Published online Oct 14, 2007. doi: 10.3748/wjg.v13.i38.5065
Revised: June 21, 2007
Accepted: June 26, 2007
Published online: October 14, 2007
AIM: To study the mechanism involved in the potentially beneficial effect of ultra low dose aspirin (ULDA) in prehepatic portal hypertension, rats were pretreated with selective COX 1 or 2 inhibitors (SC-560 or NS-398 respectively), and subsequently injected with ULDA or placebo.
METHODS: Portal hypertension was induced by portal vein ligation. Platelet activity was investigated with an in vivo model of laser induced thrombus production in mesenteric circulation and induced hemorrhagic time (IHT). Platelet aggregation induced by ADP and dosing of prostanoid products 6-keto-PGF1α, TXB2, PGE2 and LTB4 were also performed.
RESULTS: The portal hypertensive group receiving a placebo showed a decreased in vivo platelet activity with prolonged IHT, an effect that was normalized by ULDA. SC-560 induced a mild antithrombotic effect in the normal rats, and an unmodified effect of ULDA. NS-398 had a mild prothrombotic action in portal hypertensive rats, similar to ULDA, but inhibited a further effect when ULDA was added. An increased 6-keto-PGF1α was observed in portal hypertensive group that was normalised after ULDA administration. TXA2 level after ULDA, remained unchanged.
CONCLUSION: These results suggest that the effect of ULDA on platelet activity in portal hypertensive rats, could act through a COX 2 pathway more than the COX 1, predominant for aspirin at higher doses.
- Citation: Eizayaga FX, Aguejouf O, Desplat V, Belon P, Doutremepuich C. Modifications produced by selective inhibitors of cyclooxygenase and ultra low dose aspirin on platelet activity in portal hypertension. World J Gastroenterol 2007; 13(38): 5065-5070
- URL: https://www.wjgnet.com/1007-9327/full/v13/i38/5065.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i38.5065
Portal hypertension is a major complication of chronic liver disease. In its pathophysiology, increased hepatic resistance is followed by a hyperdynamic circulatory state[1]. This hyperdynamic state, in which nitric oxide (NO) and prosatcyclin (PGI2) are important vasoactive substances, induces a decreased platelet activity, even in the absence of hepatic damage. Although NO plays an important role in modifying platelet adhesion in portal hypertension[2], PGI2, a powerful vasodilator prostanoid with antithrombotic properties, was also found increased in mesenteric vascular bed[3].
Ultra low dose aspirin (ULDA) has shown proth-rombotic activity when analysed in humans and in the interface platelet-endothelial cell[4,5]. Previous papers have shown the potentially beneficial effect of ULDA in portal hypertensive animals normalizing altered platelet activity and induced hemorrhagic time[6]. Further experiments were performed to clarify if this effect was mediated mostly by a cyclooxigenase (COX) pathway or by modifying NO synthesis, the two aspirin mechanisms of action[7], although a previous study with a different model suggested that ULDA could decrease PGI2 synthesis[8]. The same in vivo Laser induced thrombus formation, was used in portal hypertensive rats to investigate the effects of Indomethacin, a non selective COX inhibitor, and L-Nitro Arginin Methyl Ester (NAME), a non selective inhibitor of NO production. The results suggested that the effects of ULDA were more influenced by COX pathway than by NO synthesis inhibition[9]. Addition of ULDA in portal hypertensive rats, when previously inhibiting COX with Indomethacin, increased number of emboli and duration of embolization but blunted the normalization of induced hemorrhagic time, suggesting probably a more selective action on COX 1 or COX 2 pathway. TXA2, the main product of arachidonic acid via the activity of COX 1 in platelets, increases platelet aggregation and its synthesis is inhibited by Aspirin. PGE2 and PGI2 are produced by COX 1 and 2 mainly in endothelial cells. PGE2 has no probable role in portal hemodynamic changes observed in portal hypertensive rats[10], but depending on its concentration, it can modulate platelet aggregation by regulating intracellular levels of cAMP[11].
The present investigation was designed to clarify the mechanism of the effects of ULDA in portal hypertension, by using previous selective inhibition of cyclooxygenase COX 1 or COX 2 (with SC-560 and NS-398 respectively). Models of Laser induced Thrombosis and IHT were used and plasmatic levels of 6-keto-PGF1α (the stable metabolite of PGI2), PGE2, TXB2 (the stable metabolite of TXA2) and LTB4 were determined.
Male Wistar rats (200-250g) purchased from Delpre Breeding Center (St. Doulchard, France) were housed separately and acclimatized before use under conditions of controlled temperature (25 ± 2°C) and illumination (12 h light/dark cycle). They were fed with standard rat chow and water ad libitum. Animals received care in compliance with the European Convention of Animal Care.
After one week of acclimatization, rats were randomized and separated into two groups: one consisted of sham-operated (Sh) rats and the other formed by portal hypertensive (PH) rats. Portal hypertension was induced by a calibrated portal vein stenosis, according to the procedure described by Vorobioff et al[1].
Rats were anesthetized with Ketamine (Panpharma, Fougères, France) 90 mg/kg body weight, i.m. and then a midline abdominal incision was made. The portal vein was located and isolated from the surrounding tissues. A ligature of 3-0 silk was placed around the vein and snugly tied to a 20 gauge blunt end needle placed along side the portal vein. The needle was subsequently removed to yield a calibrated stenosis of the portal vein. Sham-operated rats underwent an identical procedure except that portal vein was isolated but not stenosed.
Animals were housed during fourteen days after the operation to develop portal hypertension in the corresponding group.
Animals were anaesthetized with 200 mg/kg of thiopental sodium (Pentothal®, Laboratories Abbott, Rungis, France) a median laparotomy was performed. The intestinal loop was placed on the microscope table and vascular lesions were induced by Argon laser (Stabilite 2016, Spectra Physics, France). The wavelength used was 514 nm and the energy was adjusted to 120 mW. The laser beam was applied during 1/15 s. The dynamic-course of thrombus formation was continuously monitored and recorded by placing the laser beam coaxially into the inverted light beam path of the microscope (Axiovert, Zeiss, France). Microscopic images were recorded through a digital camera (DX L107, color camera CCD, Basler, Vision Technologies) to visualize and digitalize data coupled to a Dell monitor. A schematic view of the apparatus used has been previously described[12]. Arterioles between 15 and 25 μm diameter were used. The parameters assessed were the number of emboli removed by blood flow and the duration of embolisation (time between the first and the last emboli occurring during a 10 min observation period).
An experimental model of induced hemorrhagic time (IHT) was performed 10 min before thrombosis induction by laser. The tail of the rat was immersed in water for 5 min at 37°C and sectioned 6 mm from the extremity. IHT measured, corresponded to the time between the tail section and the end of bleeding, expressed in seconds.
Platelet aggregation study: Platelet aggregation was made according to the method of Cardinal and Flower on a Chrono Log 500 VS aggregometer (Coultronics, Margency, France) on the whole blood obtained from the rat after laser experimentation. Platelet aggregation was induced by ADP final concentration 5 μmol/L (Laboratoire Diagnostica, Stago, France). Two parameters were determined: Impedance, representing the maximum amplitude of aggregation expressed in Ohms. Velocity of aggregation expressed in ohms/min.
Prostanoids determinations: At the end of each experiment, blood was collected by cardiac puncture and centrifuged for 20 min at 4000 r/min to obtain Platelet Poor Plasma (PPP). Concentrations of 6-keto-PGF1α, TXB2, PGE2 and LTB4 were determined in plasma samples using competitive binding Elisa tests (R&D Systems Europe, Abingdon, UK) according to the manufacturer’s instructions.
The Aspirin solution was purchased from Boiron Laboratories (Sainte-Foy-Les-Lyon, France). ULDA was prepared as follows: 1 g of pure, finely powdered aspirin was suspended in 99 mL of alcohol (70º). After being vigorously shaken, 1 mL of this dilution was then mixed with 99 mL of distilled water and vigorously shaken. The last process was repeated 13 more times[11]. Alcohol and sterilized water following the above cited procedures without adding the Aspirin was used as control. ULDA or placebo were subcutaneously administered at a final volume of 1 mL/kg rat weight.
Selective inhibitors of COX 1, SC-560 and of COX 2, NS-398 were purchased from Cayman Chemical, (Ann Arbor Michigan, USA). They were administered per os at a dose of 10 mg/kg rat weight, suspended in Carboxymethylcellulose (CMC) 5 g/L at a final volume of 1 mL/kg rat weight. The CMC solution was used as placebo.
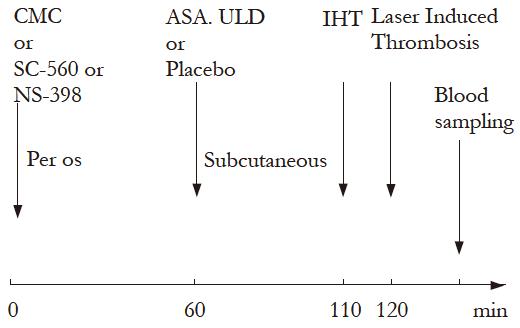
Fourteen days after the corresponding operation, 216 rats were randomly assigned in 12 groups and treated as follows:
Math 1
Groups, procedures and treatments are detailed in Table 1.
| Group | Procedure and treatments | ||
| Phase 1: 14 dbefore treatments | Phase 2: 120 minbefore experiment | Phase 3: 60 minbefore experiment | |
| ShP | Sham operated | Placebo (CMC) | Placebo (H2O) |
| PHP | Portal hypertension | Placebo (CMC) | Placebo (H2O) |
| PHPAs | Portal hypertension | Placebo (CMC) | ULDA |
| ShP | Sham operated | SC-560 | Placebo (H2O) |
| PHP | Portal hypertension | SC-560 | Placebo (H2O) |
| PHPAs | Portal hypertension | SC-560 | ULDA |
| ShP | Sham operated | NS-398 | Placebo (H2O) |
| PHP | Portal hypertension | NS-398 | Placebo (H2O) |
| PHAs | Portal hypertension | NS-398 | ULDA |
Data are expressed as mean ± SEM and compared using one way analysis of variance (ANOVA) followed by Bonferroni multiple comparison test. A value of P < 0.05 was considered significant. Statistical calculations were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego California USA, http://www.graphpad.com).
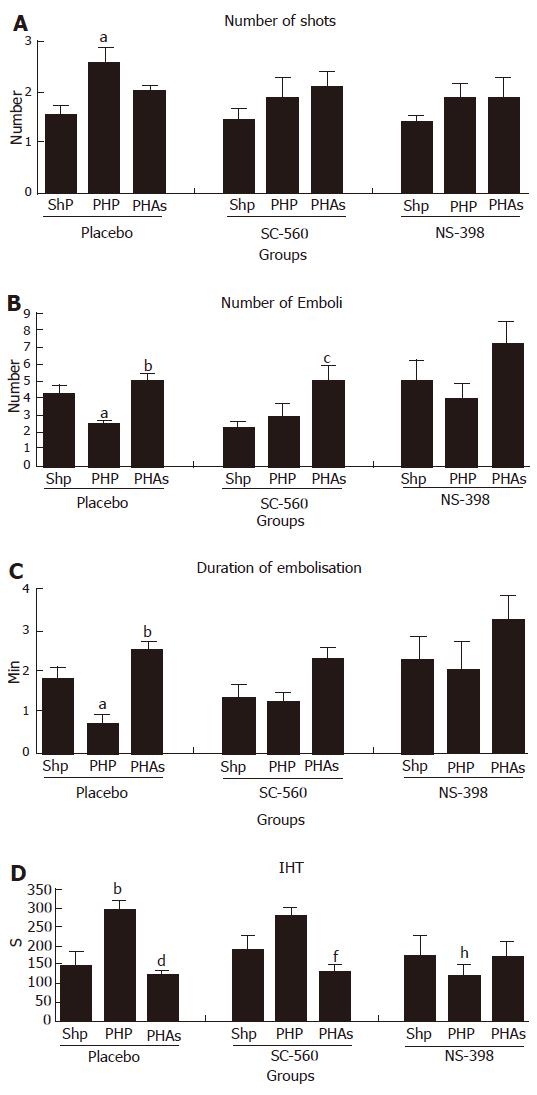
Groups with placebo (CMC): Portal hypertension decreased the number of emboli (P < 0.05) and the duration of embolisation (P < 0.05) and prolonged the Induced Hemorrhagic Time (P < 0.01). ULDA induced significant modifications to normalize these values (P < 0.01, P < 0.001 and P < 0.001 for Number of Emboli, Duration of Embolisation and IHT respectively). An increased number of shots needed to start the embolisation was observed in the portal hypertensive group, and this effect also normalised after ULDA (P < 0.05).
Effects of SC-560, selective inhibitor of COX 1: After the inhibition of COX 1, a non significant antithrombotic effect was observed in the sham operated group, and no further change in cirrhotic group. After ULDA, the prothrombotic effect remained active for Number of Emboli (P < 0.05) and IHT (P < 0.01) despite the opposite effect of pre-treatment with SC-560. No changes were observed in number of shots.
Effects of NS-398, selective inhibitor of COX 2: The pre-treatment with NS-398 induced a decreased induced hemorrhagic time in portal hypertensive rats, similar to the effect of ULDA without COX inhibitors. No effect of ULDA was seen after COX 2 inhibition (Figure 1A-D).
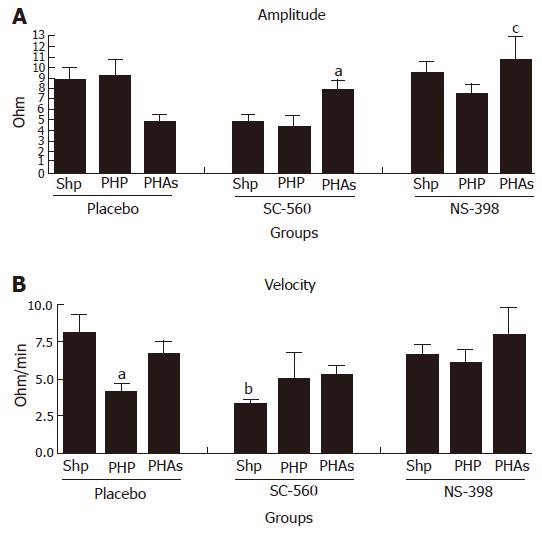
Platelet aggregation induced by ADP (Figure 2A and B): Platelet aggregation has shown a decreased velocity in portal hypertensive animals and in sham operated animals pretreated with SC-560. An increased velocity was observed in portal hypertensive animals pretreated with SC-560 or NS-398 and with ULDA. None of the observed alterations were found in Amplitude and in Velocity.
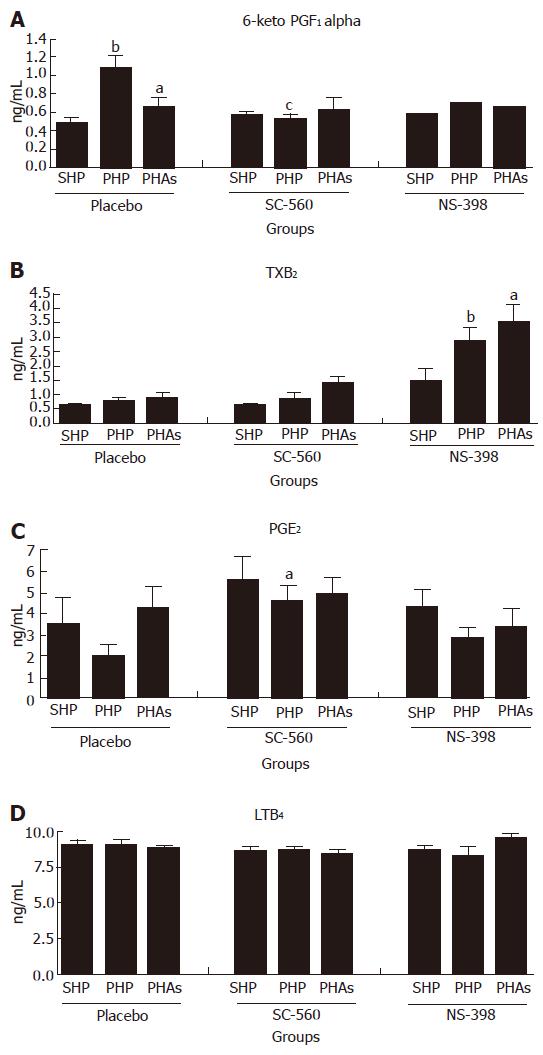
Variations in metabolites of arachidonic acid (Figure 3A-D): 6 keto PGF1α (Figure 3A): This stable metabolite of PGI2 was found increased in portal hypertensive rats (P < 0.01). This increase was normalised by ULDA (P < 0.05) or SC-560 (P < 0.05).
TXB2 (Figure 3B): Inhibition of COX 2 with NS-398 produced an increased level of TXB2 in portal hypertensive animals (XI group), that was not modified by ULDA(P < 0.05 and 0.001 respectively).
PGE2 (Figure 3C): An increase in PGE2 was observed in portal hypertensive (PHP) group, treated with SC-560 (P < 0.05).
LTB4 (Figure 3D): Dosage of LTB4, a lipoxygenase (LOX) metabolite of arachidonic acid, was performed as control of experiment. No variations of production were found when comparing the different groups.
ULDA has shown prothrombotic effects with an in vivo model, testing the interaction between platelet and endothelial cells[5,12]. Exploration of this effect in portal hypertensive rats revealed a decreased number of emboli as well as duration of embolisation and a prolonged IHT that were normalised by ULDA administration. In the search of an explanation for the mechanism involving this effect, previous publications of L-NAME (an inhibitor of Nitric Oxide synthesis) and Indomethacin (a nonselective COX inhibitor) effects on portal hypertensive rats showed that the effect of ULDA was more affected by Indomethacin than by NAME9. Moreover, Indomethacin seemed to have contradictory effects. Despite the antithrombotic effect of Indomethacin, the in vivo prothrombotic effect of ULDA (increasing number of emboli and duration of embolization) was increased, and its beneficial effect of reducing IHT in portal hypertensive rats was blocked. The present experiment was done to verify the hypothesis that these contradictory modifications produced by Indomethacin over ULDA’s prothrombotic effect were the result of different ways in which COX 1 and 2 could affect platelet-endothelial cell interaction.
The results found in this study corroborate the previously described effects of ULDA in portal hypertensive rats, normalizing number of emboli, duration of embolisation and the IHT[6,9].
In rats with portal hypertension, an increase in 6-keto PGF1α was observed, probably due to the known increased PGI2 production described for the mesenteric vascular bed in this animal model[3]. The addition of ULDA normalised this effect. As reported in an in vitro model with a vascular fragment, ULDA was active only in vascular fragments with an elevated PGI2 production[8]. This last observation is similar to our present results since the ULDA effect of decreasing 6-keto PGF1α is observed only in the portal hypertensive group.
The administration of SC-560, had a slightly antithrombotic effect in sham operated rats, decreasing non significantly the Number of Emboli. This could be explained by a decrease in platelet synthesis of TXA2[13]. There was a decrease in 6-keto PGF1α in the portal hypertensive group with COX 1 inhibition, and this effect is probably due to the inhibition of the increased production of PGI2[14,15] and COX 1 over-expression observed in this model of portal vein ligation[16-18]. It is interesting to note that COX 1 inhibition had almost not modified the prothrombotic effect of ULDA in portal hypertensive animals and observed as an increase in number of emboli (P < 0.05) and a decrease in IHT (P < 0.01).
The administration of NS-398 had not modified the in vivo parameters (NE, DE and IHT) in the Sham operated group. In the portal hypertensive group, a non significant tendency to increase NE and DE was observed, as well as a statistically significant shortened IHT. After pre-treatment with the selective COX 2 inhibitor, NS-398, ULDA induced no further effect. There is an increase in TXB2 in the portal hypertensive group with COX 2 inhibition, in which a decreased IHT was observed. Factors like trauma-hemorrhage, shear stress and pressure variations or lipopolysaccharide can modify TXA2 synthesis in the liver or in endothelial cells[19-21]. The increased TXB2 was not modified by treatment with ULDA. It is interesting to have, in the portal hypertensive group, an increased PGE2 after COX 1 inhibition and an increased TXB2 after COX 2 inhibition, as if upon COX selective inhibition, the cell switched to a prostanoid produced by the non inhibited COX enzyme.
The effect of ULDA was confirmed in this study with a prehepatic portal hypertension with a normal liver. Further research will clarify if this potentially beneficial effect is produced in rats with cirrhosis and ascities. Other complex interactions can not be evaluated by this study. For example, a recent publication has pointed out that chronic COX inhibition with Indomethacin enhances the collateral vascular responsiveness to Arginin-Vasopressin, which is also able to activate platelets[22,23].
In conclusion, despite that COX 1 inhibition caused a mild antithrombotic effect in sham operated rats, the prothrombotic effect of ULDA was not modified. COX 2 inhibition induced a mild prothrombotic effect over portal hypertensive rats, similar to that observed with ULDA alone confirming data of literature[24] and the addition of ULDA in this group produced no further changes. ULDA induced a decrease in PGI2 in portal hypertensive animals, without modifying TXA2 levels. These results suggested a predominant COX 2 inhibition by ULDA, opposite to the predominant inhibition of COX 1 commonly observed with ASA in usual doses.
Ultra Low Dose Aspirin (ULDA) has shown prothrombotic properties capable of normalizing altered platelet function found in portal hypertension. This effect is clearly the opposite of the actual main use of Aspirin as an antithrombotic drug.
The mechanism of this effect is unknown, but previous publications show changes in this effect after pretreatment with Indomethacin, a widely used non-selective COX inhibitor.
Only inhibition of NO synthesis, and perhaps Vasopressin has shown this property of modifying platelet alterations in portal hypertension.
ULDA could be useful in patients with prehepatic portal hypertension to control or decrease bleeding complications.
Aspirin is widely known as a more powerful inhibitor of COX 1 than COX 2. This is the first paper showing a positive effect of Aspirin in portal hypertension, based in a not yet explained inhibition of COX 2, rather than COX 1.
S- Editor Liu Y L- Editor Alpini GD E- Editor Yin DH
| 1. | Vorobioff J, Bredfeldt JE, Groszmann RJ. Hyperdynamic circulation in portal-hypertensive rat model: a primary factor for maintenance of chronic portal hypertension. Am J Physiol. 1983;244:G52-G57. [PubMed] [Cited in This Article: ] |
| 2. | Albornoz L, Bandi JC, Otaso JC, Laudanno O, Mastai R. Prolonged bleeding time in experimental cirrhosis: role of nitric oxide. J Hepatol. 1999;30:456-460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Hamilton G, Rosza I, Hutton R, Chow FP, Dandona P, Hobbs KE. Portal vein prostacyclin activity in experimental portal hypertension in rats. Clin Sci (Lond). 1981;60:327-329. [PubMed] [Cited in This Article: ] |
| 4. | Doutremepuich C, de Sèze O, Le Roy D, Lalanne MC, Anne MC. Aspirin at very ultra low dosage in healthy volunteers: effects on bleeding time, platelet aggregation and coagulation. Haemostasis. 1990;20:99-105. [PubMed] [Cited in This Article: ] |
| 5. | Lalanne MC, Doutremepuich C, de Sèze O, Belon P. What is the effect of acetylsalicylic acid at ultra low dose on the interaction platelets/vessel wall? Thromb Res. 1990;60:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Eizayaga FX, Aguejouf O, Desplat V, Belon P, Doutremepuich C. Modifications produced by indomethacin and L-NAME in the effect of ultralow-dose aspirin on platelet activity in portal hypertension. Pathophysiol Haemost Thromb. 2006;35:357-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Taubert D, Berkels R, Grosser N, Schröder H, Gründemann D, Schömig E. Aspirin induces nitric oxide release from vascular endothelium: a novel mechanism of action. Br J Pharmacol. 2004;143:159-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Lalanne MC, Ramboer I, de Sèze O, Doutremepuich C. In vitro platelets/endothelial cells interactions in presence of acetylsalicylic acid at various dosages. Thromb Res. 1992;65:33-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Eizayaga FX, Aguejouf O, Belon P, Doutremepuich C. Platelet aggregation in portal hypertension and its modification by ultra-low doses of aspirin. Pathophysiol Haemost Thromb. 2005;34:29-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Ackerman Z, Karmeli F, Rachmilewitz D. Longitudinal prostaglandin E(2) generation in various organs during evolution of experimental portal hypertension. Prostaglandins Leukot Essent Fatty Acids. 2002;67:197-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Fabre JE, Nguyen M, Athirakul K, Coggins K, McNeish JD, Austin S, Parise LK, FitzGerald GA, Coffman TM, Koller BH. Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. J Clin Invest. 2001;107:603-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Doutremepuich C, Aguejouf O, Pintigny D, Sertillanges MN, De Seze O. Thrombogenic properties of ultra-low-dose of acetylsalicylic acid in a vessel model of laser-induced thrombus formation. Thromb Res. 1994;76:225-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Teng XW, Davies NM. High-performance liquid chromatographic analysis of a selective cyclooxygenase-1 inhibitor SC-560 in rat serum: application to pharmacokinetic studies. J Pharm Biomed Anal. 2004;35:1143-1147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Wu Y, Burns C, Campbell KA, Sitzmann JV. Systemic and portal prostacyclin and thromboxane response to hemorrhage in portal hypertension. Shock. 1994;2:68-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Wu ZY, Chen XS, Qiu JF, Cao H. Role of PGI2 in the formation and maintenance of hyperdynamic circulatory state of portal hypertensive rats. World J Gastroenterol. 2005;11:752-755. [PubMed] [Cited in This Article: ] |
| 16. | Potenza MA, Botrugno OA, De Salvia MA, Lerro G, Nacci C, Marasciulo FL, Andriantsitohaina R, Mitolo-Chieppa D. Endothelial COX-1 and -2 differentially affect reactivity of MVB in portal hypertensive rats. Am J Physiol Gastrointest Liver Physiol. 2002;283:G587-G594. [PubMed] [Cited in This Article: ] |
| 17. | Huang HC, Wang SS, Chen YC, Lee FY, Chang FY, Lin HC, Hou MC, Chan CC, Chen CT, Wu SL. Cyclooxygenase expression in splanchnic hyposensitivity to glypressin of bleeding portal hypertensive rats. Eur J Clin Invest. 2003;33:505-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Hou MC, Cahill PA, Zhang S, Wang YN, Hendrickson RJ, Redmond EM, Sitzmann JV. Enhanced cyclooxygenase-1 expression within the superior mesenteric artery of portal hypertensive rats: role in the hyperdynamic circulation. Hepatology. 1998;27:20-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Bouaziz A, de Ficquelmont-Loïzos MM, Richert A, Caprani A. Direct physical factors and PGI2 and TXA2 secretions by a human endothelial cell line: in vitro investigation of pressure and shear stress applied independently or in synergy. Thromb Res. 1998;90:279-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Yokoyama Y, Toth B, Kitchens WC, Schwacha MG, Bland KI, Chaudry IH. Role of thromboxane in producing portal hypertension following trauma-hemorrhage. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1293-G1299. [PubMed] [Cited in This Article: ] |
| 21. | Bezugla Y, Kolada A, Kamionka S, Bernard B, Scheibe R, Dieter P. COX-1 and COX-2 contribute differentially to the LPS-induced release of PGE2 and TxA2 in liver macrophages. Prostaglandins Other Lipid Mediat. 2006;79:93-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Wun T, Paglieroni T, Lachant NA. Physiologic concentrations of arginine vasopressin activate human platelets in vitro. Br J Haematol. 1996;92:968-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Huang HC, Wang SS, Chen YC, Lee FY, Chang FY, Lin HC, Hou MC, Chang CC, Lee SD. Chronic cyclooxygenase blockade enhances the vasopressin responsiveness in collaterals of portal hypertensive rats. Scand J Gastroenterol. 2006;41:1440-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Buerkle MA, Lehrer S, Sohn HY, Conzen P, Pohl U, Krötz F. Selective inhibition of cyclooxygenase-2 enhances platelet adhesion in hamster arterioles in vivo. Circulation. 2004;110:2053-2059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |