Published online Oct 21, 2007. doi: 10.3748/wjg.v13.i39.5196
Revised: April 26, 2007
Accepted: July 15, 2007
Published online: October 21, 2007
AIM: To investigate H+, K+-ATPase inhibition, anti-H pylori, antioxidant, and the in vivo antiulcer potential of a pectic polysaccharide from Swallow root (Decalepis hamiltonii; SRPP).
METHODS: SRPP, with known sugar composition [rhamnose: arabinose: xylose: galactose in the ratio of 16:50:2:32 (w/w), with 141 mg/g of uronic acid] was examined for anti-ulcer potency in vivo against swim/ethanol stress-induction in animal models. Ulcer index, antioxidant/antioxidant enzymes, H+, K+-ATPase and gastric mucin levels were determined to assess the anti-ulcer potency. Anti-H pylori activity was also determined by viable colony count and electron microscopic studies.
RESULTS: SRPP, containing phenolics at 0.12 g GAE/g, prevented stress-induced gastric ulcers in animal models by 80%-85%. Down regulation of gastric mucin 2-3 fold, antioxidant/antioxidant enzymes and upregulation of 3 fold of H+, K+-ATPase in ulcerous animals were normalized upon treatment with SRPP. Histopathological analysis revealed protection to the disrupted gastric mucosal layer and epithelial glands. SRPP also inhibited H+, K+-ATPase in vitro, at an IC50 of 77 μg/mL as opposed to that of 19.3 μg/mL of Lansoprazole and H pylori growth at Minimum Inhibitory Concentration (MIC) of 150 μg/mL. In addition, free radical scavenging (IC50-40 μg/mL) and reducing power (3200 U/g) activities were also observed.
CONCLUSION: SRPP, with defined sugar composition and phenolics, exhibited multi-potent free radical scavenging, antioxidant, anti-H pylori, inhibition of H+, K+-ATPase and gastric mucosal protective activities. In addition, SRPP is non-toxic as opposed to other known anti-ulcer drugs, and therefore may be employed as a potential alternative for ulcer management.
-
Citation: Srikanta B, Siddaraju M, Dharmesh S. A novel phenol-bound pectic polysaccharide from
Decalepis hamiltonii with multi-step ulcer preventive activity. World J Gastroenterol 2007; 13(39): 5196-5207 - URL: https://www.wjgnet.com/1007-9327/full/v13/i39/5196.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i39.5196
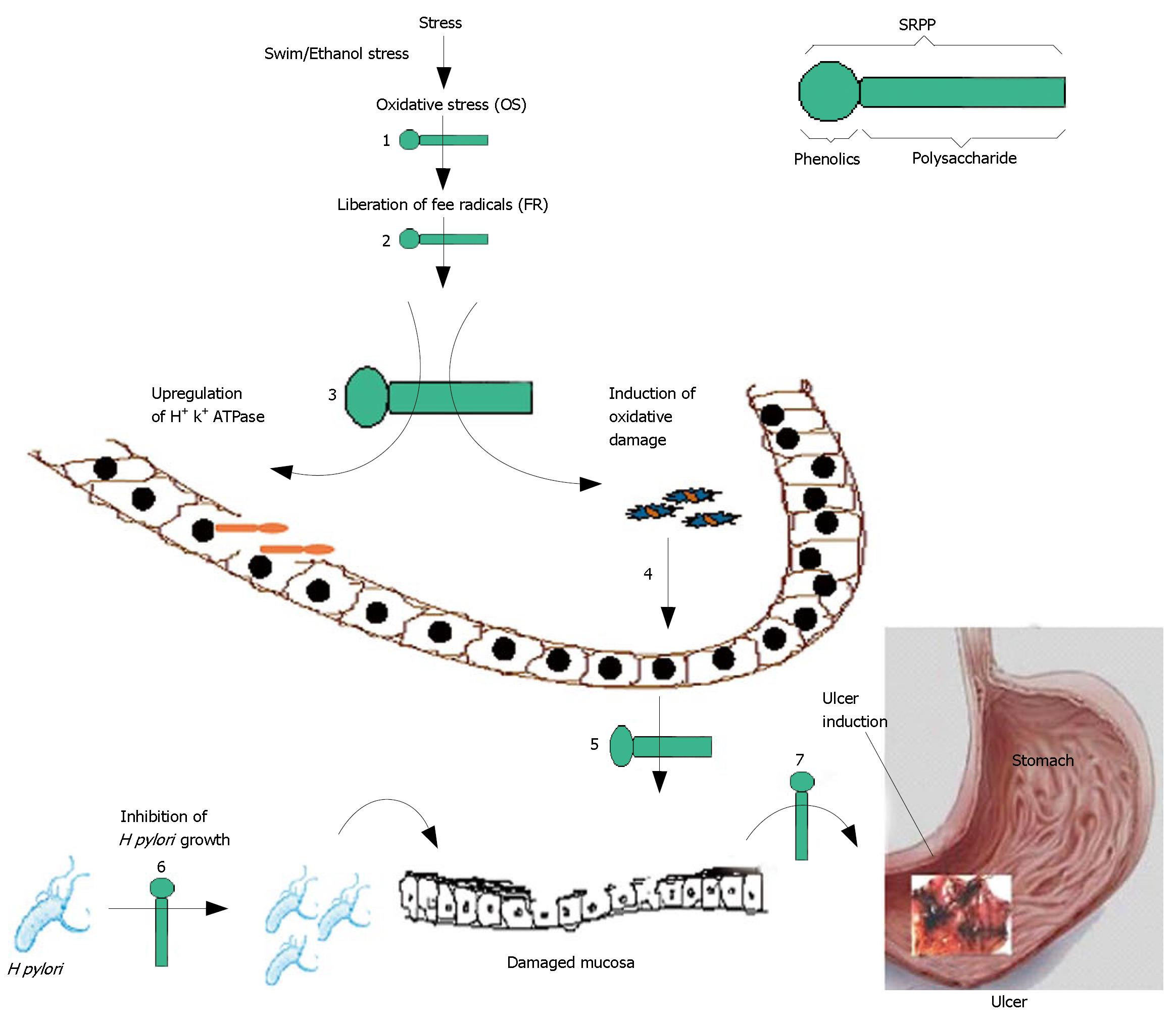
Ulcer is a common global problem with increasing incidence and prevalence. Worldwide 14.5 million people have ulcers with a mortality of 4.08 million (http://digestive.nidk.nih.gov/statistics/statistics.htm/peptic ulcer prevalence). The increasing incidence and prevalence of ulcers have been attributed to several factors encountered during day-to-day life, such as stress[1], exposure to bacterial infection[2], and use of non-steroidal anti-inflammatory drugs (NSAIDS)[3]. Indeed, NSAIDs are used daily by approximately 30 million people world wide, constituting a world market in excess of $2 billion. Associated serious side effects are ulceration and gastric bleeding, which are due to inhibiting cyclooxygenase-1 activity that is required for mucosal protection[4]. Gastric lesions develop due to loss of the delicate balance between gastro-protective and aggressive factors. Reduction in gastroprotective factors, such as mucus, bicarbonate secretion and gastric mucosa-blood flow, and enhancement of aggressive factors, such as increase of acid/pepsin secretion and H pylori infection, results in gastric ulceration[1,2]. Mucosal damage, an initial step in ulcer development, has been known to be due to oxidative stress (OS) by Reactive Oxygen Species (ROS), hypersecretion of HCl through H+, K+-ATPase action[5], harboring of H pylori on the damaged mucin layer[6], and the blockade of the cyclooxygenase enzyme system by NSAIDS[4], as depicted in Figure 1.
A modest approach to control ulceration, therefore, is via stimulation of gastric mucin synthesis, enhancement of antioxidant levels in the stomach, scavenging of ROS, inhibition of H+, K+-ATPase and H pylori growth[7]. Although the antisecretory drugs, such as H+, K+-ATPase pump inhibitors-omeprazole, lansoprazole; histamine H2-receptor blockers-ranitidine, famotidine, are being used to control acid secretion and acid related disorders; however, they are not the drugs of choice since they produce potential adverse effects on human health[8].
In light of the above, it is pertinent to study natural products from food/plants as potential anti-ulcer compounds. Due to the lack of side effects compared to synthetic drugs, approximately 60% of the world’s population relies entirely on such natural medications. In Indian traditional medicine, several plants have been employed to treat gastrointestinal disorders, including gastric ulcers[9]. Antiulcer properties have been attributed generally to phenolics[10,11] and occasionally to polysaccharides[12-14] of plant extracts.
In this paper we report a pectic polysaccharide from Decalepis hamiltonii (Swallow root) containing a sulfonamide group and phenolics as an effective antiulcer compound in vivo. We envisage a multi-potent role for this phenolic-polysaccharide in the upregulation of mucin, antioxidant levels, modulation of oxidative status, inhibition of H+, K+-ATPase activity against swim and ethanol stress-induced ulcers in experimental animal models, in addition to its ability to inhibit H pylori. This paper reveals the potency and multi-step action of phenolic polysaccharide in preventing gastric ulceration.
Monoclonal anti-gastric mucin from Sigma Chemicals (St. Louis, MO, USA), Ham’s F-12 media from HiMedia (Mumbai, India), Alkaline phosphatase conjugated-rabbit anti mouse IgG secondary antibody from GENEI (Bangalore, India). All other reagents were of analytical grade purchased from Qualigens fine chemicals (Mumbai, India).
Decalepis hamiltonii Wight & Arn. (Asclepiadaceae) roots (Batch No. 6, 2005) were procured from a local vendor at Devaraja market, Mysore, India, originally collected from the Gumballi forest range located between 11-13 N and 77-78 E, South-East corner of Mysore district in July 2005 and identified by a taxonomist in the herbarium of Vivekananda Girijana Kalyana Kendra, B.R. Hills, Chamaraja Nagar, Karnataka, India, where a voucher specimen is deposited.
Pectic polysaccharide was isolated from defatted powder of swallow root as described previously[15]. Briefly, 10 g of defatted powder were depleted with proteins, amylose and amylopectins by specific enzymatic (protease, termamylase and glucoamylase) digestions at their optimum reaction conditions and centrifuged. Further, the residue was extracted with 200 mL of 0.25% (w/v) ammonium oxalate solution and filtered; the filtrate was precipitated by ethanol at 4°C. The precipitate was resuspended in 10 mL of water and lyophilized to obtain pectic polysaccharide. Total sugar content was estimated by a Phenol-sulphuric acid method. A total yield of 6% was obtained as carbohydrate and this pectic polysaccharide of swallow root is designated as SRPP. Sugar composition analysis revealed the presence of rhamnose: arabinose: xylose: galactose in the ratio 16:50:02:32, in addition to 141 mg uronic acid/g of SRPP.
Since phenolics are generally found to be associated with polysaccharides, we evaluated the phenolic content in SRPP using a Folin-Ciocalteu reagent as described previously[16]. Gallic acid was used as a standard for the generation of a calibration curve. Total phenolic content is expressed as Gallic Acid Equivalents (GAE) in mg/g of SRPP.
The reducing power and free radical scavenging activity of SRPP was determined according to the method described previously[16]. SRPP at 10-100 μg was employed for determining the reducing power and free radical scavenging activity. Capability to scavenge the DPPH radical was calculated using the following equation.
Scavenging effect (%) =(Absorbance of control at 517 nm - Absorbance of sample at 517 nm)/ Absorbance of control at 517 nm × 100
Pectic polysaccharides, particularly with sulfur, also have been shown to exhibit antioxidant activity and SRPP was subjected to FTIR study. The samples were prepared in the form of pellets by mixing with dry KBr. Potassium bromide discs containing 1% (w/w) of film material were scanned at 4 mm/s with a resolution of 4/cm over 400-4000/cm, averaging over 128 scans for each type of film and determined the presence of sulphur group.
Fresh sheep stomach was obtained from a local slaugh-terhouse at Mysore and an enzyme extract was prepared[17]. The enzyme extract was incubated with different fractions of swallow root polysaccharide, in a reaction mixture containing 16 mmol/L Tris buffer (pH 6.5). The re-action was initiated by adding substrate (2 mmol/L ATP, 2 mmol/L MgCl2 and 10 mmol/L KCl) and after 30 min of incubation at 37°C, the reaction was stopped by the addition of an assay mixture containing 4.5% ammonium molybdate and 60% perchloric acid. Inorganic phosphate formed was measured spectrophotometrically at 400 nm. Enzyme activity was calculated as micromoles of Pi released per hour at various doses of SRPP.
Toxicity studies were carried out in Albino Wistar rats that were orally fed with SRPP at 1 mg/kg b.w. for 15 d. Analysis showed biochemical changes as described previously[17].
Wistar albino rats, weighing about 180-220 g and main-tained under standard conditions of temperature, humidity and light, were provided with standard rodent pellet diet (Amruth feeds, Bangalore, India) and water ad libitum. The study was approved by the institutional ethical committee, which follows the guidelines of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals, Reg. No. 49, 1999), Government of India, New Delhi, India.
All the animals were categorized into two sets of five groups with 6 animals in each group (n = 6). SRPP and ranitidine at indicated doses were administered orally twice daily for 14 d. At the end of 14 d, animals were fasted for 18 h and then subjected to the ulcer inducing treatment. In the first set, ulcers were induced by forced swim stress per a published protocol[18], in which rats were briefly subjected to forced swim stress by making them swim in a jar of 30 cm height and 10 cm diameter containing water up to 15 cm height for 3 h. In the second set, ulcers were induced in rats by administering 100% ethanol (5 mL/kg b.w.) for 1 h[19]. Animals were sacrificed under deep ether anesthesia and stomachs were examined for mucosal integrity and occurrence of ulcers. Lower to higher gradings were assigned to milder to severe symptoms, respectively. The following are descriptions of ulcer scores: 0.5-red coloration, 1.0-spot ulcers, 1.5-hemorrhagic streaks, 2.0-ulcers more than 3 mm and less than 5 mm, 3.0-ulcers more than 5 mm. Mean ulcer scores for each experimental group were calculated and expressed as the ulcer index (UI)[20]. Stomach/liver tissues were used for enzyme assays. Serum was collected from the blood of all animals and analyzed for various biochemical parameters.
The stomach and liver tissues were collected, weighed and homogenized in chilled Tris-buffer (10 mmol/L, pH 7.4) at a concentration of 5% (w/v). The homogenates were centrifuged at 1000 ×g at 4°C for 20 min using a high speed cooling centrifuge (REMI C 24, Mumbai, India). The clear supernatant was used to analyse biochemical parameters[21].
Gastric mucin was isolated from the glandular segments of stomach and quantitated employing a monoclonal anti-human gastric mucin antibody (MAb-GM) by ELISA[5], as well as by Alcian blue dye binding methods[22]. Histological and immunohistological evaluation was done as described previously[17]. Equal weight of gastric tissue from animals of each group was homogenized using Tris-HCl buffer pH 7.4. The gastric membrane vesicles enriched in H+, K+-ATPase were prepared and the activity was assessed as described above.
Lipid peroxidation products of serum, stomach and liver homogenates were determined as TBARS and the malondialdehyde (MDA) that formed was quantitated using the molar extinction coefficient of the MDA molecule[21].
Glutathione (GSH) content was determined as described by Das and Banerjee[21]. The activity of superoxide dismutase (SOD) was assayed by measuring the reduction in the NBT in the presence of SOD[23] and catalase (CAT) was assayed by decomposition of H2O2 in the presence of catalase at 240 nm[24]. Glutathione peroxidase (GPx) was estimated based on the degradation of H2O2 in the presence of GSH and the decrease in absorbance was read at 340 nm[25]. SOD and CAT activity was expressed as units per milligram protein per min. The activity of GPx was expressed as nanomoles of NADPH oxidized per min per milligram of protein. The protein content of the homogenate was determined by Lowry’s method[26].
H pylori is a major ulcerogen, and anti-H Pylori activity was therefore determined. H pylori was obtained by endoscopic samples of gastric ulcer patients from KCDC (Karnataka Cardio Diagnostic Centre, Mysore, India) and cultured on Ham’s F-12 agar medium supplemented with 5% FBS at 37°C for 2-3 d in a microaerophilic condition[27]. H pylori culture was characterized by specific tests as described by Siddaraju and Shylaja[16].
Anti-H pylori activity of an aqueous solution of SRPP (200 μg/mL) was determined by a viable colony count method[28]. 100 μL of SRPP treated H pylori were also processed for scanning electron microscopy (SEM)[29] and examined by SEM (Model No. LEO 425 VP, Electron microscopy LTD, Cambridge, UK) with an acceleration voltage of 20 KV. Multiple fields of visions were viewed. The MIC value was determined by a conventional broth dilution method[16]. MIC was defined as the lowest concentration to restrict the growth to less than 0.05 absorbance units at 625 nm.
All values are expressed as mean ± SE. Significance was calculated with student’s t test (parametric). When several groups were compared, significance was calculated using an ANOVA. Enzyme estimations were carried out as described and results were tabulated. After calculating means and standard deviations, Dennett’s test was performed to obtain the significance between the treated groups and the control groups. A value of P < 0.05 was considered to indicate a significant difference.
Toxicity studies with aqueous solution of SRPP were carried out in rats for safety evaluation. These studies indicated no lethal effect up to 1 g/kg b.w. when orally fed for 14 d. There were no significant differences in total protein, TBARS levels, SGPT, SGOT and ALP between normal and SRPP treated rats (Table 1), indicating no adverse effect on the major organs. After the above treat-ment schedules, animals remained as healthy as control animals with normal food and water intake, body weight gain and behavior.
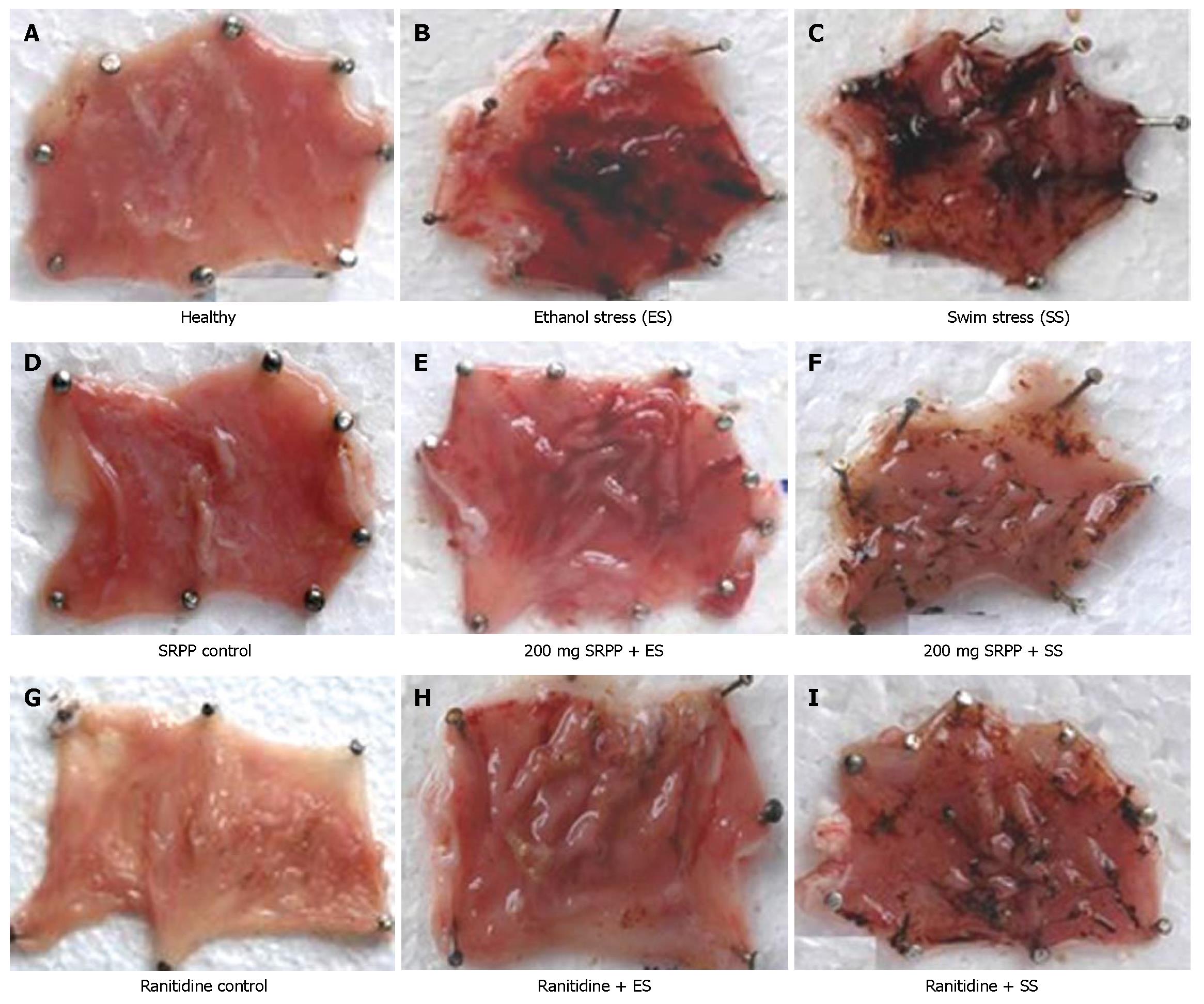
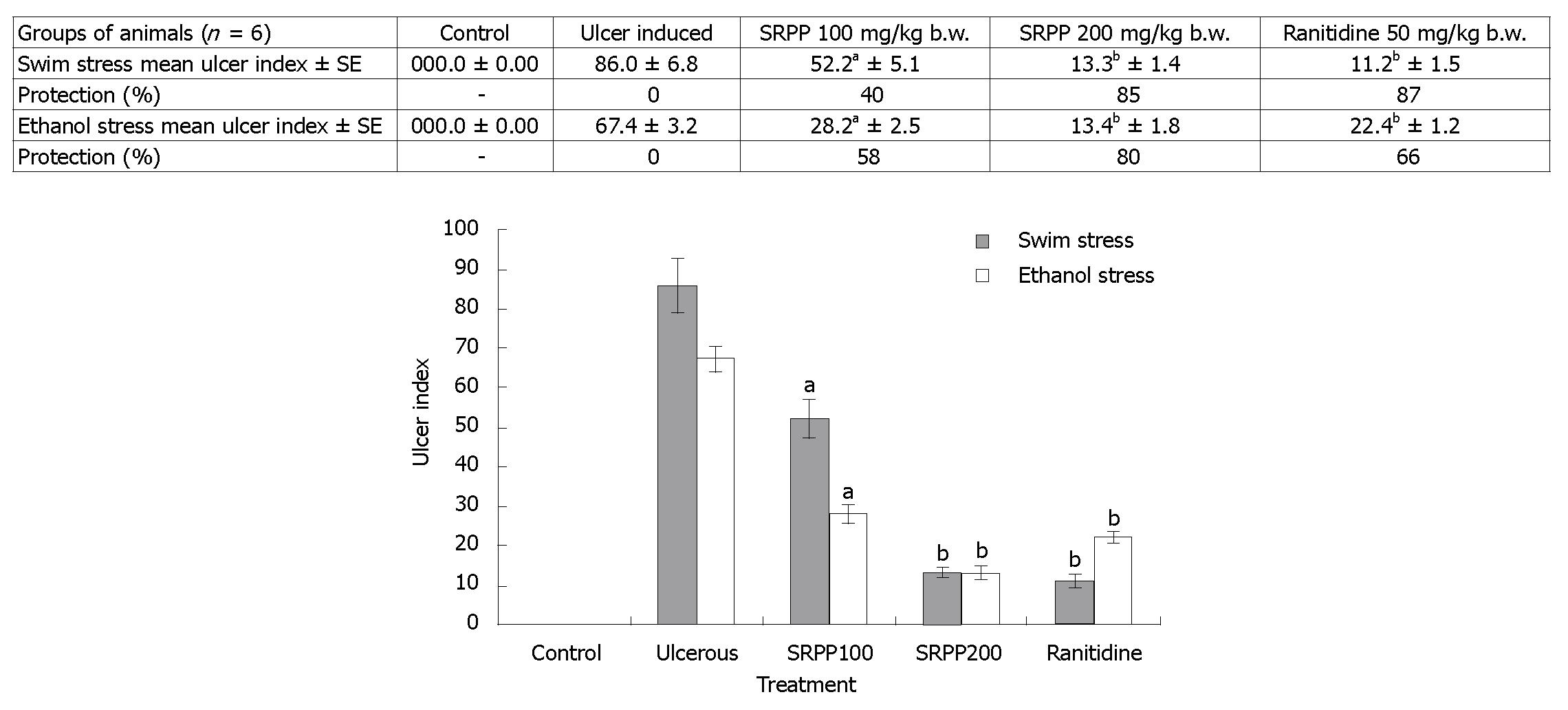
Healthy rats showed no lesions in their stomachs (Figure 2A), while rats treated with forced swim stress for 3 h or ethanol stress showed damage in the gastric wall with a hemorrhagic form of lesions and intraluminal bleeding (Figure 2B and C). Rats treated with only SRPP (Figure 2D) also showed no lesions, which is similar to the controls. Oral treatment of SRPP at 100 and 200 mg/kg b.w., as well as Ranitidine at 30 mg/kg b.w., showed protection in a dose dependent manner with no intraluminal bleeding and an insignificant number of gastric lesions (Figure 2E-I). Ranitidine protected both ethanol/swim stress-induced ulcers up to 66%-87% at 30 mg/kg b.w., while SRPP protected up to 80%-85%, respectively, indicating an ulcer preventive effect. Quantitative reduction in the ulcer index (%) in treated rats, compared to either ulcer induced or healthy, is depicted in Figure 3.
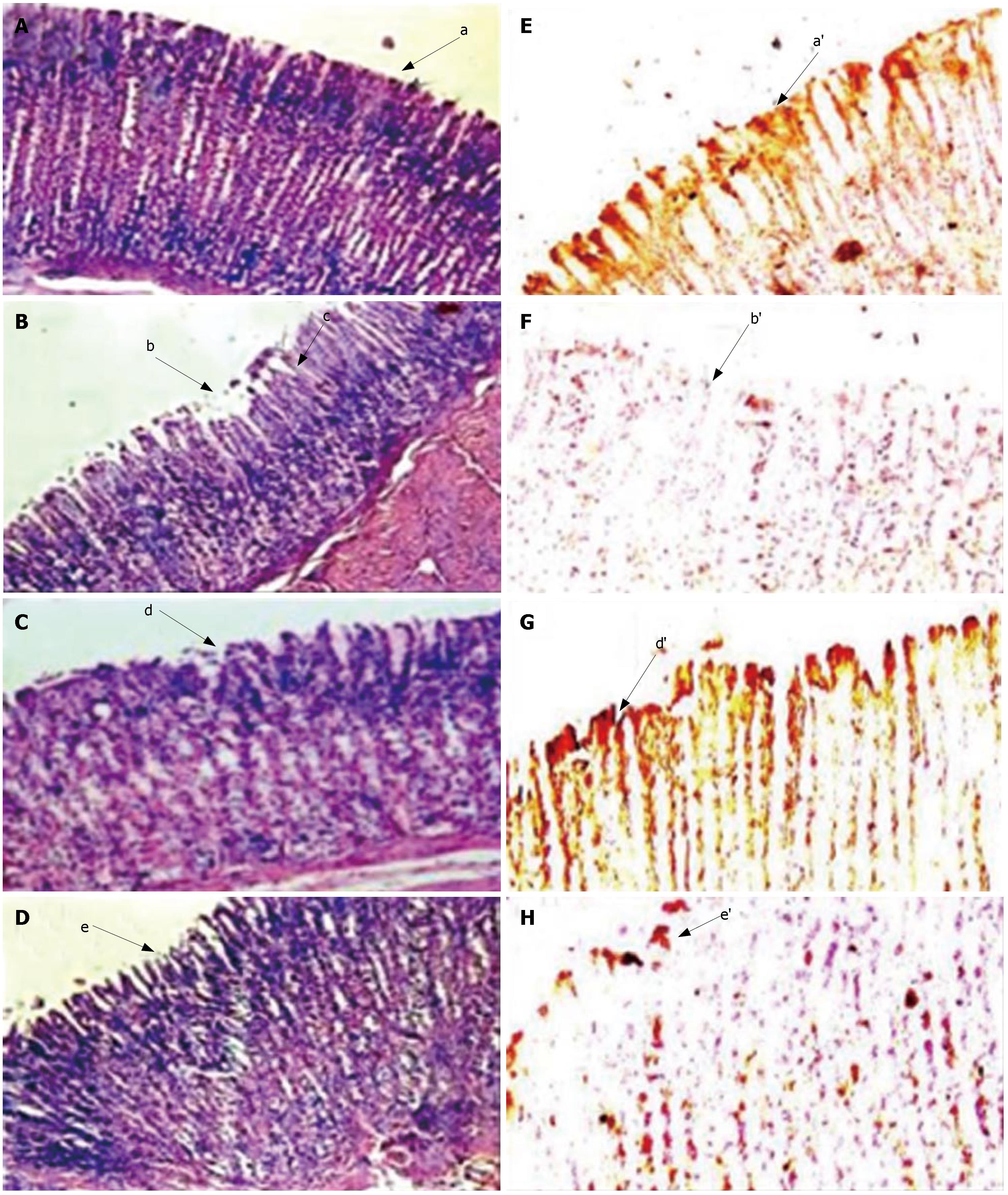
Gastric wall mucus is damaged during ulcer development and becomes the first target of stress-induced reactive oxygen species. Mucin oxidation or degradation takes place and subsequently looses the protective effect. In the current study, we evaluated the effect of in vivo ingestion of SRPP on protection of gastric wall mucus during ulceration induced by swim/ethanol stress. Since Alcian blue binds to carboxylated mucopolysaccharides as well as sulfated and carboxylated glycoproteins, any disruption results in reduction in the dye binding, which can be quantitated. The gastric mucin of stomach tissue was decreased to 17 and 16 mg/g in swim stress/ethanol stress-induced ulcerous rats, respectively, when compared to that of controls (45 mg/g) (Table 2). Results were substantiated by observing increased gastric mucin content by monoclonal antibody-based immuno histological studies (Figure 4). Hematoxylin and eosin staining of stomach tissue sections in control animals indicated intact structures (Figure 4A). Ulcer induction showed damage in the mucosal epithelium, destruction of regular glandular organization, very high inflammatory exudates, proliferated fibroblasts, infiltration of leucocytes and cellular debris (Figure 4B). SRPP and ranitidine treated rats showed recovery in the mucosal epithelium, regained glandular structure and mucosal regeneration (Figure 4C and D). Immunohistological analysis clearly revealed the intact mucosal epithelium in the control group (Figure 4E) and complete loss or eradication in ulcer-induced tissue sections (Figure 4F). Complete recovery was observed upon treatment with SRPP (Figure 4G) and partial recovery is depicted during ranitidine treatment (Figure 4H).
| Group, n = 6 | Mucin content (mg/g) | H+, K+-ATPase (μmoles Pi released/mg/h) |
| Healthy | 45.04d± 4.128 | 0.807d± 0.072 |
| Swim stress induced ulcer model | ||
| Swim stress induced | 17.78a± 2.557 | 2.209a± 0.152 |
| SRPP 100 mg/kg b.w. | 27.13b± 4.082 | 1.771b± 0.081 |
| SRPP 200 mg/kg b.w. | 35.35c± 3.221 | 1.601b± 0.091 |
| Ranitidine 50 mg/kg b.w. | 31.42b,c± 2.327 | 1.621b± 0.092 |
| Ethanol stress induced ulcer model | ||
| Ethanol stress induced | 16.32a± 3.821 | 2.621a± 0.211 |
| SRPP 100 mg/kg b.w. | 32.13b± 3.457 | 2.123b± 0.241 |
| SRPP 200 mg/kg b.w. | 39.53b,c± 3.082 | 1.512c± 0.121 |
| Ranitidine 50 mg/kg b.w. | 37.13b± 1.507 | 1.485c± 0.124 |
Tables 3 and 4 indicate antioxidant, antioxidant enzymes and TBARS levels in stomach/liver homogenate and the serum of swim/ethanol stress models. SOD and GPx levels increased in stomach (2 fold) and CAT and GSH decreased (1.8 fold) during stress-induced ulcerous conditions and were normalized upon treatment with SRPP in a dose dependent manner. An approximately 4 fold increase in TBARS levels depicts lipid peroxidation or damage of stomach tissue in ulcerous animals and was recovered up to 80% upon treatment with SRPP. Ranitidine, although showing protection against ulcer, showed no significant improvement in GSH or antioxidant enzyme levels. Similar changes in antioxidant enzymes except catalase was also observed in serum and liver homogenates. A 2 fold increase in TBARS levels was shown in the ulcer condition and, SRPP treatment at 200 mg/kg b.w. showed up to 90% recovery.
| Parameters | Protein (mg/g) | SOD (U/mg) | Catalase (U/mg) | Glutathione Peroxidase (ηmoles/g) | GSH (nmoles/mg) | TBARS ηmoles |
| Stomach | ||||||
| Healthy | 2.23c± 0.21 | 9.86a± 1.1 | 829.2c± 41.6 | 0.21a± 0.009 | 224c± 10.0 | 0.31a± 0.01 |
| Ulcerated | 1.95a± 0.13 | 19.10c± 1.8 | 462.4a± 30.2 | 0.49d± 0.01 | 121a± 18.9 | 1.12c± 0.20 |
| SRPP 100 mg/kg | 1.90a± 0.09 | 16.32b,c± 2.1 | 488.1a,b± 32.8 | 0.34b± 0.02 | 174b± 22.1 | 0.94c± 0.10 |
| SRPP 200 mg/kg | 2.10b± 0.19 | 13.06b± 2.6 | 679.6b± 9.9 | 0.22a± 0.01 | 208c± 16.5 | 0.55a,b± 0.00 |
| Ranitidine | 2.16b± 0.22 | 15.22b± 1.2 | 505.5a,b± 35.5 | 0.39c± 0.01 | 136a± 12.1 | 0.92b± 0.10 |
| Serum | ||||||
| Healthy | 6.62a± 0.51 | 112.3a± 28 | 44.20c± 4.9 | 0.221a± 0.004 | 23.6c± 3.0 | 0.165a± 0.01 |
| Ulcerated | 6.84a± 0.53 | 264.6d± 32 | 22.90a± 3.1 | 0.286c± 0.02 | 11.1a± 1.8 | 0.326d± 0.02 |
| SRPP 100 mg/kg | 6.35a± 0.59 | 201.1c± 36 | 28.63b± 2.3 | 0.298d± 0.03 | 16.5b± 2.1 | 0.261c± 0.03 |
| SRPP 200 mg/kg | 6.95a± 0.48 | 168.2b± 21 | 40.12c± 3.8 | 0.268b± 0.03 | 19.8b,c±12.9 | 0.162a± 0.01 |
| Ranitidine | 6.35a± 0.63 | 196.3b,c±23 | 30.82b± 2.9 | 0.226a± 0.02 | 12.8a± 2.6 | 0.186b± 0.01 |
| Liver | ||||||
| Healthy | 24.2c± 0.31 | 261.5b± 41 | 28.42d± 3.1 | 0.32a± 0.02 | 414c± 51 | 0.98a± 0.13 |
| Ulcerated | 21.9a± 0.23 | 142.4a± 18 | 22.18b,c± 2.6 | 0.58c± 0.05 | 221a± 26 | 2.41d± 0.23 |
| SRPP 100 mg/kg | 23.1b± 0.28 | 164.2a± 13 | 19.63b,c± 2.4 | 0.36a,b± 0.03 | 315b± 36 | 1.84c± 0.16 |
| SRPP 200 mg/kg | 23.9b± 0.28 | 361.5d± 39 | 15.54a± 2.1 | 0.28a± 0.02 | 214a± 24 | 1.26b± 0.11 |
| Ranitidine | 23.6b± 0.26 | 314.4c,d± 36 | 17.34a± 1.9 | 0.32a± 0.02 | 254a± 28 | 1.41b± 0.12 |
| Parameters | Protein (mg/g) | SOD (U/mg) | Catalase (U/mg) | Glutathione Peroxidase (ηmoles/g) | GSH (U/mg) | TBARS ηmoles |
| Stomach | ||||||
| Healthy | 2.23a± 0.21 | 09.86a± 1.1 | 829.2c± 41.6 | 0.21a± 0.009 | 224d± 23.2 | 0.31a± 0.1 |
| Ulcerated | 2.32a± 0.09 | 17.86c± 2.4 | 201.5a± 18.9 | 0.30c± 0.01 | 102a± 12.6 | 1.26d± 0.3 |
| SRPP 100 mg/kg | 2.16a± 0.16 | 16.21c± 1.0 | 193.3a± 62.5 | 0.26b± 0.01 | 162b± 15.5 | 0.92c± 0.1 |
| SRPP 200 mg/kg | 2.41a± 0.20 | 11.09b± 1.0 | 540.5b± 40.2 | 0.33c± 0.02 | 196c± 16.4 | 0.54b± 0.1 |
| Ranitidine | 2.42a± 0.19 | 12.42b±1.4 | 468.6c± 31.6 | 0.22a± 0.03 | 152b± 16.3 | 0.96c± 0.2 |
| Serum | ||||||
| Healthy | 6.62a± 0.51 | 112.3a± 28 | 44.20c± 4.9 | 0.221a± 0.04 | 23.6d± 3.0 | 0.165a± 0.01 |
| Ulcerated | 6.52a± 0.69 | 282.3d± 26 | 28.36a± 3.2 | 0.315c± 0.03 | 9.6a± 1.2 | 0.465d± 0.03 |
| SRPP 100 mg/kg | 6.35a± 0.70 | 228.4c± 32 | 34.25a,b± 3.3 | 0.286b± 0.03 | 18.6c± 2.2 | 0.321c± 0.04 |
| SRPP 200 mg/kg | 6.24a± 0.56 | 172.3b± 2 | 39.60b± 4.51 | 0.243b± 0.02 | 18.2c± 1.9 | 0.181a± 0.02 |
| Ranitidine | 6.32a± 0.69 | 210.7c±28 | 34.12ab± 4.6 | 0.252b± 0.03 | 14.6b± 1.6 | 0.214a,b± 0.02 |
| Liver | ||||||
| Healthy | 24.2a± 0.31 | 261.5b± 1.1 | 28.42c± 3.1 | 0.32b± 0.02 | 414c± 51 | 0.98a± 0.13 |
| Ulcerated | 24.3a± 0.31 | 118.1a± 16 | 19.64b± 2.2 | 0.48b,c± 0.03 | 392b,c± 41 | 2.98d± 0.31 |
| SRPP 100 mg/kg | 23.5a± 0.21 | 121.8a± 15 | 18.32b± 1.6 | 0.39b± 0.03 | 268b± 25 | 2.15c± 0.22 |
| SRPP 200 mg/kg | 26.4a± 0.41 | 325.4c± 34 | 13.17a± 1.6 | 0.29a± 0.02 | 241a,b± 28 | 1.65b± 0.14 |
| Ranitidine | 26.8a± 0.29 | 254.5b± 26 | 14.24a± 1.8 | 0.31a± 0.03 | 211a± 28 | 1.61b± 0.16 |
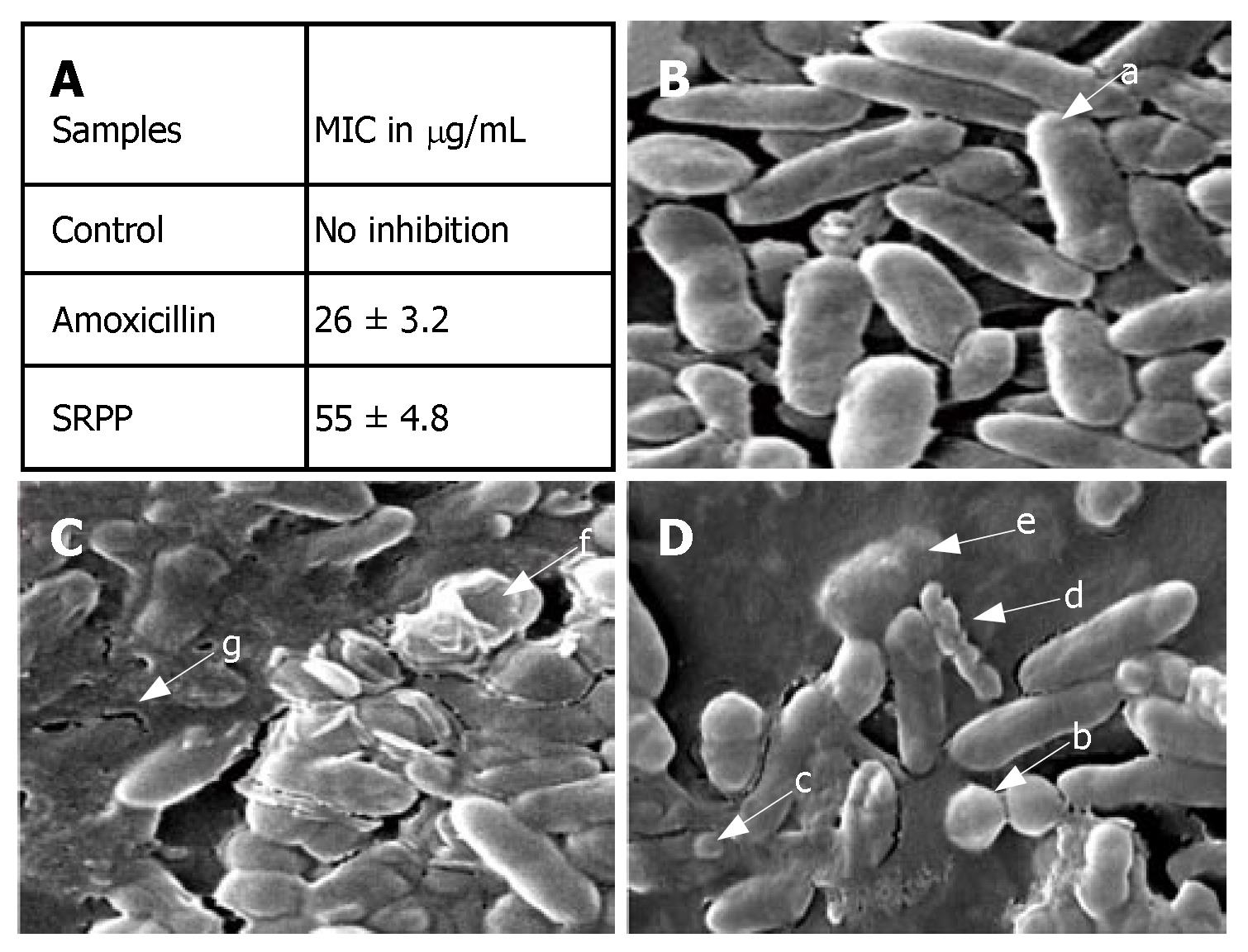
Initially, anti-H pylori activity was assayed by a viable colony count method. SRPP showed up to 95% inhibition at a 200 μg/mL concentration, which is equivalent to that of a susceptible antibiotic amoxicillin at 10 μg/mL. MIC, determined by a broth dilution method, indicated significant anti-H pylori activity at 55 μg/mL (P = 0.003) (Figure 5A).
Normal H pylori shows uniform rod shaped cells (Figure 5B), whereas the cells treated with SRPP (200 μg/mL) changed from a helical form to coccoid and became necrotic (showed in arrows in Figure 5C). A similar coccoid form was observed with H pylori treated with amoxicillin (Figure 5D) and this form has been known to result in a loss of infecti-vity[30]. A coccoid form with blebs in the bacterial surface, appearance of vacuoles, granules and an area of low electron density in the cytoplasm (shown in arrow marks) were observed in SRPP treated samples indicating the lysis of H pylori. Substantiating this viable colony test indicates the loss of more than 95% viability upon treatment with SRPP, supporting an antimicrobial nature of SRPP.
An approximately 3 fold increase in H+, K+-ATPase activity in ulcer-induced stomach homogenate was brought to normal levels in a concentration dependent manner by SRPP at 100 and 200 mg/kg b.w. Approximately 58% and 62% (1.5 fold) were reduced at 200 mg/kg b.w. in both ethanol and swim stress-induced ulcer models (Table 2).
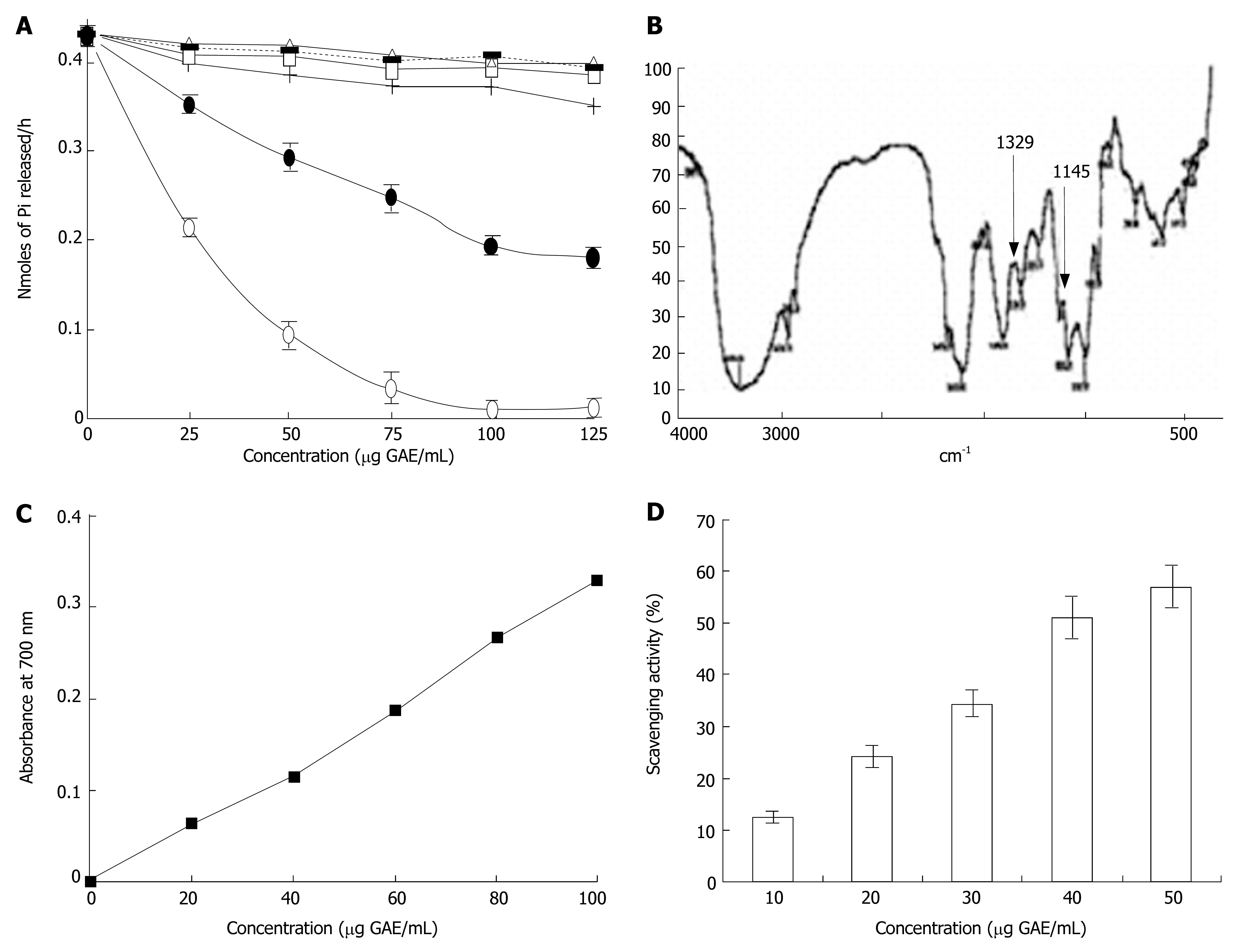
To further validate the inhibition of H+, K+-ATPase enzyme by SRPP, sheep stomach parietal cells were used. Inhibition of H+, K+-ATPase in vitro was examined with different polysaccharide fractions of swallow root including SRPP. Only SRPP inhibited H+, K+-ATPase activity, with an IC50 of 77 μg as opposed to that of Lansoprazole (19.3 μg), whereas other polysaccharide fractions did not show inhibitory activity (Figure 6A).
FTIR spectra obtained using a FTIR spectrometer (Perkin-Elmer 2000 spectrophotometer) equipped with TGS detector with solid samples at a concentration of 1-10 mg provides a signal at 1329 and 1145 cm-1 indicating the presence of sulfonamides where sulfate may be found attached to aminosuagars of pectic polysaccharide (Figure 6B).
We evaluated its phenolic content and subsequently its antioxidant property. 0.12 g GAE/g of SRPP yielding 12% of phenolics in SRPP is intriguing since this is the first report of pectic polysaccharides containing such a high level of phenolics. This could be due to the presence of higher levels of phenolics (34 mg/g) in swallow root per se. Presence of higher levels of phenolics was substantiated by expression of potent reducing power ability with 3200 absorbance Units/g of SRPP (Figure 6C). In addition, dose dependent free radical scavenging activity was also found (Figure 6D) with an IC50 of 40 μg/mL.
Recently, phytomedicines from medicinal plants and nutraceuticals from food sources have become attractive sources of new and natural drugs. However, the active ingredients and mode of action have been rarely established, which is very crucial for understanding the long-term potency of these antiulcer sources. Among the majority of identified sources, flavonoids[11,31], and occasionally polysaccharides, have frequently been implicated as antiulcer agents[12-14]. We previously reported on a non-toxic, edible antioxidant source[17] - Decalepis hamiltonii, a significant antiulcerogenic properties in vitro and in vivo. High levels of antioxidant properties, probably just little less than that found in green tea, with multiple compounds[32] may play a critical role in inhibiting oxidative induced mucosal damage in ulcers[33]. In the current paper, we report the antiulcerogenic potential of a combinational molecule, which is a pectic polysaccharide with bound phenolics from swallow root. In human nutrition, pectic polysaccharides play a key role as low energy foods and break down products have been known to have health beneficial properties.
Gastric ulcers have multiple etiopathogeneses. Stress ulcers are due to both physiological and psychological factors, which affect gastrointestinal defense and increased accumulation of acid due to influx of H+ into the lumen of the stomach by parietal cell plasma membrane bound H+, K+-ATPase leading to autodigestion of the gastric mucosa[34], and generation of free radicals. Ethanol stress, on the other hand, is known to act on the gastric mucin directly, affecting mucosal defense. Nevertheless, in both cases the causes of severe ulcerations are depicted in the current study in addition to observations from other investigators[35,1]. Our earlier studies indicated that phenolic antioxidants were efficient in inhibiting upregulated H+, K+-ATPase and recovering the depleted levels of antioxidant and antioxidant enzymes[17].
The effect of SRPP on gastric ulcers induced by swim and ethanol stress was investigated in in vivo rat models. Oral administration of 100 and 200 mg/kg b.w. reduced gastric lesions. It is evident from our data (Figures 2-4 and Tables 2-4) that swim stress and ethanol stress induced gastrointestinal effects, such as gastric erosions, gastric or duodenal ulcerations, gastrointestinal hemorrhages and perforations. These effects were modulated by the inhibition of upregulated H+, K+-ATPase, and enhancement of down regulated gastric mucin, antioxidant and antioxidant enzyme levels. Histological studies indicated that characteristic ulcerogenic pathogenicity, with a distinct ulcer margin formed by the adjacent non-necrotic mucosa, the epithelial component, and granulation tissue at the ulcer base, was normalized upon treatment with SRPP. Current data, together with the results of our previous paper[17], indicate clearly that phenolic antioxidants of SRPP may contribute to H+, K+-ATPase inhibition, rather than the polysaccharide per se since swallow root antioxidants inhibited H+, K+-ATPase at 36 μg/mL as apposed to that of SRPP (77 μg/mL).
Ethanol induced gastric lesions are thought to arise as a result of direct damage to gastric mucosal cells, resulting in the development of free radicals and hyperoxidation of lipids. Recently, it was discovered that Solanum nigrum extract provides significant antioxidant activity as one of the possible gastroprotective mechanisms against ethanol-induced gastric ulceration[19]. SRPP may also act similarly in reducing ulcerations in stomach since it showed potent antioxidant properties.
In addition, SRPP is a safer source since toxicity studies indicated no lethal effect up to an oral dose of 1 g/kg b.w. for 14 d. To understand the potential role of SRPP in gastric mucosal protection, it is important to know that mucin is an insoluble adherent mucus gel, which is quite stable and has significant buffering capacity for neutralization of luminal acid in the presence of bicarbonate. SRPP showed 2 fold upregulation of gastric mucin as revealed by immunohistological/biochemical and ELISA methods, indicating the stabilization of the mucosal layer.
Further, SRPP possessed H+, K+-ATPase inhibitory activity, although not as potent as that of phenolic fractions. Phenolics present in SRPP together with those reported in the literature revealed that phenolic antioxidants are potent H+, K+-ATPase blockers[11]. The significant levels of phenolics present in SRPP may also contribute towards inhibition of H+, K+-ATPase activity, which plays a tremendous role in reducing an acidic condition in the gastric lumen.
Results are intriguing that SRPP also showed potential anti-H pylori activity. The results are in accordance with the observation made by Lee et al[36], where inhibition of H pylori growth by pectic polysaccharide was reported. However the mechanism still needs to be established. Several mechanisms may be proposed for potential inhibition of H pylori by SRPP. SRPP phenolics may inhibit microbial activity as phenolics were thought to exert their antimicrobial effect by causing (1) hyper acidification at the plasma membrane interface of the micro organism, or (2) intracellular acidification, resulting in the disruption of H+, K+-ATPase required for ATP synthesis of microbes, or (3) may be related to inactivation of cellular enzymes causing membrane permeability changes[10,37]. The rate of inactivation of microbial cellular enzymes is dependent on the rate of penetration of phenolic antioxidants into the cell. In the case of H pylori, phenolics may be inactivating the urease enzyme, which is specifically expressed at its surface to neutralize hyperacidification to survive in the gastric environment of the stomach[38]. It is thus clear that SRPP is creating a cavity in the organism (Figure 5C) with the loss of cellular contact resulting in loss of viability of H pylori.
There are several schools of thought that indicate the ulcer healing component must be proliferative, amplify cell migration, and enhance angiogenesis in order to enhance re-epithelialization in the ulcer healing process. However, antiulcer compounds with proliferative ability, and the ability to enhance angiogenesis, may be carcinogenic also. This statement is also substantiated by observation of induction of cancer upon the usage of antiulcer drugs on a long-term basis[8]. In this context, SRPP, although found to be antiulcerogenic, has been shown to be anticancerous (Unpublished observation, 2006). Hence the treatment of ulcer by SRPP even for longer periods of time may not pose side effects.
Generally antioxidants have been known to be antimi-crobial by binding to the microbial membrane leading to disruption[39]. SRPP, by virtue of phenolics, may be antimicrobial. In addition, SRPP may also participate in enhancement of gastric mucin. The enhancement of gastric mucin contents, as measured by ELISA and Alcian blue binding, may suggest that enhancement is most probably due to prevention/protection of mucosal injury during ulceration rather than direct increase in synthesis. This is supported by no upregulation of gastric mucin in SRPP controls where animals were fed with SRPP without inducing ulcers. However, regulated synthesis might occur, which may be evaluated by tracer techniques. Antioxidant potency may also be contributed by both phenolics and sulfonamide groups containing polysaccharides[40,41]. Further, it is also possible that SRPP, by virtue of its anionic nature, may bind effectively to positively charged amino acid residues of gastric mucin as well as sucralfate and other polysaccharides[42]. This binding may avoid gastric mucin damage and subsequent ulceration. SRPP thus can be a safe and promising multi-step ulcer blocker (Figure 1).
The authors thank Dr V Prakash, Director, Central Food Technological Research Institute, Mysore for his keen interest in the work and encouragement. Authors are thankful to Karnataka Cardio Diagnostic Centre; Mysore for providing H pylori. One of the authors Dr. Shylaja M Dharmesh acknowledges Department of Biotechnology, New Delhi, India for financial assistance. Mr. Srikanta BM and Mr. Siddaraju MN thank Council of Scientific and Industrial Research, India for Senior Research fellowships.
Ulcer is a major global problem affecting day to day life in humans and the causative factors being unavoidable such as stress, use of non-steroidal anti-inflammatory drugs; and the side effects of available antiulcer drugs; alternatives that are safer, but effective in ulcer prevention must be envisaged.
Frontiers of research on development of antiulcer drugs must emphasize on (a) detection and diagnosis of H pylori-a major ulcerogen; (b) identification of ulcerogens and antiulcerogens from diet since some components of food are ulcerogens; (c) antiulcer drugs with less or no side effects, so that it can be used by subjects who are using NSAIDS and also alcohol.
Herbal/dietary sources are the challenging alternatives for potential ulcer management. Since herbal medicines include extracts from either edible or non-edible source, side effect has been a threat. Dietary sources are therefore optional. We report a novel potent multi-step ulcer blocker which inhibits acid secretion/growth and invasion of H pylori and enhances mucosal defense.
The antiulcer component identified is inexpensive, effective and nontoxic; hence can be directly applied to human health. Single compound with multi-potency implies a potential reduction in the drug load during ulcer treatment.
Ulcer, H+,K+-ATPase, pectic polysaccharide, H pylori, antioxidant, gastric mucin, mucosal injury, non-steroidal anti-inflammatory drugs.
This is a nicely done description of the effects of SRPP. Through in vivo studies, the authors concluded that SRPP with defined sugar composition and phenolics exhibited multi-potent free radical scavenging, antioxidant, anti-H pylori, inhibition of H+, K+-ATPase and gastric mucosal protective activities.
S- Editor Zhu LH L- Editor Lutze M E- Editor Liu Y
| 1. | Miller TA. Mechanisms of stress-related mucosal damage. Am J Med. 1987;83:8-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 389] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | Langman MJ, Brooks P, Hawkey CJ, Silverstein F, Yeomans N. Non-steroid anti-inflammatory drug associated ulcer: epidemiology, causation and treatment. J Gastroenterol Hepatol. 1991;6:442-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Khanna S, Madan M, Vangoori A, Banerjee R, Thaimattam R, Jafar Sadik Basha SK, Ramesh M, Casturi SR, Pal M. Evaluation of glycolamide esters of indomethacin as potential cyclooxygenase-2 (COX-2) inhibitors. Bioorg Med Chem. 2006;14:4820-4833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Phull PS, Green CJ, Jacyna MR. A radical view of the stomach: the role of oxygen-derived free radicals and anti-oxidants in gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995;7:265-274. [PubMed] [Cited in This Article: ] |
| 6. | Odenbreit S. Adherence properties of Helicobacter pylori: impact on pathogenesis and adaptation to the host. Int J Med Microbiol. 2005;295:317-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Bandyopadhyay U, Biswas K, Chatterjee R, Bandyopadhyay D, Chattopadhyay I, Ganguly CK, Chakraborty T, Bhattacharya K, Banerjee RK. Gastroprotective effect of Neem (Azadirachta indica) bark extract: possible involvement of H(+)-K(+)-ATPase inhibition and scavenging of hydroxyl radical. Life Sci. 2002;71:2845-2865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Waldum HL, Gustafsson B, Fossmark R, Qvigstad G. Antiulcer drugs and gastric cancer. Dig Dis Sci. 2005;50 Suppl 1:S39-S44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Sathyavathi GV, Gupta AK, Tandon N. Medicinal plants of India. New Delhi, India, Indian Council of Medical Research (ICMR). 1987;2. [Cited in This Article: ] |
| 10. | Sung-Sook C, Dhiraj , AV , Yuan-Tonglin , Shetty K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 2005;40:809-816. [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Reyes-Chilpa R, Baggio CH, Alavez-Solano D, Estrada-Muñiz E, Kauffman FC, Sanchez RI, Mesia-Vela S. Inhibition of gastric H+,K+-ATPase activity by flavonoids, coumarins and xanthones isolated from Mexican medicinal plants. J Ethnopharmacol. 2006;105:167-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Ye YN, So HL, Liu ES, Shin VY, Cho CH. Effect of polysaccharides from Angelica sinensis on gastric ulcer healing. Life Sci. 2003;72:925-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Matsumoto T, Sun XB, Hanawa T, Kodaira H, Ishii K, Yamada H. Effect of the antiulcer polysaccharide fraction from Bupleurum falcatum L. on the healing of gastric ulcer induced by acetic acid in rats. Phytother Res. 2002;16:91-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Gao Y, Tang W, Gao H, Chan E, Lan J, Zhou S. Ganoderma lucidum polysaccharide fractions accelerate healing of acetic acid-induced ulcers in rats. J Med Food. 2004;7:417-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Phatak L, Chang KC, Brown G. Isolation and characterization of pectin in sugar-Beet pulp. J Food Sci. 1988;53:830-833. [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 119] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Siddaraju MN, Dharmesh SM. Inhibition of gastric H+, K+-ATPase and Helicobacter pylori growth by phenolic antioxidants of Zingiber officinale. Mol Nutr Food Res. 2007;51:324-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Naik Y, Jayaram S, Harish Nayaka MA, Lakshman SM. Gastroprotective effect of swallow root (Decalepis hamiltonii) extract: possible involvement of H(+)-K(+)-ATPase inhibition and antioxidative mechanism. J Ethnopharmacol. 2007;112:173-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Brady PS, Brady LJ, Ullrey DE. Selenium, vitamin E and the response to swimming stress in the rat. J Nutr. 1979;109:1103-1109. [PubMed] [Cited in This Article: ] |
| 19. | Jainu M, Devi CS. Antiulcerogenic and ulcer healing effects of Solanum nigrum (L.) on experimental ulcer models: possible mechanism for the inhibition of acid formation. J Ethnopharmacol. 2006;104:156-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Kulkarni SK, Goel RK. Gastric antiulcer activity of UL-409 in rats. Indian J Exp Biol. 1996;34:683-688. [PubMed] [Cited in This Article: ] |
| 21. | Das D, Banerjee RK. Effect of stress on the antioxidant enzymes and gastric ulceration. Mol Cell Biochem. 1993;125:115-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 148] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Corne SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol. 1974;242:116-117. [PubMed] [Cited in This Article: ] |
| 23. | Flohé L, Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1050] [Cited by in F6Publishing: 1027] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 24. | Aebi HE. Catalase. Methods of Enzymatic Analysis. Florida: Verlag Chemie 1983; 273-286. [Cited in This Article: ] |
| 25. | Flohe L, Gunzler WA. Assay of glutathione peroxidase. Methods in Enzymology. New York: Academic Press 1984; 114-121. [Cited in This Article: ] |
| 26. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] [Cited in This Article: ] |
| 27. | Testerman TL, McGee DJ, Mobley HL. Helicobacter pylori growth and urease detection in the chemically defined medium Ham's F-12 nutrient mixture. J Clin Microbiol. 2001;39:3842-3850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | O'Mahony R, Al-Khtheeri H, Weerasekera D, Fernando N, Vaira D, Holton J, Basset C. Bactericidal and anti-adhesive properties of culinary and medicinal plants against Helicobacter pylori. World J Gastroenterol. 2005;11:7499-7507. [PubMed] [Cited in This Article: ] |
| 29. | Kai J, Satoh M, Tsukidate K. A new method for preparing electron microscopic specimens of Helicobacter pylori. Med Electron Microsc. 1999;32:62-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Kusters JG, Gerrits MM, Van Strijp JA, Vandenbroucke-Grauls CM. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect Immun. 1997;65:3672-3679. [PubMed] [Cited in This Article: ] |
| 31. | Zayachkivska OS, Konturek SJ, Drozdowicz D, Konturek PC, Brzozowski T, Ghegotsky MR. Gastroprotective effects of flavonoids in plant extracts. J Physiol Pharmacol. 2005;56 Suppl 1:219-231. [PubMed] [Cited in This Article: ] |
| 32. | Harish R, Divakar S, Srivastava A, Shivanandappa T. Isolation of antioxidant compounds from the methanolic extract of the roots of Decalepis hamiltonii (Wight and Arn.). J Agric Food Chem. 2005;53:7709-7714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Repetto MG, Llesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res. 2002;35:523-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 294] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 34. | Goel RK, Bhattacharya SK. Gastroduodenal mucosal defence and mucosal protective agents. Indian J Exp Biol. 1991;29:701-714. [PubMed] [Cited in This Article: ] |
| 35. | Hollander D, Tarnawski A, Krause WJ, Gergely H. Protective effect of sucralfate against alcohol-induced gastric mucosal injury in the rat. Macroscopic, histologic, ultrastructural, and functional time sequence analysis. Gastroenterology. 1985;88:366-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 108] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Lee JH, Shim JS, Lee JS, Kim MK, Chung MS, Kim KH. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr Res. 2006;341:1154-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Vattem DA, Lin YT, Ghaedian R, Shetty K. Cranberry synergies for dietary management of Helicobacter pylori infections. Process Biochem. 2005;40:1583-1592. [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | McGowan CC, Cover TL, Blaser MJ. Helicobacter pylori and gastric acid: biological and therapeutic implications. Gastroenterology. 1996;110:926-938. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res. 2006;40:223-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in F6Publishing: 326] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 40. | Qi H, Zhang Q, Zhao T, Chen R, Zhang H, Niu X, Li Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol. 2005;37:195-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 41. | Zhang Q, Li N, Liu X, Zhao Z, Li Z, Xu Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr Res. 2004;339:105-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Rees WD. Mechanisms of gastroduodenal protection by sucralfate. Am J Med. 1991;91:58S-63S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |