Published online Dec 14, 2007. doi: 10.3748/wjg.v13.i46.6172
Revised: September 16, 2007
Accepted: October 6, 2007
Published online: December 14, 2007
AIM: To investigate the effect of exogenous erythro-poietin (EPO) administration on acute lung injury (ALI) in an experimental model of sodium taurodeoxycholate-induced acute necrotizing pancreatitis (ANP).
METHODS: Forty-seven male Wistar albino rats were randomly divided into 7 groups: sham group (n = 5), 3 ANP groups (n = 7 each) and 3 EPO groups (n = 7 each). ANP was induced by retrograde infusion of 5% sodium taurodeoxycholate into the common bile duct. Rats in EPO groups received 1000 U/kg intramuscular EPO immediately after induction of ANP. Rats in ANP groups were given 1 mL normal saline instead. All animals were sacrificed at postoperative 24 h, 48 h and 72 h. Serum amilase, IL-2, IL-6 and lung tissue malondialdehyde (MDA) were measured. Pleural effusion volume and lung/body weight (LW/BW) ratios were calculated. Tissue levels of TNF-α, IL-2 and IL-6 were screened immunohistochemically. Additionally, ox-LDL accumulation was assessed with immune-fluorescent staining. Histopathological alterations in the lungs were also scored.
RESULTS: The mean pleural effusion volume, calculated LW/BW ratio, serum IL-6 and lung tissue MDA levels were significantly lower in EPO groups than in ANP groups. No statistically significant difference was observed in either serum or tissue values of IL-2 among the groups. The level of tumor necrosis factor-α (TNF-α) and IL-6 and accumulation of ox-LDL were evident in the lung tissues of ANP groups when compared to EPO groups, particularly at 72 h. Histopathological evaluation confirmed the improvement in lung injury parameters after exogenous EPO administration, particularly at 48 h and 72 h.
CONCLUSION: EPO administration leads to a significant decrease in ALI parameters by inhibiting polymorphonuclear leukocyte (PMNL) accumulation, decreasing the levels of proinflammatory cytokines in circulation, preserving microvascular endothelial cell integrity and reducing oxidative stress-associated lipid peroxidation and therefore, can be regarded as a cytoprotective agent in ANP-induced ALI.
- Citation: Tascilar O, Cakmak GK, Tekin IO, Emre AU, Ucan BH, Bahadir B, Acikgoz S, Irkorucu O, Karakaya K, Balbaloglu H, Kertis G, Ankarali H, Comert M. Protective effects of erythropoietin against acute lung injury in a rat model of acute necrotizing pancreatitis. World J Gastroenterol 2007; 13(46): 6172-6182
- URL: https://www.wjgnet.com/1007-9327/full/v13/i46/6172.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i46.6172
Acute pancreatitis (AP) is a life-threatening necro-inflammatory disease with significant morbidity and mortality rates, especially when complicated by systemic inflammatory response syndrome (SIRS) and multiple organ failure (MODS)[1,2]. Death occurs in 60% of the patients within the first 6 d of disease onset and pulmonary complications including acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) account for a significant number of these deaths[3]. The exact mechanisms by which diverse etiological factors induce an attack are indefinite, but once the disease process is initiated, common inflammatory and repair pathways are invoked. Within the first few days following the onset of AP, lung injury occurs as a consequence of AP, whereas sepsis is a dominant cause for lung injury and mortality in the later phase of the disease process[4]. Despite improved understanding of the pathogenesis of ARDS, pharmacological modalities are ineffective in decreasing its mortality. None of the randomized clinical trials using novel therapeutic agents has demonstrated an improvement in patient outcome. Consequently, effective therapeutic interventions are thus called for.
Erythropoietin (EPO), a 30.4-kDa glycoprotein and a member of the type I cytokine superfamily, was first introduced as a hormone that regulates erythroid progenitors within the bone marrow to mature into erythrocytes, through binding to its specific cell surface receptors[5]. Hence, EPO is approved for the treatment of anemia as a consequence of a variety of disorders. In the current era, the premise that EPO is essential only for erythropoiesis has been changed according to the researches demonstrating the existence of EPO and its receptor in other organs and tissues outside of liver and kidney, such as brain, heart, pancreas, as well as vascular, gastrointestinal and reproductive systems[6,7]. Beyond its hematopoietic properties, EPO modulates a broad array of vital cellular processes including progenitor stem cell development, cellular integrity, and angiogenesis[8,9]. Additionally, in various tissues, EPO inhibits the apoptotic mechanisms of injury, including preservation of cellular membrane asymmetry to prevent inflammation[10-12]. Experimental evidence supports a vigorous cytoprotective effect and EPO is now considered to have applicability in a variety of disorders, such as cerebral ischemia, myocardial infarction, and chronic congestive heart failure[12-15]. Wu et al[16] demonstrated that pretreatment with EPO appears to attenuate ischemia-reperfusion-induced lung injury. This function is partly related with the ability of EPO to inhibit the accumulation of polymorphonuclear leukocytes (PMNL) in lung tissue and decrease the systematic expression of tumor necrosis factor-α (TNF-α). In addition to these studies, it has been reported that EPO can attenuate different kinds of lung injuries, showing that rats exposed to hyperoxia exhibit well-maintained alveolar structure and enhanced vascularity when treated with EPO[17]. Importantly, EPO can protect the ultrastructure of tracheobronchial epithelia and pulmonary type II epithelia of rats during traumatic brain injury[18,19].
AP associated lung injury is a multifactorial pheno-menon with various phases. In the light of the above-mentioned findings, the present study was to evaluate the hypothesis that EPO administration offers pulmonary protective effect against pancreatitis induced lung injury in rats.
Forty-seven male Wistar albino rats weighing 250-300 g were housed under constant temperature (22°C) and humidity in a 12-h dark/light cycle.
The experiments were conducted following the Ethic Committee Faculty of Medicine, University of Zonguldak Karaelmas guiding principles for the care and use of laboratory animals. The animals were randomized into seven experimental groups as follows: sham group in which rats received sham operation (n = 5), 3 ANP groups in which acute necrotizing pancreatitis (ANP) was induced by retrograde infusion of sodium taurodeoxycholate and
1 mL normal saline (0.9% NaCl) was given intramuscularly immediately after induction of AP (n = 7 each), 3 EPO groups in which AP was induced by the same way and 1000 U/kg EPO (Eprex, Epoetin alfa, Janssen-Cilag AG, Sweden) was injected intramuscularly immediately after induction of AP. All animals in the ANP and EPO groups were sacrificed at postoperative 24 h, 48 h and 72 h, respectively. Histopathological, biochemical and immunohistochemical evaluations were performed.
Anesthesia was induce by injecting ketamine HCL at 100 mg/kg im and laparotomy was performed under strict sterile conditions. An upper midline abdominal incision was made to identify the common pancreaticobiliary duct. The duodenal wall was punctured at its antimesenteric aspect with a 24-gauge IV catheter (Novacath, Medipro A.Ş., Istanbul, Turkey). The catheter was advanced 5 mm into the common duct through the papilla of Vater. ANP was induced by retrograde infusion of 0.2 mL 5% sodium taurodeoxycholate (Sigma, St.Louis, MO, USA) over 3 min using an infusion pump as previously described[20] and the pacreaticobiliary duct was clamped near the liver hilum throughout the inraductal infusion in all groups, except for sham group. Animals in sham group were subjected to anesthesia, laparotomy and duodenal manipulation, but not to biliopancreatic duct cannulation. The midline incision was closed in two layers with 4/0 silk suture (Ethicon, Edinburg, UK). Rats were allowed to recover from anesthetic and returned to their cages with free access to water and food after surgery.
All the rats were sacrificed by aortic puncture method. The abdominal and thoracic cavities were entered to obtain blood and lung samples. Blood samples were centrifuged at 1800 ×g for 15 min at 4°C to obtain plasma and stored at -80°C for biochemical analysis. Then, the rats were killed with the lung removed immediately. Random cross-sections of the lung tissue were fixed in 10% neutral phosphate-buffered formalin and embedded in paraffin wax for histopathological examination. Samples of lung tissue were weighed and stored at -85°C for subsequent biochemical and immunohistochemical measurements.
The thorax was opened to collect pleural effusion (PE) by suction which was measured volumetrically. Care was also taken to eliminate blood contamination with PE. The lungs were then removed and all surrounding tissues were dissected and weighed with an analytical balance. The volume of PE (mL) and the lung weight/body weight (LW/BW) ratios were calculated and considered as an index of pulmonary edema.
Serum amylase, IL-2 and IL-6 assay: Serum amylase levels were measured by a Beckman Coulter LX-20® system analyzer (Fullerton, CA, USA) using Beckman kits (Fullerton, CA, USA), following the manufacturer's instructions. IL-2 and IL-6 levels in the serum were measured with commercially available kits (Biosource International, Commercial ELISA Kit, California, USA).
Lung tissue malondialdehyde (MDA) assay: MDA levels in the lung tissue were measured in tissue homogenate. In brief, tissue was homogenized with cold 1.15% KCl to make a 10% homogenates, and 0.2 mL of 8.1% SDS, 1.5 mL of 20% acetic acid solution adjusted to pH 3.5 with NaOH and 1.5 mL of 0.8% aqueous solution of thiobarbituric acid were added to 0.2 mL of 10% tissue homogenates. The mixture was made up to 4.0 mL with distilled water and heated in an oil bath at 95°C for 60 min. After cooling with tap water, 1.0 mL of distilled water and 5.0 mL of the mixture of n-butanol and pyridine (15:1, v/v) were added and the solution was shaken vigorously. After centrifugation at 4000 r/min for 10 min, the organic layer was taken with its absorbance measured at 532 nm on a Shimadzu UV 1601 spectrophotometer. As a standard, 1.1.3.3 tetraetoxypropane was used. MDA concentration per gram tissue was calculated (nmol/gr tissue).
Immunohistochemical method for screening IL-2, IL-6 and TNF-αin the lung tissue: Cryostat sections of lung tissue (7 μm) were fixed with absolute ethanol and stained with avidin biotin complex based immunohistochemical method. Immunohistochemistry was performed to observe peroxidase diaminobenzidine reaction. Cytokine staining was performed with biotinylated mouse anti-rat IL-2, IL-6, TNF-α antibodies (Biosource International, California, USA). Streptavidin-peroxidase (HRP) and diaminobenzidine (DAB) were purchased from DAKOCytomation (Denmark). Ethanol-fixed tissue sections were treated with biotinylated mouse anti-rat IL-2, IL-6 and TNF-α for 30 min, washed three times with PBS, incubated for an additional 30 min with streptavidin-HRP and washed three times with PBS. The sections were then treated with 0.03% 3, 3-diamino benzidine tetrahydrochloride plus 0.01% hydrogen peroxide in 50 mmol/L Tris-HCl buffer (pH 7.4) for 10 min. All incubations were performed at room temperature. The sections were examined under a light microscope by an independent observer, blind to the study.
Immune-fluorescent staining method for screening ox-LDL in the pancreas and lung tissues: Rat lung and pancreas were obtained and stored at -85°C. Slides were prepared from the 7 μm-thick frozen lung biopsy sections. Slides were further divided into two pieces: one for the test and the other for the negative control. Thirty μL human polyclonal anti-ox-LDL IgG solution as primary antibody was added only on the test slides and the control slides were manipulated with phosphate- buffered solution (PBS) as the same amount of primary antibody. After a 30-min incubation in a humid chamber at room temperature, both the control and test slides were washed with phosphate-buffered saline and 30 μL fluorescent isothiocyanate (FITC)-labeled anti human IgG was administered as a conjugate substance. The slides were incubated for a further 30 min at room temperature and washed with the standard PBS solution. After drying, the slides were covered with a mounting medium and examined under a fluorescent microscope (LEICA DMRX, Germany).
The lung tissue samples were fixed in 10% formalin immediately after removal, embedded in paraffin, sectioned at 5 μm intervals, stained with hematoxylin and eosin, and examined under a light microscope. Histopathological evaluation and scoring of the parameters were performed by a single pathologist unaware of the treatment groups. Morphometric analysis of histological sections was accomplished with the point counting technique. For this purpose, we used an optical microscope provided with an integrating eyepiece containing 100 points and 50 lines. The following parameters were evaluated as previously described[21,22].
Alveolar distension and collapse index: At a magni-fication of × 100, we analyzed 10 randomly selected fields of the proximal and 10 fields of the distal sections. We designated grades 0, 1, 2, and 3 to microscopic fields respectively as 0%, 25%, 50%, and over 50% of the area with either alveolar distension or alveolar collapse.
Alveolar edema index: At a magnification of × 400, we analyzed 10 randomly selected fields of the proximal and 10 fields of the distal sections. The relationship between the number of points of the eyepiece falling on alveolar edema and the number of points falling on the whole alveolar lumen was determined.
Alveolar cellularity index: We analyzed 10 microscopic fields from each lung slide at a magnification of × 1000. The alveolar cellularity index was obtained by the relationship between the lines of the integrating eyepiece crossing a nucleus and the lines crossing alveolar septa.
Polymorphonuclear cell (PMNL) index: We analyz-ed 10 microscopic fields from each lung slide at a magnification of × 1000. PMNL index was obtained by the relationship between the lines of the integrating eyepiece crossing a nucleus and the lines crossing alveolar septa.
Statistical analysis was performed using SPSS version 11.5 for Windows XP. The results were expressed as mean + standard deviation (SD). The differences in serum amylase, IL-2 and IL-6 were assessed by Welch test and post hoc Games-Howell test or one way ANOVA and post hoc Tukey HSD test where appropriate. The differences betweeen groups (ANP, EPO, sham), time course (three different hours) and its interaction in terms of tissue MDA levels, pulmonary effusion volume, calculated LW/BW ratio, and mean histopathological scores, were analyzed by factorial analysis of variance with a single control. P < 0.05 was considered statistically significant.
The mean pleural effusion volume (mL) and the calculated LW/BW ratio were significantly increased in ANP groups when compared to EPO groups (P < 0.0001). The mean ± SD volume of pleural effusion measured was 1.62 ± 1.08 mL, 1.5 ± 0.33 mL and 1.97 ± 0.39 mL in 3 ANP groups and 0.45 ± 0.37 mL, 0.48 ± 0.38 mL and 0.85 ± 0.13 mL in 3 EPO groups, respectively. No statistically significant difference was detected between sham and EPO groups (0.18 ± 0.08 mL vs 0.45 ± 0.37 mL, 0.48 ± 0.38 mL and 0.85 ± 0.13 mL, P > 0.05 for each). The volume of pleural effusion was statistically significant higher in ANP groups than in sham group (0.18 ± 0.08 mL vs 1.62 ± 1.08 mL, 1.5 ± 0.33 mL and 1.97 ± 0.39 mL, P < 0.001 for each).The time course of pleural effusion volume in 3 ANP groups is shown in Figure 1A. In terms of LW/BW ratio, a statistically significant difference was seen from 24 h to 72 both in 3 ANP groups (0.006 ± 0.0022 vs 0.008 ± 0.0019, P < 0.05) and in 3 EPO groups (0.004 ± 0.0008 vs 0.005 ± 0.0011, P < 0.05) and the mean calculated ratio was higher at 72 h for each. In comparison to sham group, no statistically significant difference was found in EPO groups (0.003 ± 0.0004 vs 0.004 ± 0.0008, 0.005 ± 0.0009 and 0.005 ± 0.0011, P > 0.05), where as ANP resulted in a significant increase in calculated LW/BW ratio at 24 h, 48 h, and 72 h (0.003 ± 0.0004 vs 0.006 ± 0.0022, 0.007 ± 0.0016, and 0.008 ± 0.0019, P < 0.05 for each) (Figure 1B). The pleural effusion values and LW/BW ratio are listed in Table 1.
| Groups | Sham | ANP1 | ANP2 | ANP3 | EPO1 | EPO2 | EPO3 |
| Pleural effusion (mL) | 0.18 ± 0.08 | 1.62 ± 1.08 | 1.5 ± 0.33 | 1.97 ± 0.39 | 0.45 ± 0.37 | 0.48 ± 0.38 | 0.85 ± 0.13 |
| LW/BW ratio | 0.003 ± 0.0004 | 0.006 ± 0.0022 | 0.007 ± 0.0016 | 0.008 ± 0.0019 | 0.004 ± 0.0008 | 0.005 ± 0.0009 | 0.005 ± 0.0011 |
Serum amylase, IL-2 and IL-6 assay: A statistically significant increase was detected in the mean ± SD serum levels of amylase in 3 ANP groups when compared to sham group (534 ± 124 u/L vs 3502 ± 1830 u/L, 3759 ± 1505 u/L and 5056 ± 1872 u/L, P < 0.05 for each). The mean ± SD value of serum amylase was 3502 ± 1830 u/L, 3759 ± 1505 u/L and 5056 ±1872 u/L in 3 ANP groups and 1523 ± 514 u/L, 2317 ± 311 u/L and 735 ± 454 u/L in 3 EPO groups, respectively. On the other hand, no statistically significant difference was found between the ANP and EPO groups with respect to the time intervals (P > 0.05). The serum levels of IL-6 were significantly lower in 3 EPO groups than in 3 ANP groups (P = 0.001 for each). The IL-6 value (mean ± SD) was 24.4 ± 3.26 pg/mL, 27.7 ± 3.74 pg/mL and 33.2 ± 2.1 pg/mL respectively in 3 ANP groups, and 12.2 ± 2.15 pg/mL, 12.8 ± 1.89 pg/mL and 13.8 ± 3.24 pg/mL respectively in 3 EPO groups. We did not observe any statistically significant difference in IL-2 values among these groups (7.4 ± 2.88 pg/mL vs 7.7 ± 3.17 pg/mL, 9.3 ± 2.74 pg/mL, 7.2 ± 3.1 pg/mL, 6.9 ± 2.04 pg/mL, 7.7 ± 2.93 g/mL and 10.6 ± 3.9 pg/mL, P > 0.005 for each). The mean ± SD values of serum amylase, IL-2, IL-6 and tissue MDA are listed in Table 2.
| Groups | Sham | ANP1 | ANP2 | ANP3 | EPO1 | EPO2 | EPO3 |
| Amylase (U/L) | 534 ± 124 | 3502 ± 1830 | 3759 ± 1505 | 5056 ± 1872 | 1523 ± 514 | 2317 ± 311 | 735 ± 454 |
| IL-6 (pg/mL) | 4.7 ± 2.01 | 24.4 ± 3.26 | 27.7 ± 3.74 | 33.2 ± 2.1 | 12.2 ± 2.15 | 12.8 ± 1.89 | 13.8 ± 3.24 |
| IL-2 (pg/mL) | 7.4 ± 2.88 | 7.7 ± 3.17 | 9.3 ± 2.74 | 7.2 ± 3.1 | 6.9 ± 2.04 | 7.7 ± 2.93 | 10.6 ± 3.9 |
| MDA (nmol/gr tissue) | 8.5 ± 3.1 | 45.9 ± 6.8 | 49.3 ± 9.5 | 58.8 ± 9 | 14.7 ± 2.1 | 21.2 ± 2.7 | 30.4 ± 2.1 |
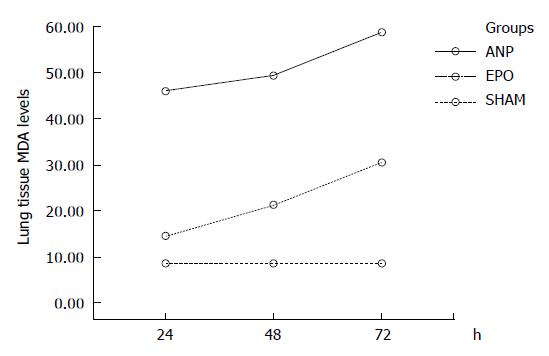
Lung tissue MDA assay: Pulmonary injury in ANP groups was characterized by an increase in lung tissue MDA levels, an indicator of lipid peroxidation. The lung tissue MDA levels were significantly reduced in EPO groups at 24 h, 48 h, and 72 h, when compared to ANP groups (P < 0.0001). The MDA value (mean ± SD) was 45.9 ± 6.8 nmol/gr tissue, 49.3 ± 9.5 nmol/gr tissue, and 58.8 ± 9 nmol/gr tissue respectively in 3 ANP groups and 14.7 ± 2.1 nmol/gr tissue, 21.2 ± 2.7 nmol/gr tissue, and 30.4 ± 2.1 nmol/gr tissue respectively in 3 EPO groups. A statistically significant increase in MDA values was noted at 24 h-72 h (45.9 ± 6.8 nmol/gr tissue vs
58.8 ± 9 nmol/gr tissue, and 14.7 ± 2.1 nmol/gr tissue vs 30.4 ± 2.1 nmol/gr tissue, P < 0.0001) and 48 h to 72 h (49.3 ± 9.5 nmol/gr tissue vs 58.8 ± 9 nmol/gr tissue and 21.2 ± 2.7 nmol/gr tissue vs 30.4 ± 2.1 nmol/gr tissue, P = 0.001) in either ANP or EPO groups. The mean MDA value was higher at 72 h. In comparison with sham group, the MDA levels were significantly higher in all the other groups (8.5 ± 3.1 nmol/gr tissue vs 45.9 ± 6.8 nmol/gr tissue, 49.3 ± 9.5 nmol/gr tissue, 58.8 ± 9 nmol/gr tissue, 21.2 ± 2.7 nmol/gr tissue, and 30.4 ± 2.1 nmol/gr tissue, P < 0.001 for each) except for EPO groups at 24 h (8.5 ± 3.1 nmol/gr tissue vs 14.7 ± 2.1 nmol/gr tissue, P = 0.224) (Figure 2).
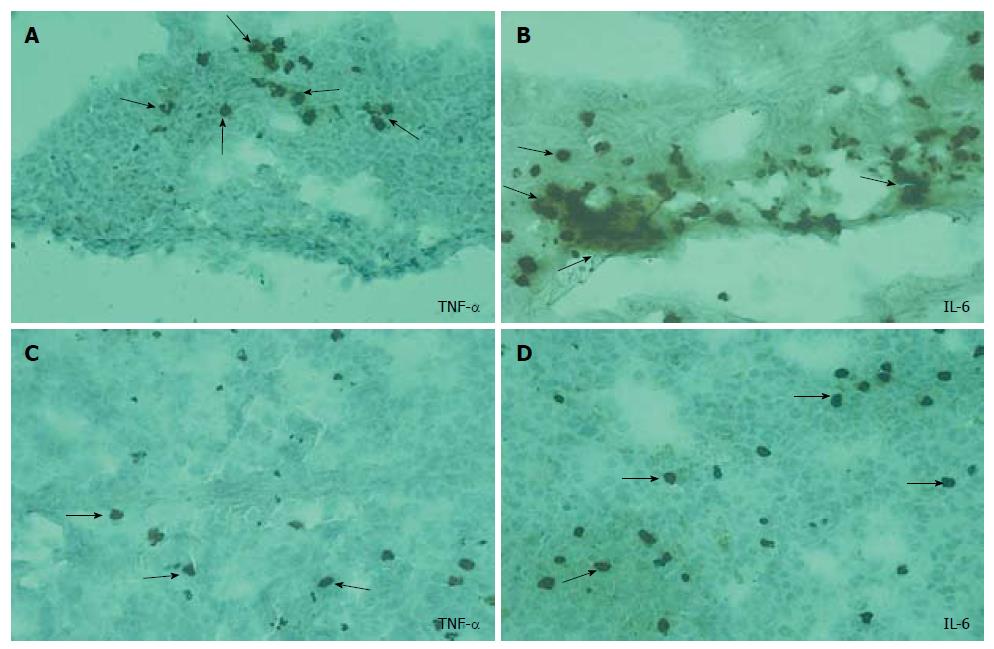
Immunohistochemical screening: The intracellular accumulation of TNF-α and IL-6 was evident in the lung tissues of ANP groups (Figure 3A and B) when compared to EPO groups, particularly at 72 h (Figure 3C and D). No significant difference in IL-2 accumulation was detected among the groups.
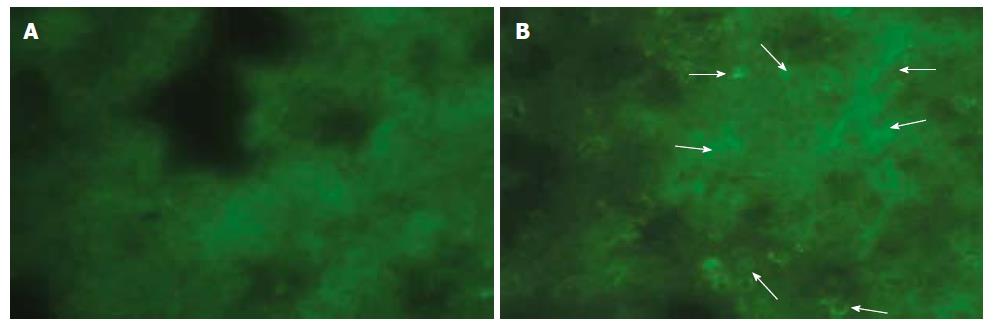
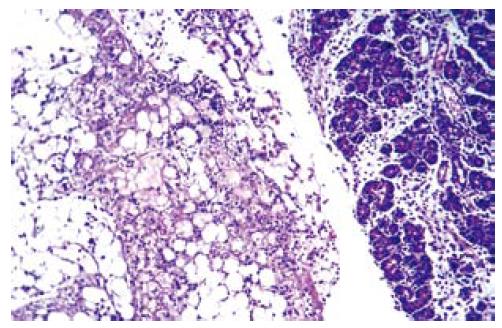
Immune-fluorescent screening of ox-LDL: As we did not observe any positive immunofluorescent staining either in pancreas or in lung tissue of sham and EPO groups, a significant positive staining for ox-LDL was determined in ANP groups, which became much evident at 72 h (Figure 4A and B).
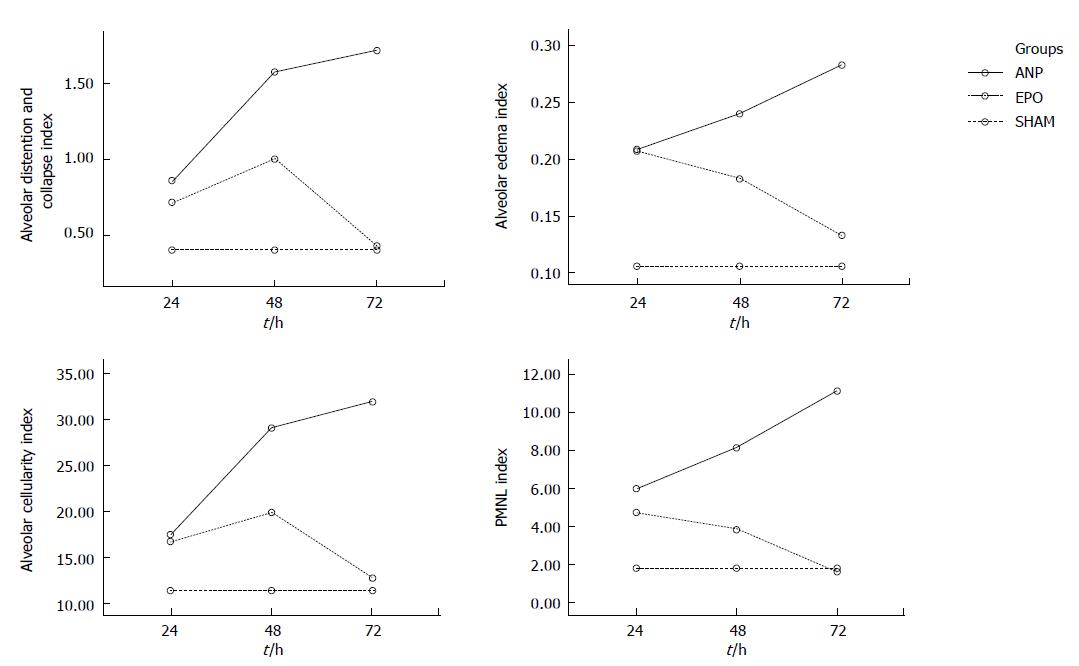
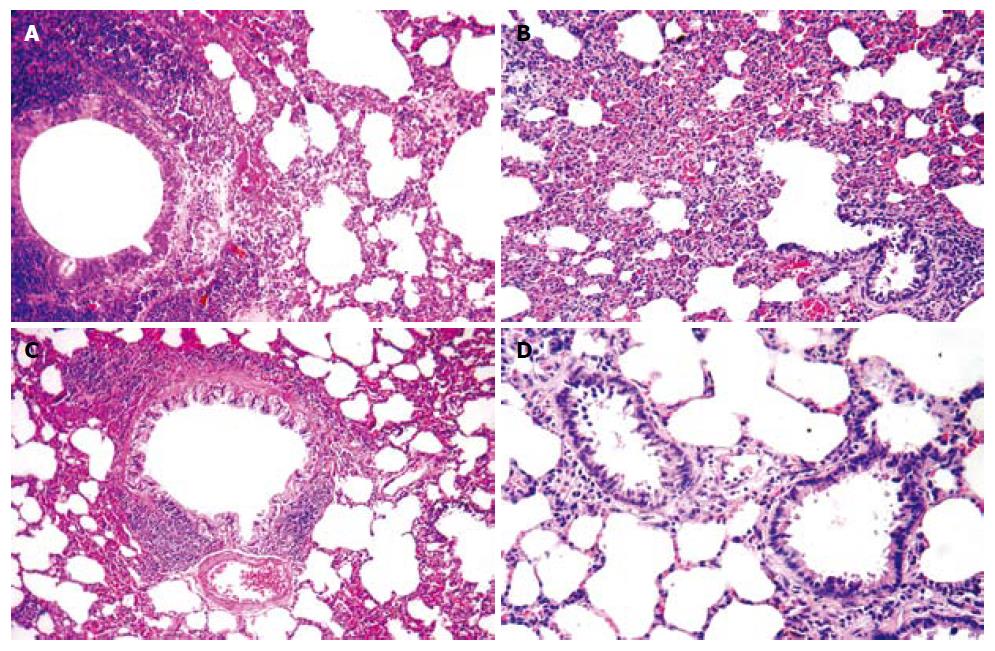
Alveolar distention and collapse: Alveolar distention and collapse were significantly intense in ANP groups at 24 h, 48 h and 72 h when compared to EPO groups (P < 0.0001). The alveolar distention and collapse scores (mean ± SD) for ANP and EPO groups calculated at 24 h, 48 h and 72 h were 0.85 ± 0.69, 1.57 ± 0.78, and 1.71 ± 0.75 vs 0.71 ± 0.75, 1 ± 0.57 and 0.42 ± 0.53 respectively. Only ANP groups demonstrated a significant difference at 72 h in comparison with sham (1.71 ± 0.75 vs 0.4 ± 0.54, P = 0.03) (Figure 5A).
Alveolar edema index: Alveolar edema index was significantly different both in ANP groups and in EPO groups depending on the time course (P = 0.002). Alveolar edema was more intense in ANP groups at 48 h and 72 h, when compared to EPO groups (0.24 ± 0.05 vs 0.18 ± 0.03, P < 0.05 and 0.28 ± 0.03 vs 0.13 ± 0.04, P < 0.01). Moreover, at 72 h the mean alveolar edema index determined was the highest in ANP groups and the lowest in EPO groups (0.28 ± 0.03 vs 0.13 ± 0.04). In comparison with sham group, ANP groups had a significantly increased mean alveolar edema index at 24 h, 48 h and 72 h (0.1 ± 0.02 vs 0.2 ± 0.08, P = 0.019; 0.1 ± 0.02 vs 0.24 ± 0.05, P = 0.001; and 0.1 ± 0.02 vs 0.28 ± 0.03, P = 0.0001; respectively). On the other hand, no statistically significant difference was detected between sham group and EPO groups at 48 h and 72 h (0.1 ± 0.02 vs 0.18 ± 0.03, P = 0.149 and 0.1 ± 0.02 vs 0.13 ± 0.04, P = 0.968), which might propose that EPO treatment could decrease alveolar edema index at 48 h and 72 h (Figure 5B).
Alveolar cellularity index: Alveolar cellularity index was significantly different in either ANP groups or in EPO groups depending on the time course (P = 0.011). There was no significant difference in alveolar cellularity index between ANP and EPO groups at 24 h (17.42 ± 9.16 vs 16.71 ± 8.61, P > 0.05), whereas the mean value for ANP groups was significantly increased at 48 h and 72 h (29.14 ± 8.39 vs 19.85 ± 5.89, P < 0.05 and 32 ± 6.42 vs 12.71 ± 7.11, P < 0.01). Additionally, the mean alveolar cellularity index at 72 h was the highest in ANP groups and the lowest in EPO groups (32 ± 6.42 vs 12.71 ± 7.11). Compared with sham group, alveolar cellularity index was significantly increased in only ANP groups at 48 h and 72 h (11.4 ± 8.14 vs 29.14 ± 8.39, P = 0.006 and 11.4 ± 8.14 vs 32 ± 6.42, P = 0.001). This might suggest that EPO administration following ANP could decrease alveolar cellularity index at 48 h and 72 h (Figure 5C).
PMNL index: A statistically significant difference was observed in PMNL index between ANP and EPO groups with respect to the time intervals (P = 0.009). PMNL index was similar either in ANP groups or in EPO groups at 24 h (6 ± 3.51 vs 4.71 ± 2.36, P > 0.05). However, EPO treatment significantly decreased the mean PMNL index at 48 h and 72 h (8.14 ± 3.48 vs 3.85 ± 2.19, P < 0.05 and 11.14 ± 5.55 vs 1.57 ± 1.61, P < 0.01). The mean ± SD value at 72 h was the greatest in ANP groups and the lowest in EPO groups (11.14 ± 5.55 vs 1.57 ± 1.61). There was no statistically significant difference in PMNL index at 24 h, 48 h and 72 h between ANP and EPO groups (1.8 ± 1.78 vs 4.71 ± 2.36, P = 0.315; 1.8 ± 1.78 vs 3.85 ± 2.19, P = 0.930; and 1.8 ±1.78 vs 1.57 ± 1.61, P = 1.00, respectively), whereas ANP induction resulted in an increased PMNL index at 48 h and 72 h (1.8 ± 1.78 vs 8.14 ± 3.48, P = 0.028 and 1.8 ± 1.78 vs 11.14 ± 5.55, P = 0.0001, respectively) but not at 24 h (1.8 ± 1.78 vs 6 ± 3.51, P = 0.725). This might be explained as EPO administration could decrease PMNL index at 48 h and 72 h (Figure 5D). The histopathological indexes of lung injury (mean ± SD) are listed in Table 3. The representative light microscopic views of lung injury at 48 h and 72 h, and inecrotizing pancreatitis with severe fatty necrosis in ANP and EPO groups are shown in Figures 6 and 7. According to the above-mentioned criteria, it might be speculated that EPO administration could alleviate pulmonary injury by decreasing alveolar edema, alveolar cellularity and PMNL indexes at 48 h and 72 h following taurocolic acid-induced pancreatitis. The effect of EPO on alveolar distention and collapse was restricted at 72 h.
| Groups | Sham | ANP1 | ANP2 | ANP3 | EPO1 | EPO2 | EPO3 |
| Alveolar distention collapse | 0.4 ± 0.54 | 0.85 ± 0.69 | 1.57 ± 0.78 | 1.71 ± 0.75 | 0.71 ± 0.75 | 1 ± 0.57 | 0.42 ± 0.53 |
| Alveolar edema index | 0.1 ± 0.02 | 0.2 ± 0.08 | 0.24 ± 0.05 | 0.28 ± 0.03 | 0.2 ± 0.04 | 0.18 ± 0.03 | 0.13 ± 0.04 |
| Alveolar cellularity index | 11.4 ± 8.14 | 17.42 ± 9.16 | 29.14 ± 8.39 | 32 ± 6.42 | 16.71 ± 8.61 | 19.85 ± 5.89 | 12.71 ± 7.11 |
| PMNL cell index | 1.8 ± 1.78 | 6 ± 3.51 | 8.14 ± 3.48 | 11.14 ± 5.55 | 4.71 ± 2.36 | 3.85 ± 2.19 | 1.57 ± 1.61 |
ANP is an inflammatory disorder with various systemic complications. ALI and ARDS are the most dreadful complications of ANP and impending catastrophe which is difficult to deal with clinically. Various medications directed at key stages of the pathophysiology are not clinically efficacious as indicated in the preceding experimental trials[23]. Therefore, therapies for preventing or reversing lung injury would be ideal for the treatment of AP[1,2,21,24-26].
Randomized studies of AP in the clinical setting do have limitations. In this regard, reliable AP animal models are of paramount importance. Taurocholate infusion model is a well-established ANP rat model that induces multiple organ failure involving the lung[27]. Moreover, Milani et al[28] found that mechanical and morphologic alterations in pancreatitis-associated pulmonary injury in rats are similar to those observed in humans.
The pathophysiology of ALI/ARDS and most of other pulmonary complications is multifactorial in ANP. The major pathway is the induction of a strong inflammatory response both in experimental models and in patients[29]. Regardless of the priming process, the disease progression can be viewed as three phases in continuum: local inflammation of the pancreas (a generalized inflammation stage and SIRS), and the final stage of multiorgan dysfunction[24,25]. The first sign of MODS is often the impaired lung function that manifests itself clinically as ARDS[1,2,24,25,30]. SIRS is one of the crucial reasons for pancreatitis-associated lung injury and PMNL plays a central role with various inflammatory cytokines and reactive oxygen species (ROS)[2,29,31]. Many researchers have focused their efforts on preventing AP-induced lung injury by pharmacologic interventions. Attractively, recent works have discovered the potential role of EPO as a multifunctional endogenous mediator offering cytoprotective effect against injury in various tissues including lung[16-19]. In multiple species including humans, many tissues injured by ischemia, mechanical trauma, excitotoxins, and other stressors are significantly improved by administration of EPO following injury[32]. The presence of a therapeutic window dictates specific time constraints for efficacious administration of exogenous EPO as a cytoprotectant[5]. According to this hypothesis we administered EPO immediately following the induction of pancreatitis and evaluated its effect in three different time courses.
The principle mechanism by which EPO confers tissue protection involves the modulation of cellular apoptosis. EPO inhibits the apoptotic mechanisms of injury, including preservation of cellular membrane asymmetry to prevent inflammation, can therefore be regarded as a general tissue-protective cytokine[11,12,14]. Agents that can prevent apoptosis can be effective long after the occurrence of injury[5]. This phenomenon might describe the long protective effect of EPO on lung injury in our study, particularly at 48 h and 72 h. We would also like to emphasize that, in patients with a severe attack, the effects of distant organ damage including lung injury, are often not fully established and become apparent only over the following 48 h. There is thus a therapeutic window between hospital presentation and development of distant organ dysfunction. As an obvious time window existed in this process, therapeutic approach should focus on it during this period. From this assumption, the animals were sacrificed on postoperative hours 24, 48 and 72 for histopathological and biochemical evaluations in our study.
Another crucial determinant for observing the cytoprotective effect of EPO is the serum concentration. The serum concentration of EPO required for tissue protection is higher than that required for erythropoiesis. Preclinical data suggest that the minimum therapeutic level needed for protection against tissue injury appears to be 300-500 mIU/kg body weight (intravenously or intraperitoneally) for the organs to be adequately investigated. EPO administration (100-1000 U/kg body weight) achieves possible systemic protective effects whereas high doses of EPO (3000-5000 U/kg body weight) are necessary for cardioprotection and neuroprotectin[33]. According to this, we administered EPO at the dose of 1000 mIU/kg body weight to observe the cytoprotective effects.
EPO plays a dual role in vascular protection by preserving endothelial cell integrity[6,8,9], thus playing a role in maintaining the integrity of microvasculature[11]. One of the major factors for the development of alveolar edema in ANP is the increased microvascular permeability. The experimental protocol we performed let us to measure the amount of edema within alveoli. We used alveolar edema index and alveolar distension and collapse index as markers of ALI according to the previous observations suggesting that histologic evidence of pulmonary tissue injury can appear before the development of clinically relevant respiratory mechanical changes[22]. We prefer three different time courses, since pulmonary injury indexes are quite intense in taurocolic acid induced acute pancreatitis on d 1 and 3, some of which persist through d 8[22]. In the present study, pulmonary edema, alveolar cellularity index and PMNL index (pulmonary injury index) were significantly reduced in EPO groups at 48 h and 72 h, suggesting that EPO can preserve endothelial cell integrity.
Oxidative stress has been implicated as a crucial landmark by increasing endothelial permeability in ARDS[1]. ROS scavengers possess protective effect against local acute pancreatitis-associated with lung injury[21,34,35]. In addition to other effects, EPO has been demonstrated in various tissues to be an antioxidant as it can decrease the plasma iron concentration and increase the ability of plasma to inhibit lipid peroxidation[36,37]. In the present study, we determined the tissue levels of thiobarbituric acid reactant MDA, which is considered a good indicator of lipid peroxidation, and found a significant decrease in EPO group when compared to ANP groups in all three time courses. This might be attributed to the antioxidant effect of EPO. Furthermore, the tissue damage induced by ANP was associated with a significant ox-LDL accumulation either in pancreas samples or in lung tissue specimens. Ox-LDL is an early product of lipid peroxidation and ox-LDL accumulation in pancreatitis is associated with lung injury.
At present, the role of inflammatory mediators in the pathogenesis of ARDS has become a hot issue in the research field. EPO has been demonstrated to prevent cellular inflammation by inhibiting several proinflammatory cytokines, such as IL-6, TNF-α, and monocyte chemoattractant protein[7,38]. Attractively, these effects of EPO can be mediated by both hormonal and paracrine modalities[38]. There is mounting evidence that proinflammatory cytokines are the agents behind the systemic complications of AP[1,2]. It was reported that systemic inflammation plays a role in development of ALI triggered by pancreatitis[30]. The critical players of this process include proinflammatory cytokines including IL-1β, TNF-α, IL-6, IL-8, and platelet activating factor (PAF)[29]. Among these, the serum and/or tissue levels of TNF-α, IL-2 and IL-6 were analyzed in this study. Regardless of the model of acute pancreatitis, inhibition of the potent cytokine TNF-α might decrease organ injury and improve survival[39]. The tissue levels of TNF-α in the lungs were analyzed with immunohistochemical staining. Since no quantitative analysis was possible unavailable techniques, we evaluated this parameter not statistically but morphologically. IL-6 is another proinflammatory cytokine, and its high circulating level has been shown to be an excellent predictor of the severity of ARDS with different etiologies, including AP[40]. Moreover, IL-6 has been proposed to be one of the best prognostic parameters for pulmonary failure in human AP[30,41]. Mayer et al[41] have confirmed the important role of soluble IL-2 receptors (a lymphocyte activation marker), as a marker for severe AP, especially when complicated by lung or kidney failure or sepsis during lethal course of the disease[41]. In the present study, pulmonary injury in ANP groups was characterized by the increased serum or tissue IL-6 and TNF-α level. EPO treatment significantly decreased IL-6 and TNF-α level which might be due to the antiinflammatory properties of its molecule. However, we did not determine a statistically significant difference in the IL-2 level among the groups. This result might reflect the ineffectiveness of EPO on lymphocyte activity.
In conclusion, EPO administration plays a crucial role in preventing histological changes of ALI induced by experimental ANP. Moreover, it can significantly reduce the circulating and tissue levels of proinflammatory cytokines which have been considered the key factors for ALI. Additionally, oxidative stress markers are decreased particularly at 72 h following the induction of pancreatitis that might be attributed to the long-lasting antioxidant effect of EPO. All these findings show that EPO can attenuate ANP-induced lung injury by inhibiting PMNL accumulation, decreasing the circulating levels of proinflammatory cytokines, preserving microvascular endothelial cell integrity and reducing oxidative stress-associated lipid peroxidation. Years of clinical application in patients with anemia and chronic renal disease indicate that EPO is safe and well tolerated and can act as an ideal cytoprotective agent[7,42,43]. Nevertheless, the issue which should also be taken into consideration is that EPO is not an absolutely innocent agent with subsequent clinical toxic effects. Therefore, it would be of value to investigate its pharmacodynamics, pharmacokinetics, side-effects, administration routes and doses before used as a potential candidate for the treatment of ANP-associated ALI in routine clinical practice. In other words, this is a preliminary study and more experiments are necessary for the efficacy and potentially cytoprotective mechanisms of EPO action.
Pulmonary complication is the major cause for mortality in acute necrotizing pancreatitis (ANP). Since no absolutely effective treatment is available at present, therapies for preventing or reversing lung injury would be ideal for the treatment of AP.
Erythropoietin (EPO) has long been known as a glycoprotein hormone that regulates erythropoiesis in mammals. Beyond its hematopoietic properties, EPO modulates a broad array of vital cellular processes including progenitor stem cell development, cellular integrity, and angiogenesis. EPO has recently been demonstrated to play a role in prolonging cell survival by acting as an antiapopitotic agent. EPO inhibits the apoptotic mechanisms of injury including preservation of cellular membrane asymmetry to prevent inflammation, and can therefore be regarded as a general tissue-protective cytokine. Additionally, experimental evidence supports a vigorous cytoprotective effect of EPO, which is now considered to have applicability in a variety of disorders, such as cerebral ischemia, myocardial infarction, and chronic congestive heart failure.
The present study was an experimental study addressing the beneficial effects of EPO on lung injury. We cited several articles from other investigators reporting researches of EPO action on various tissues including lungs.
Recent works have discovered the potential role of EPO as a multifunctional endogenous mediator offering cytoprotective effect against injury in various tissues including the lungs. Pretreatment with EPO appears to attenuate ischemia-reperfusion-induced lung injury and hyperoxic lung injury in neonatal rats. From this point of view we evaluated the potantial protecting effects of EPO against acute lung injury in a rat model of ANP. Our data show that EPO administration can alleviate pulmonary injury parameters in experimental pancreatitis.
The impending catastrophe in ANP is generally preceded by acute lung injury. Despite improved understanding of the pathogenesis of ARDS, pharmacological modalities are ineffective in decreasing its mortality. None of the randomized clinical trials using novel therapeutic agents has demonstrated an improvement in patient outcome. The verification of cytoprotective effects of EPO on acute lung injury in a model of experimental pancreatitis might shed some valuable light on the novel effective therapeutic interventions.
Erythropoietin (EPO), a 30.4-kDa glycoprotein and a member of the typeIcytokine superfamily, was first introduced as a hormone that regulates erythroid progenitors within the bone marrow to mature into erythrocytes, through binding to its specific cell surface receptors. Acute necrotizing pancreatitis (ANP) is a life-threatening necroinflammatory disease of pancreas with significant morbidity and mortality rates. Acute lung injury (ALI) is one of the most dreadful complications of AP which might be described as the continuum of pathological responses to pulmonary parenchymal injury. Acute respiratory distress syndrome (ARDS) is a severe form of ALI and acute pulmonary inflammation syndrome and resultant increased capillary endothelial permeability with clinical features of severe dyspnea and extreme hypoxemia refractory to a high inspired oxygen concentration.
This is a well-designed and interesting study about the beneficial effects of EPO on lung injury in an experimental model of ANP. Since this is a preliminary study as discussed by the authors, more comprehensive experiments should be carried out to reveal the underlying cellular mechanisms of EPO's cytoprotective action against lung injury.
S- Editor Liu Y L- Editor Wang XL E- Editor Ma WH
| 1. | Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 810] [Cited by in F6Publishing: 846] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 2. | Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 359] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 3. | Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Pastor CM, Matthay MA, Frossard JL. Pancreatitis-associated acute lung injury: new insights. Chest. 2003;124:2341-2351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Coleman T, Brines M. Science review: recombinant human erythropoietin in critical illness: a role beyond anemia? Crit Care. 2004;8:337-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002;11:863-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Genc S, Koroglu TF, Genc K. Erythropoietin as a novel neuroprotectant. Restor Neurol Neurosci. 2004;22:105-119. [PubMed] [Cited in This Article: ] |
| 8. | Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973-2979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 301] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, cytochrome c, and caspase-9 form the critical elements for cerebral vascular protection by erythropoietin. J Cereb Blood Flow Metab. 2003;23:320-330. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20:299-315. [PubMed] [Cited in This Article: ] |
| 11. | Ghezzi P, Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11 Suppl 1:S37-S44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 251] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 12. | Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 404] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 13. | Kumral A, Uysal N, Tugyan K, Sonmez A, Yilmaz O, Gokmen N, Kiray M, Genc S, Duman N, Koroglu TF. Erythropoietin improves long-term spatial memory deficits and brain injury following neonatal hypoxia-ischemia in rats. Behav Brain Res. 2004;153:77-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Savino R, Ciliberto G. A paradigm shift for erythropoietin: no longer a specialized growth factor, but rather an all-purpose tissue-protective agent. Cell Death Differ. 2004;11 Suppl 1:S2-S4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 430] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Wu H, Ren B, Zhu J, Dong G, Xu B, Wang C, Zheng X, Jing H. Pretreatment with recombined human erythropoietin attenuates ischemia-reperfusion-induced lung injury in rats. Eur J Cardiothorac Surg. 2006;29:902-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Ozer EA, Kumral A, Ozer E, Yilmaz O, Duman N, Ozkal S, Koroglu T, Ozkan H. Effects of erythropoietin on hyperoxic lung injury in neonatal rats. Pediatr Res. 2005;58:38-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Yildirim E, Ozisik K, Solaroglu I, Kaptanoglu E, Beskonakli E, Sargon MF, Kilinc K, Sakinci U. Protective effect of erythropoietin on type II pneumocyte cells after traumatic brain injury in rats. J Trauma. 2005;58:1252-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Yildirim E, Solaroglu I, Okutan O, Ozisik K, Kaptanoglu E, Sargon MF, Sakinci U. Ultrastructural changes in tracheobronchial epithelia following experimental traumatic brain injury in rats: protective effect of erythropoietin. J Heart Lung Transplant. 2004;23:1423-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Chen CC, Wang SS, Tsay SH, Lee FY, Lu RH, Chang FY, Lee SD. Effects of gabexate mesilate on serum inflammatory cytokines in rats with acute necrotizing pancreatitis. Cytokine. 2006;33:95-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Leme AS, Lichtenstein A, Arantes-Costa FM, Landucci EC, Martins MA. Acute lung injury in experimental pancreatitis in rats: pulmonary protective effects of crotapotin and N-acetylcysteine. Shock. 2002;18:428-433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Lichtenstein A, Milani R Jr, Fernezlian SM, Leme AS, Capelozzi VL, Martins MA. Acute lung injury in two experimental models of acute pancreatitis: infusion of saline or sodium taurocholate into the pancreatic duct. Crit Care Med. 2000;28:1497-1502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Udobi KF, Childs E, Touijer K. Acute respiratory distress syndrome. Am Fam Physician. 2003;67:315-322. [PubMed] [Cited in This Article: ] |
| 24. | Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 25] [Reference Citation Analysis (0)] |
| 25. | Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy. 2002;1:343-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2005;288:L3-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 224] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 27. | Chan YC, Leung PS. Acute pancreatitis: animal models and recent advances in basic research. Pancreas. 2007;34:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Milani Júnior R, Pereira PM, Dolhnikoff M, Saldiva PH, Martins MA. Respiratory mechanics and lung morphometry in severe pancreatitis-associated acute lung injury in rats. Crit Care Med. 1995;23:1882-1889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24 Suppl 1:45-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 182] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 30. | Takala A, Jousela I, Takkunen O, Kautiainen H, Jansson SE, Orpana A, Karonen SL, Repo H. A prospective study of inflammation markers in patients at risk of indirect acute lung injury. Shock. 2002;17:252-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Browne GW, Pitchumoni CS. Pathophysiology of pulmonary complications of acute pancreatitis. World J Gastroenterol. 2006;12:7087-7096. [PubMed] [Cited in This Article: ] |
| 32. | Erbayraktar S, Grasso G, Sfacteria A, Xie QW, Coleman T, Kreilgaard M, Torup L, Sager T, Erbayraktar Z, Gokmen N. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci USA. 2003;100:6741-6746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 33. | Bogoyevitch MA. An update on the cardiac effects of erythropoietin cardioprotection by erythropoietin and the lessons learnt from studies in neuroprotection. Cardiovasc Res. 2004;63:208-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Demols A, Van Laethem JL, Quertinmont E, Legros F, Louis H, Le Moine O, Devière J. N-acetylcysteine decreases severity of acute pancreatitis in mice. Pancreas. 2000;20:161-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Eşrefoğlu M, Gül M, Ateş B, Yilmaz I. Ultrastructural clues for the protective effect of ascorbic acid and N-acetylcysteine against oxidative damage on caerulein-induced pancreatitis. Pancreatology. 2006;6:477-485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Bany-Mohammed FM, Slivka S, Hallman M. Recombinant human erythropoietin: possible role as an antioxidant in premature rabbits. Pediatr Res. 1996;40:381-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev. 2004;27:113-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Chong ZZ, Kang JQ, Maiese K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. J Cereb Blood Flow Metab. 2002;22:503-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Denham W, Yang J, Wang H, Botchkina G, Tracey KJ, Norman J. Inhibition of p38 mitogen activate kinase attenuates the severity of pancreatitis-induced adult respiratory distress syndrome. Crit Care Med. 2000;28:2567-2572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 367] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 41. | Mayer J, Rau B, Gansauge F, Beger HG. Inflammatory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 290] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 42. | Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann Hematol. 2004;83:673-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |