M. Green tea catechins inhibit VEGF-induced angiogenesis in vitro through suppression of VE-cadherin phosphorylation and inactivation of Akt molecule. Int J Cancer. 2003;106:871-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003;523-524:201-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Chen ZP, Schell JB, Ho CT, Chen KY. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;129:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 295] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, Tan W, Fitch TR, Rowland KM, Young CY. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442-1446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |