Published online Sep 28, 2007. doi: 10.3748/wjg.v13.i36.4824
Revised: July 2, 2007
Accepted: July 9, 2007
Published online: September 28, 2007
Hepatitis C virus (HCV) is a major cause of hepatitis world-wide. The majority of infected individuals develop chronic hepatitis which can then progress to liver cirrhosis and hepatocellular carcinoma. Spontaneous viral clearance occurs in about 20%-30% of acutely infected individuals and results in resolution of infection without sequaelae. Both viral and host factors appear to play an important role for resolution of acute infection. A large body of evidence suggests that a strong, multispecific and long-lasting cellular immune response appears to be important for control of viral infection in acute hepatitis C. Due too the lack of convenient neutralization assays, the impact of neutralizing responses for control of viral infection had been less defined. In recent years, the development of robust tissue culture model systems for HCV entry and infection has finally allowed study of antibody-mediated neutralization and to gain further insights into viral targets of host neutralizing responses. In addition, detailed analysis of antibody-mediated neutralization in individual patients as well as cohorts with well defined viral isolates has enabled the study of neutralizing responses in the course of HCV infection and characterization of the impact of neutralizing antibodies for control of viral infection. This review will summarize recent progress in the understanding of the molecular mechanisms of antibody-mediated neutralization and its impact for HCV pathogenesis.
- Citation: Zeisel MB, Fafi-Kremer S, Fofana I, Barth H, Stoll-Keller F, Doffoël M, Baumert TF. Neutralizing antibodies in hepatitis C virus infection. World J Gastroenterol 2007; 13(36): 4824-4830
- URL: https://www.wjgnet.com/1007-9327/full/v13/i36/4824.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i36.4824
With an estimated 170 million infected individuals, hepatitis C virus (HCV) has a major impact on public health[1]. HCV is a major cause of hepatitis world-wide. The majority of infected individuals develop chronic hepatitis which can then progress to liver cirrhosis and hepatocellular carcinoma. Treatment options for chronic HCV infection are limited and a vaccine to prevent HCV infection is not available.
HCV is a small enveloped positive-strand RNA virus that belongs to the genus Hepacivirus of the Flaviviridae family. This virus exhibits high genetic heterogeneity and has been classified into six genotypes and several subtypes. The HCV genome encodes a single precursor polyprotein of about 3000 amino acids that is cleaved co- and post-translationally by host and viral proteases into functional structural and non-structural proteins. The virion is composed of three different structural proteins: The core protein forming the viral nucleocapsid and two envelope glycoproteins, E1 and E2.
In vivo, HCV infects only humans and chimpanzees[2]. Each individual is infected with a mixture of distinct but closely related HCV genomes, termed quasispecies. The liver is the primary target organ of HCV, and the hepatocyte is its primary target cell. Replication of the HCV genome has been demonstrated in vivo and in vitro in liver hepatocytes, and hematopoietic cells including dendritic cells and B lymphocytes[3,4]. HCV establishes persistent infection in the majority of infected individuals despite the fact that it is recognized and targeted by the host’s immune system[5].
Viral proteins are recognized as non-self by the host’s immune system and induce the production of antibodies. During the natural course of infection, a large number of antibodies targeting epitopes of both structural and non-structural proteins are produced. The vast majority of antibodies induced have no antiviral activity, either because they are elicited by intracellular, degraded or incompletely processed proteins released from dying cells or because they are directed against epitopes that do not play any role in the virus entry process[6,7]. A small proportion of antibodies termed “neutralizing antibodies” are able to target exposed epitopes of the viral structural proteins and neutralize the infectious virus by preventing or controlling viral infection. This review will summarize the current knowledge about host neutralizing responses in HCV infection. It starts with a brief description of the current model systems allowing the study of neutralizing responses, followed by viral targets of neutralizing antibodies. Finally, neutralizing responses in the course of HCV infection and the impact of neutralizing antibodies for HCV pathogenesis are discussed.
For many years, studies of host neutralizing responses against HCV had been hampered by the lack of a convenient tissue culture system for HCV entry and infection. In recent years, several in vitro models have been developed to study defined aspects of HCV host cell interaction and antibody-mediated virus neutralization: These include recombinant HCV envelope glycoproteins[8,9], HCV-like particles[10], HCV pseudotyped particles[11-13], and, more recently, cell-culture derived infectious HCV[14-16]. Recombinant HCV envelope glycoproteins have been successfully used as a surrogate model to study virus-host cell interaction leading to the identification of putative HCV receptor candidates including CD81[8], scavenger receptor class B typeI(SR-BI)[9] and heparan sulfate[17] as well as antibodies inhibiting cellular binding of envelope glycoproteins[18]. HCV-like particles (HCV-LP) generated by self-assembly of the HCV structural proteins in insect cells have been shown to exhibit morphologic, biophysical, and antigenic properties similar to putative virions isolated from HCV-infected patients[10]. In contrast to individually expressed envelope glycoproteins E1 and E2, E1/E2 heterodimers of HCV-LPs are presumably presented in a native, virion-like conformation. HCV-LPs have been shown to bind and enter human hepatoma cells as well as primary hepatocytes and dendritic cells in a receptor-mediated manner, therefore representing a useful model system for the study of HCV-host cell interaction including the characterization of antibodies interfering with cellular binding of particles[17,19-25].
Retroviral HCV pseudotyped particles (HCVpp) represent a convenient and elegant approach to study viral entry and antibody-mediated neutralization[11,12]. Infectious HCVpp consist of unmodified HCV envelope glycoproteins E1 and E2 assembled onto retroviral or lentiviral core particles[11,12]. HCVpp are produced by transfecting cells with expression vectors encoding the full-length E1/E2 polyprotein, retroviral or lentiviral core proteins, and a packaging-competent retro- or lentiviral genome carrying a marker gene. The presence of a green fluorescent protein or luciferase reporter gene packaged within these HCVpp allows reliable and fast determination of infectivity mediated by the envelope glycoproteins. HCVpp are infectious for certain cell lines of hepatocyte origin, principally Huh-7 cells, as well as for human primary hepatocytes[11,12]. This system has been extremely useful in identifying neutralizing antibodies as well as characterization of the molecular mechanisms of antibody-mediated neutralization[12,25-30]. Vesicular stomatitis viruses (VSV)/HCV pseudotypes expressing HCV E1 or E2 chimeric proteins containing transmembrane and cytoplasmic domains of the VSV G glycoprotein have been developed as another HCV pseudotype model system to study HCV entry and antibody-mediated neutralization[13,31]. VSV/HCV pseudotypes infect human hepatoma cell lines and sera from HCV-infected chimpanzees or humans neutralize the pseudotype virus infectivity[13,32]. In contrast to retroviral HCV pseudotypes demonstrating strong tropism for liver-derived cell lines, VSV/HCV pseudotypes are generated in relatively lower titer and can infect a broad range of mammalian cell lines, including cell lines not derived from the liver.
Most recently, several laboratories succeeded in establishing the efficient production of infectious HCV particles using a unique clone derived from a viral isolate of a Japanese patient with fulminant hepatitis C (JFH-1)[14-16]. Successful infection of naïve Huh-7 and Huh-7-derived hepatoma cells with cell-culture derived HCV (HCVcc) was demonstrated by detection of viral proteins and a highly reproducible time-dependent increase of viral RNA in infected cells[14-16]. Virus production in Huh-7 cells was dependent on an active viral polymerase and expression of a functional viral envelope containing the HCV envelope glycoproteins E1 and E2[14-16]. Inoculation of naïve chimpanzees with JFH-1 or chimeric J6/JFH-1-derived HCV particles synthesized in vitro resulted in viral infection in vivo demonstrating the biological significance of this model system[14,33]. The ability to generate infectious HCVcc of different genotypes – such as the development of chimeric HCVcc or HCVcc derived from HCV genotype prototype 1a strain H77 certainly improves the scope of the cell culture system for HCV infection[34,35]. Infection of HCVcc has been shown to be efficiently neutralized by anti-HCV antibodies derived from human sera[14] as well as polyclonal anti-envelope antibodies[34].
The chimpanzee remains the only natural occurring animal model for the study of HCV infection in vivo. The clinical course of infection is usually milder in chimpanzees than in humans. However, these animals have provided unique opportunities to study adaptive immune responses to HCV[36]. Using the chimpanzee model, antibodies with neutralizing properties have first been described[37,38]. These antibodies were directed against epitopes in the envelope glycoprotein E2 hypervariable region 1 (HVR-1) of HCV and appeared to be isolate-specific. The chimpanzee model has also been used to study protective immunity against re-exposure. Vaccination studies[39] and passive immunization with rabbit anti-sera[38] have shown some protection but infection of chimpanzees with HCV does not provide complete protective immunity against re-infection with homologous or heterologous virus[40-44].
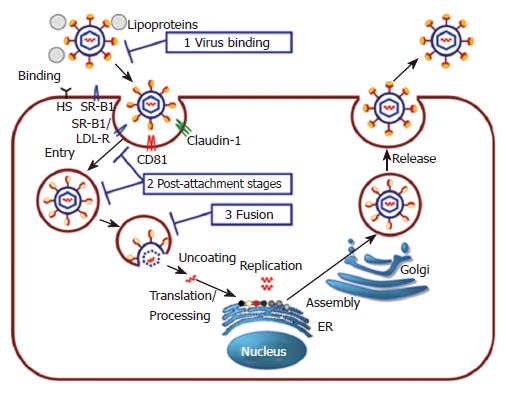
In recent years, rapid progress has been made in the understanding of the molecular mechanisms of HCV life cycle (Figure 1). Attachment of the virus to the target cell is mediated through binding of HCV envelope glycoproteins to binding factors present on the host cell surface, such as the glycosaminoglycan heparan sulfate[17,25]. Binding and entry of HCV is believed to be a multistep process involving several attachment and entry factors, such as CD81, SR-BI and claudin-1[45-47]. HCV is most certainly internalized in a clathrin-dependent manner and HCV genome delivery into the host cell cytosol prior to HCV replication is pH dependent[48-51]. In analogy to other viral infections, antibodies neutralizing HCV may render virions non-infectious by interfering with different steps of the viral life cycle[52,53]. Binding of the antibodies to the virus may directly block attachment of the virus with the host cell and thus inhibit dissemination of infection. Neutralizing antibodies may also interfere with post-binding steps such as interaction of the virus with host entry factors. If endocytosis is an obligate replicative step, internalization of the virus into the host cell by endocytosis may also cause neutralization. Neutralization of viruses by antibodies may also take place during fusion at the cell surface or in endosomes: Neutralizing antibodies may directly interfere with the fusogenic protein, hinder conformational changes necessary for the fusion process or simply obstruct contact between cellular and viral membranes. In addition, neutralizing antibodies may also interfere with viral uncoating or the first steps necessary for viral replication (Figure 1). The identification and characterization of antibodies targeting distinct steps of viral entry is thus an important strategy for the understanding of the molecular mechanisms of antibody-mediated neutralization.
Using the above described model systems, it could be demonstrated that envelope glycoproteins E1 and E2 are critical for host cell entry and thus represent important targets for virus neutralization. Monoclonal or polyclonal antibodies targeting both linear and conformational epitopes of envelope glycoprotein E2 have been shown to inhibit cellular binding of HCV-LP binding, entry of HCVpp and infection of HCVcc (Table 1)[10-12,14-16,19,20]. Several viral epitopes targeted by neutralizing antibodies have already been identified: epitopes of the E2 HVR-1 region (aa 384-410)[12,18,27], two epitopes adjacent to the N-terminal HVR-1 region (aa 408-422 and aa 412-419)[22,54,55], the E2 CD81 binding region (aa 474-494 and aa 522-551)[12,54,56,57] and conformational epitopes within glycoprotein E2[58-61]. These epitopes may represent potential candidate targets for antibodies in passive immunoprophylaxis. Indeed, two studies have demonstrated that monoclonal antibodies directed against conformational epitopes[60] or epitope aa 412-423 exhibited broad cross-neutralizing activity among all major genotypes of HCVpp entry[54] as well as HCVcc infectivity[62]. Most recently, at least three epitopes (aa 270-284, 416-430, 600-620) playing a role in membrane fusion processes have been identified in the envelope glycoproteins E1 and E2[63]. Since one epitope (aa 416-430) has been shown to represent a target for monoclonal antibodies efficiently neutralizing HCV infection[54], it is conceivable that membrane fusion may represent another target for anti-HCV antibodies with neutralizing properties.
| Envelopeglycoprotein | Epitope(Amino acids) | Potential function | Model system |
| E1 | |||
| 192-226 | HCV-LP binding[85] | ||
| 197-207 | HCV-LP binding[24] | ||
| 270-284 | Membrane fusion | HCVpp entry[63] | |
| 313-332 | HCV-LP binding[85] | ||
| E2 | |||
| HVR-1 | SR-BI/heparan sulfate binding | Chimpanzee[38] | |
| HVR-1 | SR-BI/heparan sulfate binding | E2 binding, HCVpp entry[18,27] | |
| 396-407 | HCVpp entry[12] | ||
| 408-422 | HCV-LP binding[22] | ||
| 412-419 | HCVpp entry[55] | ||
| 412-423 | HCVpp entry, HCVcc infection[12,57] | ||
| 416-430 | Membrane fusion | HCVpp entry[63] | |
| 432-443 | HCVpp entry[12] | ||
| 436-447 | HCVpp entry[12] | ||
| 474-494 | CD81 binding | E2 binding, HCVpp entry[56,86] | |
| 522-551 | CD81 binding | E2 binding, HCVpp entry[56,86] | |
| 600-620 | Membrane fusion | HCVpp entry[63] | |
| 640-653 | HCV-LP binding[24] | ||
| 644-655 | HCVpp entry[12] | ||
| CD | E2 binding, HCVpp entry[58-61] |
HCV RNA is detectable already one week following infection. Despite the rapid onset of viral replication, there is a delay in the appearance of HCV-specific T-lymphocytes and HCV-specific antibodies which only appear several weeks after infection. Patients who spontaneously clear HCV infection have been described to mount a vigorous multi-epitope-specific CD4 and CD8 T-cell response[64,65]. Antibody-mediated neutralization occurs during HCV infection in vivo but the role of antibodies for the control of HCV infection has been difficult to study. Antibody-mediated neutralization has been suggested by study of patients undergoing liver transplantation for HCV- and hepatitis B virus (HBV)-related liver cirrhosis. Infusion of anti-HBs hyperimmune globulin containing anti-HCV appeared to reduce HCV infection in the transplanted liver[66]. In addition, HCV-infected patients with primary antibody deficiencies have been reported to have accelerated rates of disease progression[67,68]. Moreover, passive protection against HCV has been demonstrated in a cohort of patients that had been administered immunoglobulin preparations derived from HCV RNA-positive plasma but containing HCV-neutralizing antibodies[69]. However, in the majority of patients, HCV infection is established despite the induction of an humoral immune response that targets various epitopes of the HCV envelope glycoproteins[22,26,28,70,71].
Until recently, functional studies analyzing the neutralizing antibody response during acute and chronic HCV infection using HCV model systems demonstrated a lack of neutralizing antibodies in the majority of patients with acute HCV infection[22,26,70,72]. These studies were limited by the fact that the viral surrogate ligand was derived from a different isolate than the virus present in the infected patient thus precluding the detection of isolate-specific antibodies. Most recently, studies using well defined nosocomial or single-source HCV outbreaks with a defined inoculum enabled to study the role of isolate-specific neutralizing antibodies for control of HCV infection in humans. Using the HCVpp model system, two studies have demonstrated that neutralizing antibodies are induced in the early phase of infection by patients who subsequently clear the virus[29] or control viral infection[73]. In a well characterized single-source outbreak of hepatitis C, viral clearance was associated with a rapid induction of neutralizing antibodies in the early phase of infection. In contrast, chronic HCV infection was characterized by absent or low-titer neutralizing antibodies in the early phase of infection[29]. In addition, patients with resolution of infection were shown to exhibit a broader cross-neutralizing activity of antibodies in the early phase of infection. An impaired ability to cross-neutralize viral variants rapidly emerging during acute infection may thus contribute to viral evasion from neutralizing responses in persistent HCV infection[29]. These results suggest that a strong early broad neutralizing antibody response may contribute to control of HCV in the acute phase of infection and assist cellular immune responses in viral clearance. This conclusion is further supported by recent findings for HIV demonstrating that neutralizing antibodies act in concert with antiviral cellular responses for control of HIV infection[74-76]. Furthermore, experimental data obtained in animal models have demonstrated that immune control of poorly cytopathic viruses, such as lymphocytic choriomeningitis virus (LCMV) or simian immunodeficiency virus requires a collaboration of both the cellular and humoral arms of the immune system[7,77]. Indeed, gene-targeted mice expressing the immunoglobulin heavy chain of virus-neutralizing antibodies exhibit an accelerated LCMV clearance[75]. Applying these findings to HCV infection- another prototype of persistent-prone non-cytopathic viruses- it is conceivable, that both cellular[5,64,65,78,79] and neutralizing responses[29,73] may contribute to control of HCV infection during the very early phase of viral infection.
Patients who do not clear the virus develop high-titer and even cross-neutralizing antibodies during the chronic phase of infection[14,22,26,29,70,80]. Paradoxically, these antibodies are not able to control HCV infection. Viral escape from antibody-mediated neutralization in these patients may occur on several levels: (1) HCV exists as a quasispecies with distinct viral variants in infected individuals changing constantly over time and his variability has been shown to represent a mechanism of escape from antibody-mediated neutralization in the chimpanzee model[26]; (2) the interplay of HCV glycoproteins with high-density lipoprotein and SR-BI has been shown to mediate protection from neutralizing antibodies present in sera of acute and chronic HCV-infected patients[27,74]; (3) as shown for other viruses such as human immunodeficiency virus (HIV), escape from neutralizing antibodies may occur through a combination of different mechanisms, for instance point mutations, insertions/deletions or changes in glycosylation patterns of the viral envelope[30,81] or conformational masking of receptor binding sites following envelope-antibody interaction[82] preventing neutralizing antibody binding[83].
Most recently, it has been shown for a chronic HCV patient who has been meticulously followed-up for 30 years that HCV continuously escapes the host’s immune system by repeated mutational changes resulting in loss of recognition of the HCV envelope glycoproteins by antibodies[80]. In fact, neutralization of heterologous strains does not reflect neutralization of the viral variants present in the patient’s serum at the time of sampling[80]. These data suggest that the neutralizing antibody response of the host lags behind the rapidly evolving HCV envelope glycoprotein sequences of the quasispecies population. The fact that envelope glycoprotein sequences and neutralizing antibody specificity change over time suggest that neutralizing antibodies exert selective pressure on HCV evolution. In line with this hypothesis, it has been shown that HCV quasispecies complexity is associated with the inability to clear HCV infection and development of chronic disease[84].
The development of robust tissue culture model systems for HCV infection within recent years has finally allowed for the study of antibody-mediated neutralization in HCV infection. Rapid progress has since then been made in determining the kinetic and targets of host neutralizing responses in the course of HCV infection. The novel model systems and patient cohorts with well defined viral isolates will now allow the identification of the molecular mechanisms of antibody-mediated neutralization as well as mechanisms of viral escape from host neutralizing responses. The elucidation of these mechanisms will be crucial for the understanding of HCV pathogenesis as well as the development of novel preventive and therapeutic strategies for control of HCV infection.
S- Editor Ma N L- Editor Alpini GD E- Editor Yin DH
| 1. | Chisari FV. Unscrambling hepatitis C virus-host interactions. Nature. 2005;436:930-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 175] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Lindenbach BD, Thiel HJ and Rice CM. Flaviviridae: the viruses and their replication. Fields Virology. Philadelphia: Lippincott-Raven 2007; 1101-1152. [Cited in This Article: ] |
| 3. | Sung VM, Shimodaira S, Doughty AL, Picchio GR, Can H, Yen TS, Lindsay KL, Levine AM, Lai MM. Establishment of B-cell lymphoma cell lines persistently infected with hepatitis C virus in vivo and in vitro: the apoptotic effects of virus infection. J Virol. 2003;77:2134-2146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Goutagny N, Fatmi A, De Ledinghen V, Penin F, Couzigou P, Inchauspé G, Bain C. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J Infect Dis. 2003;187:1951-1958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1202] [Cited by in F6Publishing: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 6. | Parren PW, Burton DR. The antiviral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 208] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 8. | Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G. Binding of hepatitis C virus to CD81. Science. 1998;282:938-941. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1572] [Cited by in F6Publishing: 1521] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 9. | Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017-5025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 888] [Cited by in F6Publishing: 868] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 10. | Baumert TF, Ito S, Wong DT, Liang TJ. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J Virol. 1998;72:3827-3836. [PubMed] [Cited in This Article: ] |
| 11. | Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197:633-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 885] [Cited by in F6Publishing: 868] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 12. | Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci USA. 2003;100:7271-7276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 655] [Cited by in F6Publishing: 636] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 13. | Lagging LM, Meyer K, Owens RJ, Ray R. Functional role of hepatitis C virus chimeric glycoproteins in the infectivity of pseudotyped virus. J Virol. 1998;72:3539-3546. [PubMed] [Cited in This Article: ] |
| 14. | Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami M. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2241] [Cited by in F6Publishing: 2239] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 15. | Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1843] [Cited by in F6Publishing: 1817] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 16. | Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294-9299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1427] [Cited by in F6Publishing: 1441] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 17. | Barth H, Schafer C, Adah MI, Zhang F, Linhardt RJ, Toyoda H, Kinoshita-Toyoda A, Toida T, Van Kuppevelt TH, Depla E. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J Biol Chem. 2003;278:41003-41012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 368] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 18. | Rosa D, Campagnoli S, Moretto C, Guenzi E, Cousens L, Chin M, Dong C, Weiner AJ, Lau JY, Choo QL. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA. 1996;93:1759-1763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 268] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Barth H, Cerino R, Arcuri M, Hoffmann M, Schürmann P, Adah MI, Gissler B, Zhao X, Ghisetti V, Lavezzo B. Scavenger receptor class B type I and hepatitis C virus infection of primary tupaia hepatocytes. J Virol. 2005;79:5774-5785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Wellnitz S, Klumpp B, Barth H, Ito S, Depla E, Dubuisson J, Blum HE, Baumert TF. Binding of hepatitis C virus-like particles derived from infectious clone H77C to defined human cell lines. J Virol. 2002;76:1181-1193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Barth H, Ulsenheimer A, Pape GR, Diepolder HM, Hoffmann M, Neumann-Haefelin C, Thimme R, Henneke P, Klein R, Paranhos-Baccalà G. Uptake and presentation of hepatitis C virus-like particles by human dendritic cells. Blood. 2005;105:3605-3614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Steinmann D, Barth H, Gissler B, Schürmann P, Adah MI, Gerlach JT, Pape GR, Depla E, Jacobs D, Maertens G. Inhibition of hepatitis C virus-like particle binding to target cells by antiviral antibodies in acute and chronic hepatitis C. J Virol. 2004;78:9030-9040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Triyatni M, Saunier B, Maruvada P, Davis AR, Ulianich L, Heller T, Patel A, Kohn LD, Liang TJ. Interaction of hepatitis C virus-like particles and cells: a model system for studying viral binding and entry. J Virol. 2002;76:9335-9344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Triyatni M, Vergalla J, Davis AR, Hadlock KG, Foung SK, Liang TJ. Structural features of envelope proteins on hepatitis C virus-like particles as determined by anti-envelope monoclonal antibodies and CD81 binding. Virology. 2002;298:124-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Barth H, Schnober EK, Zhang F, Linhardt RJ, Depla E, Boson B, Cosset FL, Patel AH, Blum HE, Baumert TF. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J Virol. 2006;80:10579-10590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Bartosch B, Bukh J, Meunier JC, Granier C, Engle RE, Blackwelder WC, Emerson SU, Cosset FL, Purcell RH. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci USA. 2003;100:14199-14204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Bartosch B, Verney G, Dreux M, Donot P, Morice Y, Penin F, Pawlotsky JM, Lavillette D, Cosset FL. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol. 2005;79:8217-8229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 28. | Meunier JC, Engle RE, Faulk K, Zhao M, Bartosch B, Alter H, Emerson SU, Cosset FL, Purcell RH, Bukh J. Evidence for cross-genotype neutralization of hepatitis C virus pseudo-particles and enhancement of infectivity by apolipoprotein C1. Proc Natl Acad Sci USA. 2005;102:4560-4565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 29. | Pestka JM, Zeisel MB, Bläser E, Schürmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci USA. 2007;104:6025-6030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 417] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 30. | Helle F, Goffard A, Morel V, Duverlie G, McKeating J, Keck ZY, Foung S, Penin F, Dubuisson J, Voisset C. The neutralizing activity of anti-hepatitis C virus antibodies is modulated by specific glycans on the E2 envelope protein. J Virol. 2007;81:8101-8111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 31. | Buonocore L, Blight KJ, Rice CM, Rose JK. Characterization of vesicular stomatitis virus recombinants that express and incorporate high levels of hepatitis C virus glycoproteins. J Virol. 2002;76:6865-6872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Meyer K, Beyene A, Bowlin TL, Basu A, Ray R. Coexpression of hepatitis C virus E1 and E2 chimeric envelope glycoproteins displays separable ligand sensitivity and increases pseudotype infectious titer. J Virol. 2004;78:12838-12847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, McKeating JA, Lanford RE, Feinstone SM, Major ME, Leroux-Roels G. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci USA. 2006;103:3805-3809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 34. | Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, Abid K, Negro F, Dreux M, Cosset FL. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci USA. 2006;103:7408-7413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 602] [Cited by in F6Publishing: 593] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 35. | Yi M, Villanueva RA, Thomas DL, Wakita T, Lemon SM. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc Natl Acad Sci USA. 2006;103:2310-2315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 327] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 36. | Bukh J. A critical role for the chimpanzee model in the study of hepatitis C. Hepatology. 2004;39:1469-1475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Farci P, Alter HJ, Wong DC, Miller RH, Govindarajan S, Engle R, Shapiro M, Purcell RH. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci USA. 1994;91:7792-7796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 364] [Cited by in F6Publishing: 375] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 38. | Farci P, Shimoda A, Wong D, Cabezon T, De Gioannis D, Strazzera A, Shimizu Y, Shapiro M, Alter HJ, Purcell RH. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci USA. 1996;93:15394-15399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 447] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 39. | Choo QL, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 413] [Cited by in F6Publishing: 421] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 40. | Farci P, Alter HJ, Govindarajan S, Wong DC, Engle R, Lesniewski RR, Mushahwar IK, Desai SM, Miller RH, Ogata N. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992;258:135-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 546] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 41. | Prince AM, Brotman B, Huima T, Pascual D, Jaffery M, Inchauspé G. Immunity in hepatitis C infection. J Infect Dis. 1992;165:438-443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 167] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Bassett SE, Guerra B, Brasky K, Miskovsky E, Houghton M, Klimpel GR, Lanford RE. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33:1479-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Major ME, Mihalik K, Puig M, Rehermann B, Nascimbeni M, Rice CM, Feinstone SM. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol. 2002;76:6586-6595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Lanford RE, Guerra B, Chavez D, Bigger C, Brasky KM, Wang XH, Ray SC, Thomas DL. Cross-genotype immunity to hepatitis C virus. J Virol. 2004;78:1575-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | Koutsoudakis G, Kaul A, Steinmann E, Kallis S, Lohmann V, Pietschmann T, Bartenschlager R. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J Virol. 2006;80:5308-5320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 46. | Zeisel MB, Koutsoudakis G, Schnober EK, Haberstroh A, Blum HE, Cosset FL, Wakita T, Jaeck D, Doffoel M, Royer C. Scavenger receptor class B type I is a key host factor for hepatitis C virus infection required for an entry step closely linked to CD81. Hepatology. 2007;46:1722-1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 47. | Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wölk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 930] [Cited by in F6Publishing: 915] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 48. | Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouillé Y. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964-6972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 418] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 49. | Meertens L, Bertaux C, Dragic T. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J Virol. 2006;80:11571-11578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 50. | Codran A, Royer C, Jaeck D, Bastien-Valle M, Baumert TF, Kieny MP, Pereira CA, Martin JP. Entry of hepatitis C virus pseudotypes into primary human hepatocytes by clathrin-dependent endocytosis. J Gen Virol. 2006;87:2583-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Tscherne DM, Jones CT, Evans MJ, Lindenbach BD, McKeating JA, Rice CM. Time- and temperature-dependent activation of hepatitis C virus for low-pH-triggered entry. J Virol. 2006;80:1734-1741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 52. | Barth H, Liang TJ, Baumert TF. Hepatitis C virus entry: molecular biology and clinical implications. Hepatology. 2006;44:527-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 53. | Klasse PJ, Sattentau QJ. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol. 2002;83:2091-2108. [PubMed] [Cited in This Article: ] |
| 54. | Owsianka A, Tarr AW, Juttla VS, Lavillette D, Bartosch B, Cosset FL, Ball JK, Patel AH. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol. 2005;79:11095-11104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 234] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 55. | Zhang P, Wu CG, Mihalik K, Virata-Theimer ML, Yu MY, Alter HJ, Feinstone SM. Hepatitis C virus epitope-specific neutralizing antibodies in Igs prepared from human plasma. Proc Natl Acad Sci U S A. 2007;104:8449-8454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Zhang J, Randall G, Higginbottom A, Monk P, Rice CM, McKeating JA. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J Virol. 2004;78:1448-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 57. | Owsianka AM, Timms JM, Tarr AW, Brown RJ, Hickling TP, Szwejk A, Bienkowska-Szewczyk K, Thomson BJ, Patel AH, Ball JK. Identification of conserved residues in the E2 envelope glycoprotein of the hepatitis C virus that are critical for CD81 binding. J Virol. 2006;80:8695-8704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 58. | Habersetzer F, Fournillier A, Dubuisson J, Rosa D, Abrignani S, Wychowski C, Nakano I, Trépo C, Desgranges C, Inchauspé G. Characterization of human monoclonal antibodies specific to the hepatitis C virus glycoprotein E2 with in vitro binding neutralization properties. Virology. 1998;249:32-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Keck ZY, Op De Beeck A, Hadlock KG, Xia J, Li TK, Dubuisson J, Foung SK. Hepatitis C virus E2 has three immunogenic domains containing conformational epitopes with distinct properties and biological functions. J Virol. 2004;78:9224-9232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Schofield DJ, Bartosch B, Shimizu YK, Allander T, Alter HJ, Emerson SU, Cosset FL, Purcell RH. Human monoclonal antibodies that react with the E2 glycoprotein of hepatitis C virus and possess neutralizing activity. Hepatology. 2005;42:1055-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Op De Beeck A, Voisset C, Bartosch B, Ciczora Y, Cocquerel L, Keck Z, Foung S, Cosset FL, Dubuisson J. Characterization of functional hepatitis C virus envelope glycoproteins. J Virol. 2004;78:2994-3002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 62. | Tarr AW, Owsianka AM, Timms JM, McClure CP, Brown RJ, Hickling TP, Pietschmann T, Bartenschlager R, Patel AH, Ball JK. Characterization of the hepatitis C virus E2 epitope defined by the broadly neutralizing monoclonal antibody AP33. Hepatology. 2006;43:592-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Lavillette D, Pécheur EI, Donot P, Fresquet J, Molle J, Corbau R, Dreux M, Penin F, Cosset FL. Characterization of fusion determinants points to the involvement of three discrete regions of both E1 and E2 glycoproteins in the membrane fusion process of hepatitis C virus. J Virol. 2007;81:8752-8765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 64. | Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, Jung MC, Eichenlaub D, Pape GR. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 532] [Cited by in F6Publishing: 544] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 65. | Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395-1406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 899] [Cited by in F6Publishing: 884] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 66. | Féray C, Gigou M, Samuel D, Ducot B, Maisonneuve P, Reynès M, Bismuth A, Bismuth H. Incidence of hepatitis C in patients receiving different preparations of hepatitis B immunoglobulins after liver transplantation. Ann Intern Med. 1998;128:810-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Chapel HM, Christie JM, Peach V, Chapman RW. Five-year follow-up of patients with primary antibody deficiencies following an outbreak of acute hepatitis C. Clin Immunol. 2001;99:320-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Christie JM, Healey CJ, Watson J, Wong VS, Duddridge M, Snowden N, Rosenberg WM, Fleming KA, Chapel H, Chapman RW. Clinical outcome of hypogammaglobulinaemic patients following outbreak of acute hepatitis C: 2 year follow up. Clin Exp Immunol. 1997;110:4-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Yu MY, Bartosch B, Zhang P, Guo ZP, Renzi PM, Shen LM, Granier C, Feinstone SM, Cosset FL, Purcell RH. Neutralizing antibodies to hepatitis C virus (HCV) in immune globulins derived from anti-HCV-positive plasma. Proc Natl Acad Sci USA. 2004;101:7705-7710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci USA. 2004;101:10149-10154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 333] [Cited by in F6Publishing: 324] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 71. | Hadlock KG, Gish R, Rowe J, Rajyaguru SS, Newsom M, Warford A, Foung SK. Cross-reactivity and clinical impact of the antibody response to hepatitis C virus second envelope glycoprotein (E2). J Med Virol. 2001;65:23-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Netski DM, Mosbruger T, Depla E, Maertens G, Ray SC, Hamilton RG, Roundtree S, Thomas DL, McKeating J, Cox A. Humoral immune response in acute hepatitis C virus infection. Clin Infect Dis. 2005;41:667-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 73. | Lavillette D, Morice Y, Germanidis G, Donot P, Soulier A, Pagkalos E, Sakellariou G, Intrator L, Bartosch B, Pawlotsky JM. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J Virol. 2005;79:6023-6034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 209] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 74. | Dreux M, Pietschmann T, Granier C, Voisset C, Ricard-Blum S, Mangeot PE, Keck Z, Foung S, Vu-Dac N, Dubuisson J. High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J Biol Chem. 2006;281:18285-18295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 75. | Hangartner L, Senn BM, Ledermann B, Kalinke U, Seiler P, Bucher E, Zellweger RM, Fink K, Odermatt B, Bürki K. Antiviral immune responses in gene-targeted mice expressing the immunoglobulin heavy chain of virus-neutralizing antibodies. Proc Natl Acad Sci USA. 2003;100:12883-12888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Igarashi T, Brown C, Azadegan A, Haigwood N, Dimitrov D, Martin MA, Shibata R. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell-free virions from blood plasma. Nat Med. 1999;5:211-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 139] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 77. | Ciurea A, Klenerman P, Hunziker L, Horvath E, Senn BM, Ochsenbein AF, Hengartner H, Zinkernagel RM. Viral persistence in vivo through selection of neutralizing antibody-escape variants. Proc Natl Acad Sci USA. 2000;97:2749-2754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 78. | Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 563] [Cited by in F6Publishing: 548] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 79. | Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 607] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 80. | von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 81. | Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1944] [Cited by in F6Publishing: 1879] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 82. | Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 719] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 83. | Srivastava IK, Ulmer JB, Barnett SW. Role of neutralizing antibodies in protective immunity against HIV. Hum Vaccin. 2005;1:45-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, Strazzera A, Chien DY, Munoz SJ, Balestrieri A. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288:339-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 680] [Cited by in F6Publishing: 632] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 85. | Fournillier A, Wychowski C, Boucreux D, Baumert TF, Meunier JC, Jacobs D, Muguet S, Depla E, Inchauspé G. Induction of hepatitis C virus E1 envelope protein-specific immune response can be enhanced by mutation of N-glycosylation sites. J Virol. 2001;75:12088-12097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |